
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Atul Bhattad | -- | 2455 | 2023-04-17 19:07:08 | | | |
| 2 | Camila Xu | Meta information modification | 2455 | 2023-04-18 03:22:48 | | | | |
| 3 | Atul Bhattad | + 192 word(s) | 2647 | 2023-04-28 18:31:11 | | | | |
| 4 | Camila Xu | Meta information modification | 2647 | 2023-05-04 02:50:24 | | |
Video Upload Options
Nanofluids are colloidal mixtures of nanosized particles (10–100 nm) suspended in base fluids. They possess good physical or chemical properties and thermal or rheological properties. Hybrid nanofluids are suspensions of a mixture of dissimilar nanoparticles or nanocomposites infused in the conventional base fluid, which yield better thermal conductivity and heat transfer characteristics due to hybridization.
1. Hybrid Nanofluids
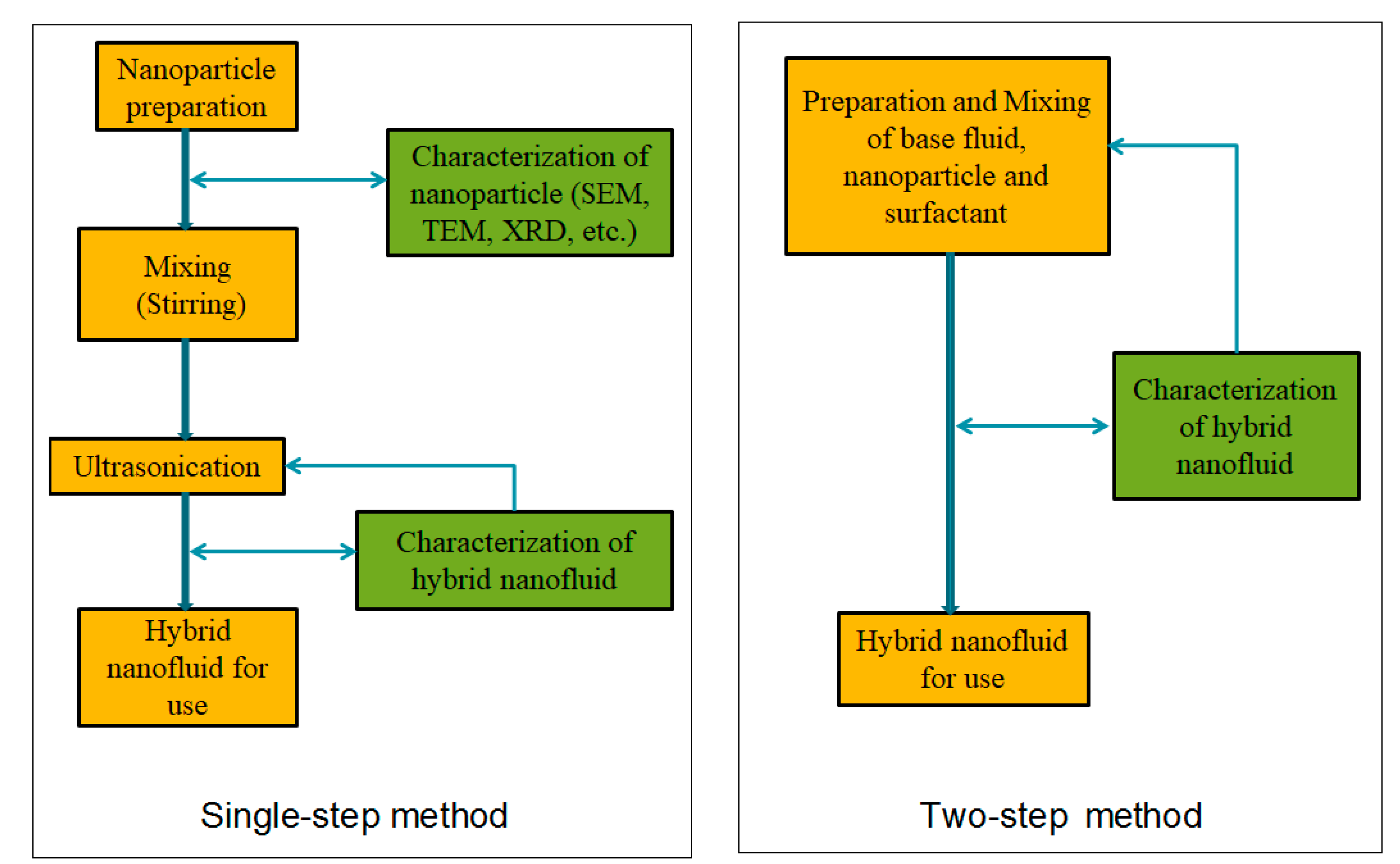
2. Preparation of Mono/Hybrid Nanofluids
| Author(s) | Nanoparticle | Base Fluid |
|---|---|---|
| Jana et al. [41] | Au–CNT, Cu–CNT | Water |
| Han et al. [42] | Sphere–CNT | Oil |
| Turcu et al. [43] | Fe3O4–polypyrrole | Water |
| Jha and Ramaprabhu [44] | Cu–MWCNT | Water/EG |
| Han and Rhi [45] | Ag–Al2O3 | Water |
| Baby and Sundara [46] | CuO–HEG | Water/EG |
| Paul et al. [47] | Al–Zn | EG |
| Suresh et al. [48] | Al2O3–Cu | Water |
| Botha et al. [49] * | Ag–SiO2 | Oil |
| Ho et al. [50] | Al2O3–PCM | Water |
| Baby and Sundara [51] | Ag–HEG | Water/EG |
| Amiri et al. [52] | Ag–MWCNT | Water |
| Chen et al. [53] | Ag–MWCNT | Water |
| Aravind and Ramaprabhu [54] | Graphene–MWCNT | Water and EG |
| Bhosale and Borse [55] | Al2O3–CuO | Water |
| Balla et al. [56] | CuO–Cu | Water |
| Abbasi et al. [57] | ϒ-Al2O3–MWCNT | Water |
| Nine et al. [58] | Cu–Cu2O | Water |
| Munkhbayar et al. [59] * | Ag–MWCNT | Water |
| Sundar et al. [60] | Nanodiamond–nickel | Water/EG |
| Parameshwaran et al. [61] | Ag–TiO2 | PCM |
| Batmunkh et al. [62] | Ag–TiO2 | Water |
| Madhesh et al. [63] | Cu–TiO2 | Water |
| Chen et al. [64] | MWCNT–Fe3O4 | Water |
| Parekh [65] | Mn0.5Zn0.5Fe2O4 | Oil |
| Luo et al. [66] | Al2O3–TiO2 | Lubricating oil |
| Madhesh and Kalaiselvam [67] | Cu–TiO2 | Water |
| Zubir et al. [68] | Graphene oxide–CNT | Water |
| Qadri et al. [69] | Graphene–Cu2O | Water/EG |
| Karimi et al. [70] | NiFe2O4 | Water |
