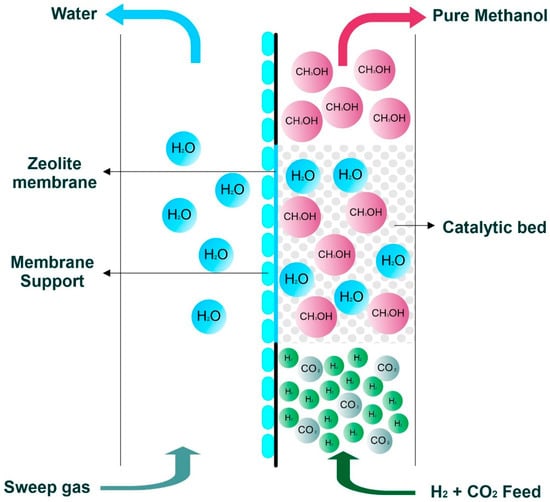
Clean methanol can play an important role in achieving net zero emission targets by decarbonizing the energy and chemical sectors. Conventionally, methanol is produced by using fossil fuel as raw material, which releases a significant amount of greenhouse gases (GHGs) into the environment. Clean methanol, which is produced by hydrogen (H2) from renewable sources (green H2) and captured carbon dioxide (CO2), is totally free from the influence of fossil fuel. Due to its vast applications, clean methanol has potential to substitute for fossil fuels while preventing further GHGs emissions. Renewable energy sources such as solar, wind and biomass should be utilized for producing green H2, while CO2 captured from air, and more likely from point emission sources, can be recycled to produce clean methanol. After producing methanol from CO2 and H2, the removal of by-product water by distillation is a big challenge due its high energy consumption. An alternative approach for this methanol-water separation is membrane technology, which is an energy saving option. Water-selective zeolite membranes can separate water post-synthesis, as well as during the synthesis. Production efficiency of methanol can be enhanced by utilizing zeolite membranes inside the methanol synthesis reactor. Furthermore, CO2 conversion as well as methanol selectivity, purity and yield can also be increased significantly by selectively removing by-product water using a zeolite membrane reactor.
- methanol
- carbon dioxide
- hydrogenation
- zeolite
- membrane reactor
1. Feasibility of Methanol as Clean Source of Energy
2. Key Ingredients for Clean Methanol
2.1. Production of Green Hydrogen
About 95% of global hydrogen (H
2
) is produced from fossil fuel sources, such as natural gas, coal and oil. The production of H
2
has been classified into grey, blue and green H
2
, based on source and production strategy. Grey H
2 is produced by steam reforming of fossil fuel and termed as “grey” due to substantial GHGs emissions during the process [47]. The source of blue H
is produced by steam reforming of fossil fuel and termed as “grey” due to substantial GHGs emissions during the process [89]. The source of blue H
2
is also fossil fuel, but the production process must be equipped with efficient CO
2
capturing facility (
~100%) and have minimal GHGs impact on environment [48]. Green H
100%) and have minimal GHGs impact on environment [90]. Green H
2 is a totally environmentally-friendly process with zero GHGs emissions [49][50], produced by water electrolysis. The electrolyzer must be powered with renewable energy sources to avoid any GHG emission into the atmosphere during its entire production process [51]. This green H
is a totally environmentally-friendly process with zero GHGs emissions [91,92], produced by water electrolysis. The electrolyzer must be powered with renewable energy sources to avoid any GHG emission into the atmosphere during its entire production process [93]. This green H
2 is the key approach towards achieving zero emission target of 2050 set by global climate communities. However, despite of the zero emissions from electrolysis operation, an electrolyzer manufacturing process could inevitably lead to GHG emissions, particularly if critical way materials are used, which should be taken into account for net zero emission target [52].
is the key approach towards achieving zero emission target of 2050 set by global climate communities. However, despite of the zero emissions from electrolysis operation, an electrolyzer manufacturing process could inevitably lead to GHG emissions, particularly if critical way materials are used, which should be taken into account for net zero emission target [94].