| Chakraborty et al. [71] | Cu–Al | Water |
| Megatif et al. [72] | CNT–TiO2 | Water |
| Abbasi et al. [73] | MWCNT–TiO2 | Water |
| Toghraie et al. [74] | ZnO–TiO2 | EG |
| Bhanvase et al. [75] | PANI–CuO | Water |
| Asadi et al. [76] | CuO–TiO2 | Water |
| Chen et al. [77] | Al2O3 | Liquid paraffin |
| Asadi et al. [78] | MWCNT | Water |
| Gulzar et al. [79] | Al2O3–TiO2 | Therminol-55 |
| Alarifi et al. [80] | MWCNT–TiO2 | Oil |
| Akram et al. [81] | CGNP | DI Water |
| Sharafeldin and Grof [82] | WO3 | Water |
| Chen et al. [83] | MWCNT | Water |
| Ali et al. [84] | Al | Water |
| Mahbubul et al. [85] | Al2O3 | Water |
| Mahyari et al. [86] | GO–SiC | Water/EG |
| Chen et al. [87] | Fe3O4–MWCNT | Brine water |
| Okonkwo et al. [88] | Al2O3–Fe | Water |
| Terueal et al. [89] | MoSe2 | Water |
| Li et al. [90] | SiO2 | Liquid paraffin |
| Geng et al. [91] | ZnO–MWCNT | Oil |
| Li et al. [92] | SiO2 | EG |
3. Characterization and Stability of Mono/Hybrid Nanofluids
| Author(s) | Nanoparticle | Base Fluid | Surfactant (s) |
|---|---|---|---|
| Xian et al. [122] | COOH-GnP, TiO2 | DW/EG | SDC, CTAB *, SDBS |
| Almanassra et al. [123] | CNT | Water | GA *, PVP, SDS |
| Cacua et al. [124] | Al2O3 | Water | CTAB, SDBS * |
| Kazemi et al. [121] | SiO2, graphene | Water | CMC * |
| Ouikhalfan et al. [125] | TiO2 | DW | CTAB *, SDS |
| Siddiqui et al. [126] | Cu-Al2O3 | DI water | |
| Cacua et al. [120] | Al2O3 | DI water | CTAB, SDBS * |
| Etedali et al. [127] | SiO2 | DI water | CTAB *, SLS * |
| Giwa et al. [128] | Al2O3-Fe2O3 | DW | SDS *, NaDBS * |
| Kazemi et al. [129] | G-SiO2 | DW | CMC * |
| Gallego et al. [130] | Al2O3 | Water | SDBS * |
| Shah et al. [131] | (rGO) | EG | CTAB *, SDBS, and SDS |
| Ilyas et al. [132] | GnP | Saline water | SDS * |
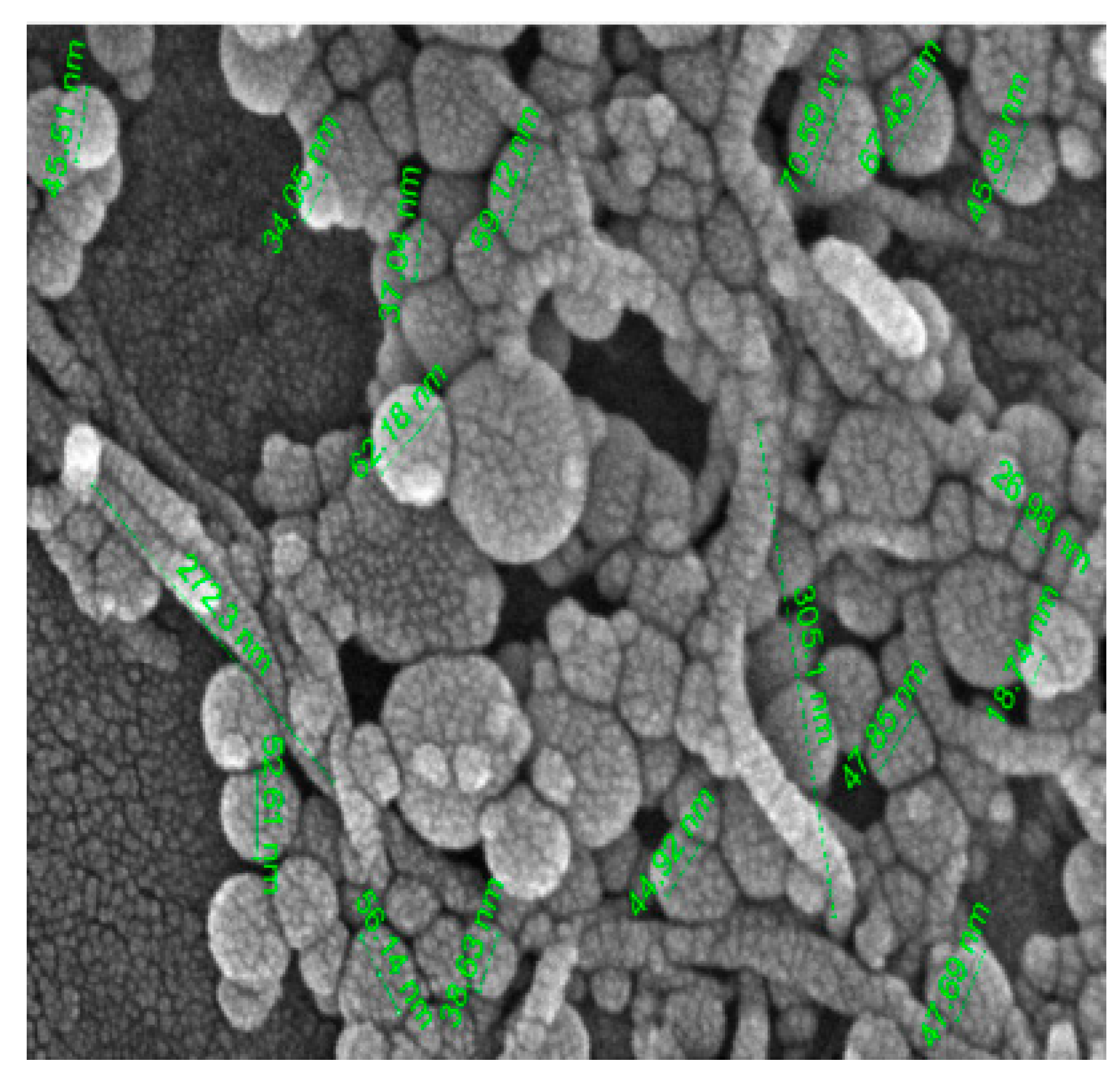
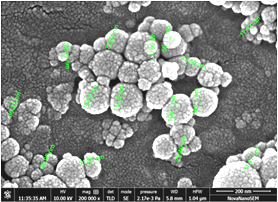
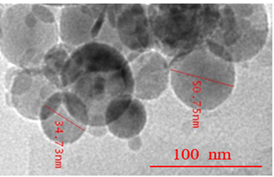
SEM (Scanning electron microscopy) and TEM (Transmission electron microscopy) tests were performed and measured the mean size of Al2O3 and TiO2 nanoparticles by ImageJ 2.0.0-rc-3 as 45 nm and 20 nm, respectively. The small-size particles in Figure 3 represent TiO2 nanoparticles, whereas larger ones are the Al2O3 nanoparticles. Both types of nanoparticles were found to be spherical, with a shape factor of 1. One of the key challenges in studying nanofluids is ensuring their stability and homogeneity. A stability test involving gravitational settling was performed to address this issue, and images of the test tube were taken at different intervals (Figure 4). The results showed that there was no sedimentation throughout the 7-day investigation.
(a)
(b)
Figure 3. (a). SEM image of Al2O3- TiO2/water hybrid nanofluid; (b). TEM image of Al2O3- TiO2/water hybrid nanofluid.

Figure 4. Stability analysis of a sample showing no sedimentation for 7 days.
References
- Rostami, S.; Shahsavar, A.; Kefayati, G.; Goldanlou, A.S. Energy and exergy analysis of using turbulator in a parabolic trough solar collector filled with mesoporous silica modified with copper nanoparticles hybrid nanofluid. Energies 2020, 13, 2946.
- Choi, S. Enhancing Thermal Conductivity of Fluids with Nanoparticles, Developments and Applications of Non-Newtonian Flows; ASME: New York, NY, USA, 1995; pp. 99–105.
- Kumar, P.M.; Palanisamy, K.; Vijayan, V. Stability analysis of heat transfer hybrid/water nanofluids. Mater. Today Proc. 2020, 21, 708–712.
- Okonkwo, E.; Wole-Osho, I.; Almanassra, I.; Abdullatif, Y.; Al-Ansari, T. An updated review of nanofluids in various heat transfer devices. J. Therm. Anal. 2021, 145, 2817–2872.
- Sarkar, J.; Ghosh, P.; Adil, A. A review on hybrid nanofluids: Recent research, development and applications. Renew. Sustain. Energy Rev. 2015, 43, 164–177.
- Sahin, A. Performance enhancement of solar energy using nanofluids: An update review. Renew Energy 2020, 145, 1126–1148.
- Zayed, M.; Du, Y.; Kabeel, A.; Shalaby, S. Factors affecting the thermal performance of the plate solar collector using nanofluids: A review. Sol. Energ. 2019, 182, 382–396.
- Wahab, A.; Hassan, A.; Qasim, M.; Ali, H.; Babar, H.; Sajid, M. Solar energy systems—Potential of nanofluids. J. Mol. Liq. 2019, 289, 111049.
- Pordanjani, A.; Aghakhani, S.; Afrand, M.; Mahmoudi, B.; Mahian, O.; Wongwises, S. An updated review on application of nanofluids in heat exchangers for saving energy. Energy Convers. Manag. 2019, 198, 111886.
- Sajid, M.; Ali, H. Recent advances in application of nanofluids in heat transfer devices: A critical review. Renew. Sustain. Energy Rev. 2019, 103, 556–592.