The PEMEL and AEL are low temperature electrolysis strategies to split water in acidic and alkaline media, respectively. In PEMEL, water is fed at the anode and oxidized to OSolid oxide electrolysis (SOEL) is performed in a solid oxide electrolysis cell (SOEC) which works on the reverse principle of a solid oxide fuel cell, used for electricity production from pure H
2
and O
2 [58]. In SOEC, steam is fed at cathode and it undergoes reduction at high temperatures of 600–1000 °C to produce H
[102]. In SOEC, steam is fed at cathode and it undergoes reduction at high temperatures of 600–1000 °C to produce H
2 and oxide ions [59]. Due to the high temperature, oxide ions can travel from cathode to anode via some oxide-conducting electrolyte medium, where it captures an electron from an outer circuit and generates oxygen gas. This process of H
and oxide ions [103]. Due to the high temperature, oxide ions can travel from cathode to anode via some oxide-conducting electrolyte medium, where it captures an electron from an outer circuit and generates oxygen gas. This process of H
2 production has a good efficiency of more than 80% [58], however, it has the drawback of high-temperature operation [60]. To overcome this issue, researchers have made efforts to develop electrolytes for SOEC which can transfer ions at comparatively at low temperatures. Ishihara et al. performed steam electrolysis at 600 °C in SOEC having LaGaO
production has a good efficiency of more than 80% [102], however, it has the drawback of high-temperature operation [104]. To overcome this issue, researchers have made efforts to develop electrolytes for SOEC which can transfer ions at comparatively at low temperatures. Ishihara et al. performed steam electrolysis at 600 °C in SOEC having LaGaO
3
-based electrolytes and successfully achieved H
2 production rate of 70 L/min at 1.8 V of applied potentia [61].
production rate of 70 L/min at 1.8 V of applied potentia [105].
2.1.1. Solar to Hydrogen
Hydrogen (H-holes
(h+)
pairs under sunlight irradiation. These
e−/h+
pairs migrate towards the active sites of respective electrodes to produce H
2
and O
2 [65][66][67]. In spite of direct exposure to sunlight in PC technology, the drawbacks include slow kinetics, unfavorable thermodynamics and some safety issues [68]. For instance, the separation of H
[112,113,114]. In spite of direct exposure to sunlight in PC technology, the drawbacks include slow kinetics, unfavorable thermodynamics and some safety issues [115]. For instance, the separation of H
2
/O
2
from the PC cell is needed to avoid H
2 explosion in contrast with photovoltaic-electrocatalysis (PV-EC) and PEC [69]. Subsequently, research efforts have been focused to overcome these issues for better efficiency of converting solar energy to H
explosion in contrast with photovoltaic-electrocatalysis (PV-EC) and PEC [116]. Subsequently, research efforts have been focused to overcome these issues for better efficiency of converting solar energy to H
2
by improving the semiconductor materials. For example, Wang et al. have developed Pt co-catalyzed BaTaO
2
N photocatalyst that produced 100 times more efficient H
2 production compared to previous studies [70].
production compared to previous studies [117].
2.1.2. Wind to Hydrogen
Solar-driven hydrogen (H2.1.3. Biomass to Hydrogen
2.2. Recycled Carbon Dioxide
The recycled CO2 from direct air capture (DAC) or industrial emission can complete the carbon cycle through methanol production. Based on an analysis report from NOAA’s Global Monitoring Laboratory, the global average atmospheric CO2 reached 414.72 ppm in 2021. It has reached a new high-level record amount of CO2 in spite of decelerated human activities due to COVID-19 pandemic [85][165]. Presently, the average value of CO2 is 417.2 ppm which is more than 50 percent above pre-industrial levels [86][166]. The increasing concentration of CO2 depicts that its natural sinks (that removes CO2 from atmosphere), which are plants and oceans, are not taking out an equivalent amount as that being emitted. This imposes the need of the installation of other CO2 capturing points and at the same time reducing the further emissions to balance the atmospheric concentration. Methanol production from captured CO2 will reduce further emissions in the atmosphere [87][88][17,167].2.2.1. CO2 from Direct Air Captured
CO2 from the atmosphere is directly captured from air using well-developed DAC technology. Currently, there are 19 DAC plants operational worldwide, capturing around 0.01 Mt CO2/year [89][172]. In comparison with the available CO2 in atmosphere and net zero target of 2050, this installed capacity is too low and needs to increase up to 60 Mt CO2/year by the end of 2030. To meet this target, several large capacity DAC plants are under development and will be operational in the near future, including a 1.0 Mt capacity plant in US which will be operational in mid 2020s [90][173]. Technologies used in DAC are based on suitable solid (s-DAC) or liquid (l-DAC) sorbent to selectively extract CO2 from the ambient air [91][174]. Ambient air is brought in contact with these sorbents at certain temperature and pressure, depending upon the physiochemical nature of the sorbent. CO2 is attracted towards the sorbent while leaving behind the rest of the gases. The CO2 rich sorbent is then processed under reverse operating conditions to desorb the attached CO2 and regenerated for the next cycle. The sorption of CO2 on the sorbent can be either chemical (chemisorption) or physical (physiosorption). In chemisorption, CO2 is attached to the sorbent via chemical bonds which require a considerably high level of energy for their regeneration [92][175].2.2.2. CO2 Captured from Industrial Emissions