- Kumar, A.; Subudhi, S. Preparation, characterization and heat transfer analysis of nanofluids used for engine cooling. Appl. Therm. Eng. 2019, 160, 114092.
- Rahman, S.; Ashraf, M.; Amin, A.; Bashar, M.; Ashik, M.; Kamruzzaman, M. Tuning nanofluids for improved lubrication performance in turning biomedical grade titanium alloy. J. Clean. Prod. 2019, 206, 180–196.
- Panithasan, M.; Gopalakichenin, D.; Veeraraagavan, S. Impact of rice husk nanoparticle on the performance and emission aspect of a diesel engine running on blends on pine oildiesel. Env. Sci. Pollut. Res. 2019, 26, 282–291.
- Pinto, R.; Fiorelli, F. Review of the mechanisms responsible for heat transfer enhancement using nanofluids. Appl. Therm. Eng. 2016, 108, 720–739.
- Radomska, E.; Mika, L.; Sztekler, K. The impact of additives on the main properties of phase change materials. Energies 2020, 13, 3064.
- Suhaimi, N.; Din, M.; Hamid, M.; Amin, N.; Zamri, W.; Wang, J. Optimum electrical and dielectric performance of multi-walled carbon nanotubes doped disposed transformer oil. Energies 2020, 13, 3181.
- Sekar, A.; Jayabalan, T.; Muthukumar, H.; Chandrasekaran, N.; Mohamed, S.; Matheswaran, M. Enhancing power generation and treatment of dairy waste water in microbial fuel cell using Cu-doped iron oxide nanoparticles decorated anode. Energy 2019, 172, 173–180.
- Said, Z.; Gupta, M.; Khan, A.; Jamil, M.; Bellos, E. A comprehensive review on minimum quantity lubrication (MQL) in machining processes using nano-cutting fluids. Int. J. Adv. Manuf. Technol. 2019, 105, 2057–2086.
- Huminic, G.; Huminic, A. The influence of hybrid nanofluids on the performances of elliptical tube: Recent research and numerical study. Int. J. Heat Mass Transf. 2019, 129, 132–143.
- Elsaid, A. Experimental study on the heat transfer performance and friction factor characteristics of Co3O4 and Al2O3 based H2O/(CH2OH)2 nanofluids in a vehicle engine radiator. Int. Commun. Heat Mass Transf. 2019, 108, 104263.
- Al-Shdaifat, M.; Zulkifli, R.; Sopian, K.; Salih, A. Thermal and hydraulic performance of CuO/water nanofluids: A review. Micromachines 2020, 11, 416.
- Eshgarf, H.; Kalbasi, R.; Maleki, A.; Shadloo, M.; Karimipour, A. A review on the properties, preparation, models and stability of hybrid nanofluids to optimize energy consumption. J. Therm. Anal. Calorim. 2021, 144, 1959–1983.
- Zainon, S.; Azmi, W. Recent progress on stability and thermo-physical properties of mono and hybrid towards green nanofluids. Micromachines 2021, 12, 176.
- Pavia, M.; Alajami, K.; Estellé, P.; Vigolo, B. A critical review on thermal conductivity enhancement of graphene-based nanofluids. Adv. Colloid. Interface Sci. 2021, 294, 102452.
- Sreelakshmy, K.; Aswathy, S.; Vidhya, K.; Saranya, T.; Sreeja, C. An overview of recent nanofluid research. Int. Res. J. Pharm. 2014, 5, 239–243.
- Nor-Azwadi, C.; Adamu, I.; Jamil, M. Preparation methods and thermal performance of hybrid nanofluids. J. Adv. Rev. Sci. Res. 2016, 24, 13–23.
- Sidik, N.; Adamu, I.; Jamil, M.; Kefayati, G.; Mamat, R.; Najafi, G. Recent progress on hybrid nanofluids in heat transfer applications: A comprehensive review. Int. Commun. Heat Mass Transf. 2016, 78, 68–79.
- Nabil, M.; Azmi, W.; Hamid, K.; Zawawi, N.; Priyandoko, G.; Mamat, R. Thermo-physical properties of hybrid nanofluids and hybrid nanolubricants: A comprehensive review on performance. Int. Commun. Heat Mass Transf. 2017, 83, 30–39.
- Sundar, L.; Sharma, K.; Singh, M.; Sousa, A. Hybrid nanofluids preparation, thermal properties, heat transfer and friction factor–A review. Renew. Sustain. Energy Rev. 2017, 68, 185–198.
- Babu, J.; Kumar, K.; Rao, S. State-of-art review on hybrid nanofluids. Renew. Sustain. Energy Rev. 2017, 77, 551–565.
- Sidik, N.; Jamil, M.; Aziz-Japar, W.; Adamu, I. A review on preparation methods, stability and applications of hybrid nanofluids. Renew. Sustain. Energy Rev. 2017, 80, 1112–1122.
- Kumar, D.; Arasu, A. A comprehensive review of preparation, characterization, properties and stability of hybrid nanofluids. Renew. Sustain. Energy Rev. 2018, 81, 1669–1689.
- Gupta, M.; Singh, V.; Kumar, S.; Said, Z. Up to date review on the synthesis and thermophysical properties of hybrid nanofluids. J. Clean. Prod. 2018, 190, 169–192.
- Jamkhande, P.; Bamer, A.; Kalaskar, M. Metal nanoparticles synthesis: An overview on methods of preparation, advantages and disadvantages, and applications. J. Drug Deliv. Sci. Technol. 2019, 53, 101174.
- Asadi, A.; Aberoumand, S.; Moradikazerouni, A.; Pourfattah, F.; Żyła, G.; Estellé, P.; Mahian, O.; Wongwises, S.; Nguyen, H.M.; Arabkoohsar, A. Recent advances in preparation methods and thermophysical properties of oil-based nanofluids: A state of the art review. Powder Technol. 2019, 352, 209–226.
- Arshad, A.; Jabbal, M.; Yan, Y.; Reay, D. A review on graphene based nanofluids: Preparation, characterization and applications. J. Mol. Liq. 2019, 279, 444–484.
- Mishra, P.; Sen, S.; Amin, R.; Biring, S. Effect of annealing on structure, optoelectronic and photoresponsivity properties of sol–gel prepared ZnO nanoparticles. Mater. Today Proc. 2019, 17, 261–265.
- Esmaeili, E.; Rounaghi, S.; Gruner, W.; Eckert, J. The preparation of surfactant-free highly dispersed ethylene glycol-based aluminum nitride-carbon nanofluids for heat transfer application. Adv. Powder Technol. 2019, 30, 2032–2041.
- Das, N.; Naik, P.; Reddy, D.; Mallik, B.; Bose, S.; Banerjee, T. Experimental and molecular dynamic insights on the thermophysical properties for MWCNT-Phosphonium based eutectic thermal media. J. Mol. Liq. 2022, 354, 118892.
- Bhattad, A.; Sarkar, J.; Ghosh, P. Use of Hybrid Nanofluids in Plate Heat Exchanger for Low Temperature Applications. Ph.D. Thesis, Indian Institute of Technology Banaras Hindu University, Varanasi, Uttar Pradesh, India, 2019.
- Jana, S.; Khojin, A.; Zhong, W. Enhancement of fluid thermal conductivity by the addition of single and hybrid nano-additives. Thermochim. Acta 2007, 462, 45–55.
- Han, Z.; Yang, B.; Kim, S.; Zachariah, M. Application of hybrid sphere/ carbon nanotube particles in nanofluids. Nanotechnology 2007, 18, 105701.
- Turcu, R.; Pana, O.; Nan, A.; Craciunescu, I.; Chauvet, O.; Payen, C. Polypyrrole coated magnetite nanoparticles from water based nanofluids. J. Phys. Appl. Phys. 2008, 41, 245002.
- Jha, N.; Ramaprabhu, S. Synthesis and thermal conductivity of copper nanoparticle decorated multiwalled carbon nanotubes based nanofluids. J. Phys. Chem. C 2008, 112, 9315–9319.
- Han, W.; Rhi, S. Thermal characteristics of grooved heat pipe with hybrid nanofluids. Therm. Sci. 2011, 15, 195–206.
- Baby, T.; Sundara, R. Synthesis and transport properties of metal oxide decorated graphene dispersed nanofluids. J. Phys. Chem. C 2011, 115, 8527–8533.
- Paul, G.; Philip, J.; Raj, B.; Das, P.; Manna, I. Synthesis, characterization and thermal property measurement of nano-Al95Zn05 dispersed nanofluid prepared by a two-step process. Int. J. Heat Mass Transf. 2011, 54, 3783–3788.
- Suresh, S.; Venkitaraj, K.; Selvakumar, P.; Chandrasekar, M. Synthesis of Al2O3-Cu/water hybrid nanofluids using two step method and its thermo physical properties. Colloids Surfaces A Physicochem. Eng. Asp. 2011, 388, 41–48.
- Botha, S.S.; Ndungu, P.; Bladergroen, B.J. Physicochemical properties of oil-based nanofluids containing hybrid structures of silver nanoparticles supported on silica. Ind. Eng. Chem. Res. 2011, 50, 3071–3077.
- Ho, C.; Huang, J.; Tsai, P.; Yang, Y. On laminar convective cooling performance of hybrid water-based suspensions of Al2O3 nanoparticles and MEPCM particles in a circular tube. Int. J. Heat Mass Transf. 2011, 54, 2397–2407.
- Baby, T.; Sundara, R. Synthesis and nanofluid application of silver nanoparticles decorated graphene. J. Mater. Chem. 2011, 21, 9702–9709.
- Amiri, A.; Shanbedi, M.; Eshghi, H.; Heris, S.; Baniadam, M. Highly dispersed multiwalled carbon nanotubes decorated with Ag nanoparticles in water and experimental investigation of the thermophysical properties. J. Phys. Chem. C 2012, 116, 3369–3375.
- Chen, L.; Yu, W.; Xie, H. Enhanced thermal conductivity of nanofluids containing Ag/MWNT composites. Powder Technol. 2012, 231, 18–20.
- Aravind, S.; Ramaprabhu, S. Graphene wrapped multiwalled carbon nanotubes dispersed nanofluids for heat transfer applications. J. Appl. Phys. 2012, 112, 124304–124309.
- Bhosale, G.; Borse, S. Pool boiling CHF enhancement with Al2O3–CuO/H2O hybrid nanofluid. Int. J. Eng. Res. Technol. 2013, 2, 946–950.
- Balla, H.; Abdullah, S.; MohdFaizal, W.; Zulkifli, R.; Sopian, K. Numerical study of the enhancement of heat transfer for hybrid CuO-Cu nanofluids flowing in a circular pipe. J. Oleo Sci. 2013, 62, 533–539.
- Abbasi, S.; Rashidi, A.; Nemati, A.; Arzani, K. The effect of functionalisation methodon the stability and the thermal conductivity of nanofluid hybrids of carbon nanotubes/gamma alumina. Ceram. Int. 2013, 39, 3885–3891.