Currently, there are three main approaches available to capture CO2 from large-scale fossil fueled industrial sectors: (1) post-combustion capture; (2) pre-combustion capture; and (3) oxy-fuel combustion capture. Among all three technologies, post-combustion carbon capture is the oldest one, due to its ease of operation and installation without any significant changes in existing plant [93][168]. This technology is based on removal of CO2 from combustion flue gases, mainly consisting of CO2, O2, N2, H2O, SOx and NOx, depending upon the nature of the fuel burned. Around 10–15% of the total volume of flue gases subjected to post-combustion treatment is CO2 [94][194]. The second technology, pre-combustion carbon capture, refers to the removal of CO2 from fossil fuels before feeding to combustion process [95][195]. In this technology, fossil fuel is first passed through a gasification process to convert it into syngas (CO, H2 and CO2) which is then subjected to water gas shift reaction to convert CO to CO2 and H2. The final mixture of CO2 and H2 having 15 to 50% CO2, is then fed to a CO2 removal process and clean H2 is used for combustion purpose, while rejecting clean flue gases [96][196]. Pre-combustion capture technology utilizes a highly concentrated stream of CO2 for carbon capture and hence can perform more efficient removal. The third technology, namely oxy-fuel combustion is one of the leading technologies for carbon capture and utilizes pure oxygen instead of air for fossil fuel burning [97][197]. An air separation unit separates oxygen from air while leaving behind the major portion of N2. Flue gases produced from this oxy-fuel combustion are more concentrated in CO2, which is then removed from it in a flue gas processing unit [98][198].
2.2.3. CO2 Produced from Biomass
The gasification of biomass is mainly performed to convert it into clean H2 fuel along with a side product, CO2, which is generated in very abundant amount during this process due to the organic nature of subjected biomass. In this scenario, CO2 is captured from biogas in order to produce value-added H2 fuel [99][100][207,208]. Biomass usage is not only limited to H2 production but also used for producing syngas, bio-methane and other light hydrocarbons fuels [101][209]. In any gasification process, excess CO2 must be removed via either in situ or post process techniques, as it imparts negative value to any produced fuel. Dinca et al. produced syngas by biomass gasification while capturing the CO2 by using liquid absorption method [102][210]. Dashtestani et al. used novel Ca/Fe based sorbents for efficient removal of CO2 via calcination-carbonation looping from biomass gasification [103][211]. They also studied the effectiveness of sorbent material over number of regeneration cycles, which is very important stability criteria for a newly developed sorbent.
In view of this, CO2 captured from its different point sources or directly from air can contribute to the circular economy by producing synthetic fuels such as methanol [104][216], as shown in Figure 14.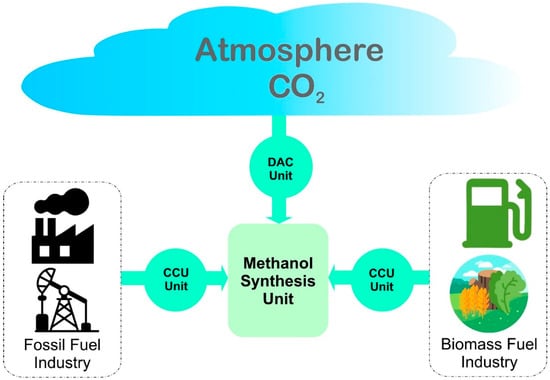
3. Production of Clean Methanol
An important method for the production of e-methanol by utilizing these sources, is nameed heterogeneous catalysis. E-methanol is produced by reacting green H2 with captured CO2 from industrial sources or ambient air [6][42]. Because of the green H2 from renewable energy and captured CO2, e-methanol is an alternative and net-carbon-neutral fuel. There are several processing routes for producing methanol from captured CO2, including a well mature catalytic hydrogenation of CO2 with green H2, which is responsible for e-methanol production [105][217]. In catalytic hydrogenation of CO2, both gases are processed over Cu/Zn based bimetallic or other multi-component catalysts at certain operating conditions. The operating conditions vary depending upon the nature and composition of catalyst employed. The normal operating conditions were in the range of 200–250 °C temperature and 3–10 MPa of pressure. The following reaction scheme is followed during catalytic hydrogenation of CO2 [106][218],4. Post-Synthesis Methanol/Water Separation
4.1. Challenges and Improvement in Methanol/Water Separation