- Nine, M.; Munkhbayar, B.; Rahman, M.; Chung, H.; Jeong, H. Highly productive synthesis process of well dispersed Cu2O and Cu/Cu2O nanoparticles and its thermal characterization. Mater. Chem. Phys. 2013, 141, 636–642.
- Munkhbayar, B.; Tanshen, M.; Jeoun, J.; Chung, H.; Jeong, H. Surfactant-free dispersion of silver nanoparticles into MWCNT-aqueous nanofluids prepared by one-step technique and their thermal characteristics. Ceram. Int. 2013, 39, 6415–6425.
- Sundar, L.; Singh, M.; Ramana, E.; Sing, B.; Gracio, J.; Sousa, A. Enhanced thermal conductivity and viscosity of nanodiamond–nickel nanocomposite nanofluids. Sci. Rep. 2014, 4, 4039.
- Parameshwaran, R.; Deepak, K.; Saravanan, R.; Kalaiselvam, S. Preparation, thermal and rheological properties of hybrid nanocomposite phase change material for thermal energy storage. Appl. Energy 2014, 115, 320–330.
- Batmunkh, M.; Tanshen, M.; Nine, M.; Myekhlai, M.; Choi, H.; Chung, H.; Jeong, H. Thermal conductivity of TiO2 nanoparticles based aqueous nanofluids with an addition of a modified silver particle. Ind. Eng. Chem. Res. 2014, 53, 8445–8451.
- Madhesh, D.; Parameshwaran, R.; Kalaiselvam, S. Experimental investigation on convective heat transfer and rheological characteristics of Cu-TiO2 hybrid nanofluids. Exp. Therm. Fluid Sci. 2014, 52, 104–115.
- Chen, L.; Cheng, M.; Yang, D.; Yang, L. Enhanced thermal conductivity of nanofluid by synergistic effect of multi-walled carbon nanotubes and Fe2O3 nanoparticles. Appl. Mech. Mater. 2014, 548, 118–123.
- Parekh, K. Thermo-magnetic properties of ternary polydispersed Mn0.5Zn0.5Fe2O4 ferrite magnetic fluid. Solid State Commun. 2014, 187, 33–37.
- Luo, T.; Wei, X.; Zhao, H.; Cai, G.; Zheng, X. Tribology properties of Al2O3/TiO2 nanocomposites as lubricant additives. Ceram. Int. 2014, 40, 10103–10109.
- Madhesh, D.; Kalaiselvam, S. Experimental study on heat transfer and rheological characteristics of hybrid nanofluids for cooling applications. J. Exp. Nanosci. 2015, 10, 1194–1213.
- Zubir, M.; Badarudin, A.; Kazi, S.; Huang, N.; Misran, M.; Sadeghinezhad, E.; Mehrali, M.; Syuhada, N.; Gharehkhani, S. Experimental investigation on the use of reduced graphene oxide and its hybrid complexes in improving closed conduit turbulent forced convective heat transfer. Exp. Therm. Fluid Sci. 2015, 66, 290–303.
- Qadri, M.; Chandra, R.; Ravindra, S.; Velmurugan, V. Synthesis and testing of graphene /cuprous oxide composite based nano fluids for engine coolants. Mater. Today Proc. 2015, 2, 4640–4645.
- Karimi, A.; Sadatlu, M.; Saberi, B.; Shariatmadar, H. Experimental investigation on thermal conductivity of water based nickel ferrite nanofluids. Adv. Powder Technol. 2015, 26, 1529–1536.
- Chakraborty, S.; Sarkar, I.; Haldar, K.; Pal, S.; Chakraborty, S. Synthesis of Cu–Al layered double hydroxide nanofluid and characterization of its thermal properties. Appl. Clay Sci. 2015, 107, 98–108.
- Megatif, L.; Ghozatloo, A.; Arimi, A.; Niasar, M. Investigation of laminar convective heat transfer of a novel TiO2-carbon nanotube hybrid water-based nanofluid. Exp. Heat Transf. 2015, 6152, 1–15.
- Abbasi, S.; Zebarjad, S.; Baghban, S.; Youssefi, A.; Ekrami-Kakhki, M. Experimental investigation of the rheological behavior and viscosity of decorated multi-walled carbon nanotubes with TiO2 nanoparticles/water nanofluids. J. Therm. Anal. Calorim. 2016, 123, 81–89.
- Toghraie, D.; Chaharsoghi, V.; Afrand, M. Measurement of thermal conductivity of ZnO–TiO2/EG hybrid nanofluid. J. Therm. Anal. Calorim. 2016, 125, 527–535.
- Bhanvase, B.; Kamath, S.; Patil, U.; Patil, H.; Pandit, A.; Sonawane, S. Intensification of heat transfer using PANI nanoparticles and PANI-CuO nanocomposite based nanofluids. Chem. Eng. Process. 2016, 104, 172–180.
- Asadi, A.; Alarifi, I.; Foong, L. An experimental study on characterization, stability and dynamic viscosity of CuO-TiO2/water hybrid nanofluid. J. Mol. Liq. 2020, 307, 112987.
- Chen, Z.; Shahsavar, A.; Al-Rashed, A.; Afrand, M. The impact of sonication and stirring durations on the thermal conductivity of alumina-liquid paraffin nanofluid: An experimental assessment. Powder Technol. 2020, 360, 1134–1142.
- Asadi, A.; Alarifid, I.; Ali, V.; Nguyen, H. An experimental investigation on the effects of ultrasonication time on stability and thermal conductivity of MWCNTwater nanofluid: Finding the optimum ultrasonication time. Ultrason. Sonochem. 2019, 58, 104639.
- Gulzar, O.; Qayoum, A.; Gupta, R. Experimental study on stability and rheological behavior of hybrid Al2O3—TiO2 Therminol-55 nanofluids for concentrating solar collectors. Powder Technol. 2019, 352, 436–444.
- Alarifi, I.; Alkouh, A.; Ali, V.; Nguyen, H.; Asadi, A. On the rheological properties of MWCNT-TiO2/oil hybrid nanofluid: An experimental investigation on the effects of shear rate, temperature, and solid concentration of nanoparticles. Powder Technol. 2019, 355, 157–162.
- Akram, N.; Sadri, R.; Kazi, S.N.; Ahmed, S.M.; Zubir, M.N.M.; Ridha, M.; Soudagar, M.; Ahmed, W.; Arzpeyma, M.; Tong, G.B. An experimental investigation on the performance of a flat-plate solar collector using eco-friendly treated graphene nanoplatelets–water nanofluids. J. Therm. Anal. Calorim. 2019, 138, 609–621.
- Sharafeldin, M.; Grof, G. Efficiency of evacuated tube solar collector using WO3/Water nanofluid. Renew. Energy 2019, 134, 453–460.
- Chen, W.; Zou, C.; Li, X. Application of large-scale prepared MWCNTs nanofluids in solar energy system as volumetric solar absorber. Sol. Energy Mater. Sol. Cells 2019, 200, 109931.
- Ali, N.; Teixeira, J.; Addali, A. Aluminum nanofluids stability: A comparison between the conventional two-step fabrication approach and the controlled sonication bath temperature method. J. Nanomater. 2019, 2019, 3930572.
- Mahbubul, I.; Elcioglu, E.; Amalina, M.; Saidur, R. Stability, thermophysical properties and performance assessment of alumina–water nanofluid with emphasis on ultrasonication and storage period. Powder Technol. 2019, 345, 668–675.
- Mahyari, A.; Karimipour, A.; Afrand, M. Effects of dispersed added Graphene Oxide-Silicon Carbide nanoparticles to present a statistical formulation for the mixture thermal properties. Phys. A Stat. Mech. Its Appl. 2019, 521, 98–112.
- Chen, W.; Zou, C.; Li, X.; Liang, H. Application of recoverable carbon nanotube nanofluids in solar desalination system: An experimental investigation. Desalination 2019, 451, 92–101.
- Okonkwo, E.; Wole-Osho, I.; Kavaz, D.; Abid, M. Comparison of experimental and theoretical methods of obtaining the thermal properties of alumina/iron mono and hybrid nanofluids. J. Mol. Liq. 2019, 292, 111377.
- Teruel, M.; Aguilar, T.; Martínez-Merino, P.; Carrillo-Berdugo, I.; Gallardo-Bernal, J.J.; Gómez-Villarejo, R.; Alcántara, R.; Fernández-Lorenzo, C.; Navas, J. 2D MoSe2-based nanofluids prepared by liquid phase exfoliation for heat transfer applications in concentrating solar power. Sol. Energy Mater. Sol. Cells 2019, 200, 109972.
- Li, Z.; Asadi, S.; Karimipour, A.; Abdollahi, A.; Tlili, I. Experimental study of temperature and mass fraction effects on thermal conductivity and dynamic viscosity of SiO2-oleic acid/ liquid paraffin nanofluid. Int. Commun. Heat Mass Transf. 2020, 110, 104436.
- Geng, Y.; Al-Rashed, A.; Mahmoudi, B.; Alsagri, A.; Shahsavar, A.; Alebizadehsardari, P. Characterization of the nanoparticles, the stability analysis and the evaluation of a new hybrid nano-oil thermal conductivity. J. Therm. Anal. Calorim. 2020, 139, 1553–1564.
- Li, Y.; Kalbasi, R.; Nguyen, Q.; Afrand, M. Effects of sonication duration and nanoparticles concentration on thermal conductivity of silica-ethylene glycol nanofluid under different temperatures: An experimental study. Powder Technol. 2020, 367, 464–473.
- Bhattad, A.; Sarkar, J.; Ghosh, P. Experimentation on effect of particle ratio on hydrothermal performance of plate heat exchanger using hybrid nanofluid. Appl. Therm. Eng. 2019, 162, 114309.
- Yang, L.; Ji, W.; Mao, M.; Huang, J. An updated review on the properties, fabrication and application of hybrid-nanofluids along with their environmental effects. J. Clean. Prod. 2020, 257, 120408.
- Sezer, N.; Atieh, M.A.; Koç, M. A comprehensive review on synthesis, stability, thermophysical properties, and characterization of nanofluids. Powder Technol. 2019, 344, 404–431.
- Asadi, A.; Pourfattah, F.; Szilágyi, I.; Afrand, M.; Żyła, G.; Ahn, H.; Wongwises, N.H.; Arabkoohsar, A.; Mahian, O. Effect of sonication characteristics on stability, thermophysical properties, and heat transfer of nanofluids: A comprehensive review. Ultrason. Sonochem. 2019, 58, 104701.
- Lu, Y.; Lu, X.; Mayers, B.; Herricks, T.; Xia, Y. Synthesis and characterization of magnetic Co nanoparticles: A comparison study of three different capping surfactants. J. Solid State Chem. 2008, 181, 1530–1538.
- Saeedinia, M.; Akhavan-Behabadi, M.; Razi, P. Thermal and rheological characteristics of CuO-Base oil nanofluidflow inside a circular tube. Int. Commun. Heat Mass Tran. 2012, 39, 152–159.
- Khan, A.; Arasu, A. A review of influence of nanoparticle synthesis and geometrical parameters on thermophysical properties and stability of nanofluids. Therm. Sci. Eng. Prog. 2019, 11, 334–364.