In distillation operation, re-boiler and condenser are major energy consumers, and it accounts for approximately 40% of total energy requirement of a chemical industry [112][113][225,226]. To have better process efficiency and energy economy, several improvements were made in existing distillation process including heat integrated distillation, multi-effect distillation and heat pump assisted distillation [114][115][116][117][118][227,228,229,230,231]. Liang et al. worked on making distillation process energy efficient by combining preconcentration and entrainer recovery columns instead of 3-column configuration of conventional extractive distillation which is good energy and capital cost saving [119][232].4.2. Membrane Based Methanol/Water Separation
Membrane technology has a great potential to replace any distillation process by incorporating suitable membrane materials. Different organic and inorganic membrane materials have been proposed and tested for methanol-water dehydration following vapor-permeation or pervaporation mechanisms [120][121][20,21]. In vapor-permeation method, the feed is evaporated and enters in the membrane module in vapor form. In this case, both species (methanol and water) are in vapor form when they come in contact with membrane, which allows one vapor to pass through [122][234]. Conversely, in the pervaporation method, the feed is preheated to a required temperature and passes through the membrane module in liquid form. Vacuum is generated on the permeate side (either by condensing the incoming vapors or by a vacuum pump) for in situ evaporation of selective species from feed and pass-through membrane [123][235]. For most of the methanol-water separation studies using membranes, a pervaporation scheme is used, along with organophilic or hydrophilic membrane material.4.2.1. Zeolite Membranes for Methanol/Water Separation
Zeolites are porous crystalline aluminosilicates having successful applications in ion exchange, adsorption and catalysis [124][238]. Zeolite membranes being hydrophilic in nature has been widely used for selective water removal applications, especially from aqueous-organic mixtures [125][126][236,239]. Due to fine crystalline porous structure, the zeolite-based molecular sieves were used for separating species based on size such as separation of ethanol/water or iso-propanol/water mixtures [127][128][240,241]. As for methanol-water separation, the simple molecular sieving mechanism itself is not enough for effective separation due to comparable sizes of both components [122][234]. Therefore, structural tuning and modifications were performed to make zeolite applicable for methanol-water separation.4.2.2. Synthesis of Zeolite Membranes
In membrane separation processes, the thickness of the selective layer has a great influence on the mass transfer resistance to the permeating species. During fabrication of zeolite membranes, the thickness is kept at a few microns in order to minimize the mass transfer resistance. This thin layer can be very fragile; therefore, these membranes are prepared on a porous supports of different materials (alumina or stainless steel) in different configurations such as tubular, flat, and hollow fiber [129][130][131][254,255,256]. Several methods have been developed for synthesizing zeolite membranes on these supports using in situ crystallization and secondary-growth [132][133][134][135][257,258,259,260]. Both processes work on the principle of hydrothermal crystallization of zeolite particles on a well-prepared support material. A synthesis solution is prepared by mixing required amounts of aluminate and silicate solutions along with other structural directing agents. These agents upon post calcination are removed from inner cavities of zeolite leaving behind porous crystalline structure [136][261]. For in-situ crystallization, the support is directly immersed in synthesis solution/gel contained in a Teflon lined stainless steel autoclave and kept at a certain temperature. The zeolite crystals grow on support surface by thermally driven crystallization followed by in situ nucleation [132][133][257,258]. This method produces non-uniform zeolite layers and is not reproducible, as nucleation and crystal growth processes happen simultaneously. The second method of secondary-growth crystallization is the most reliable for producing a uniform zeolite layer by separately performing the nucleation and crystal growth steps [134][135][259,260], as shown in Figure 25. Nucleation is done by depositing zeolite nuclei (seeds) on support by dip coating or rubbing, before doing final crystallization. This step is more crucial in the secondary growth method and must be carried out carefully to ensure evenly distributed crystal seeds. Many factors such as seed concentration, seed size, coating cycles, coating time, pH and temperature of seed solution are optimized to prepare an optimized seeded support [137][138][262,263]. This seeded support is then subjected to hydrothermal treatment similar to in situ crystallization, which provides the seeds as active sites for further growth of zeolite crystals and hence results in a smooth and uniform layer. After successful deposition of zeolite layer on the support, the prepared membrane is subjected to high temperature calcination to strengthen and stabilize the zeolite layer on the support [139][140][22,32].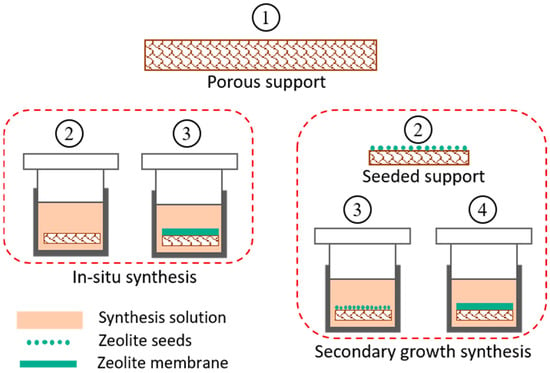
5. Zeolite Membrane Reactors for Methanol Synthesis
5. Zeolite Membrane Reactors for Methanol Synthesis