- Singh, S. Review on the stability of the nanofluids. In Pipeline Engineering; Rushd, S., Ismail, M.A., Eds.; IntechOpen: London, UK, 2022.
- Safiei, W.; Rahman, M.; Yusoff, A.; Radin, M. Preparation, stability and wettability of nanofluid: A review. J. Mech. Eng. Sci. 2020, 14, 7244–7257.
- Amin, A.; Hamzah, W.; Oumer, A. Thermal conductivity and dynamic viscosity of mono and hybrid organic- and synthetic-based nanofluids: A critical review. Nanotechnol. Rev. 2021, 10, 1624–1661.
- Malika, M.; Sonawane, S. Effect of nanoparticle mixed ratio on stability and thermo-physical properties of CuO-ZnO/water-based hybrid nanofluid. J. Indian Chem. Soc. 2020, 97, 414–419.
- Sahoo, R.; Kumar, V. Comparative analysis of Viscosity and Thermal Conductivity for Al2O3/CuO Hybrid Nanofluid in Binary Base Fluids. Nanotechnol. Mater. Sci. 2019, 6, 34–42.
- Ramadhan, A.; Azmi, W.; Mamat, R.; Hamid, K.; Norsakinah, S. Investigation on stability of tri-hybrid nanofluids in water- ethylene glycol mixture. IOP Conf. Ser. Mater. Sci. Eng. 2019, 469, 012068.
- Afshari, F.; Manay, E.; Rahimpour, S.; Sahin, B.; Muratçobanoglu, B.; Teimuri-Mofrad, R. A review study on factors affecting the stability of nanofluids. Heat Transf. Res. 2022, 53, 77–91.
- Arora, N.; Gupta, M. Stability Evaluation and Enhancement Methods in Nanofluids: A Review. AIP Conf. Proc. 2021, 2341, 040022.
- Wcislik, S. Efficient stabilization of mono and hybrid nanofluids. Energies 2020, 13, 3793.
- Bumataria, R.; Chavda, N.; Panchal, H. Current research aspects in mono and hybrid nanofluid based heat pipe technologies. Heliyon 2019, 5, e01627.
- Ali, A.; Salam, B. A review on nanofluid: Preparation, stability, thermophysical properties, heat transfer characteristics and application. SN Appl. Sci. 2020, 2, 1636.
- Xu, Q.; Liu, L.; Feng, J.; Qiao, L.; Yu, C.; Shi, W.; Ding, C.; Zang, Y.; Chang, C.; Xiong, Y.; et al. A comparative investigation on the effect of different nanofluids on the thermal performance of two-phase closed thermosiphon. Int. J. Heat Mass Transf. 2020, 149, 119189.
- Said, Z.; Abdelkareem, M.; Rezk, H.; Nassef, A.; Atwany, H. Stability, thermophysical and electrical properties of synthesized carbon nanofiber and reduced-graphene oxidebased nanofluids and their hybrid along with fuzzy modeling approach. Powder Technol. 2020, 364, 795–809.
- Muthoka, M.; Xuelai, Z.; Xioafeng, X. Experimental investigation on supercooling, thermal conductivity and stability of nanofluid based composite phase change material. J. Energy Storage 2018, 17, 47–55.
- Said, Z.; Allagui, A.; Abdelkareem, M.; Alawadhi, H.; Elsaid, K. Acid functionalized carbon nanofibers for high stability, thermoelectrical and electrochemical properties of nanofluids. J. Colloid Interface Sci. 2018, 18, 30190–30195.
- Alawi, O.; Mallah, A.; Kazi, S.; Sidik, N.; Najafi, G. Thermophysical properties and stability of carbon nanostructures and metallic oxides nanofluids. J. Therm. Anal. Calor. 2019, 135, 1545–1562.
- Akbari, A.; Saidi, M. Experimental investigation of nanofluid stability on thermal performance and flow regimes in pulsating heat pipe. J. Therm. Anal. Calor. 2019, 135, 1835–1847.
- Boroomandpour, A.; Toghraie, D.; Hashemian, M. A comprehensive experimental investigation of thermal conductivity of a ternary hybrid nanofluid containing MWCNTs- titania zinc oxide/water water ethylene (80:20) as well as binary and mono nanofluids. Synth. Met. 2020, 268, 116501.
- Uysal, A. Investigation of flank wear in MQL milling of ferritic stainless steel by using nano graphene reinforced vegetable cutting fluid. Ind. Lubr. Tribol. 2016, 68, 446–451.
- Al-Waeli, A.H.A.; Chaichan, M.T.; Kazem, H.A.; Sopian, K. Evaluation and analysis of nanofluid and surfactant impact on photovoltaic-thermal systems. Case Stud. Therm. Eng. 2019, 13, 100392.
- Cacua, K.; Ordoñez, F.; Zapata, C.; Herrera, B.; Pabón, E.; Buitrago-Sierra, R. Surfactant concentration and pH effects on the zeta potential values of alumina nanofluids to inspect stability. Colloids Surf. A Physicochem. Eng. Asp. 2019, 583, 123960.
- Kazemi, I.; Sefid, M.; Afrand, M. A novel comparative experimental study on rheological behavior of mono & hybrid nanofluids concerned graphene and silica nano-powders: Characterization, stability and viscosity measurements. Powder Technol. 2020, 366, 216–229.
- Xian, H.; Sidik, N.; Saidur, R. Impact of different surfactants and ultrasonication time on the Stability and thermophysical properties of hybrid nanofluids. Int. Commun. Heat Mass Transf. 2020, 110, 104389.
- Almanassra, I.; Manasrah, A.; Al-Mubaiyedh, U.; Al-Ansari, T.; Malaibari, Z.; Atieh, M. An experimental study on stability and thermal conductivity of water/CNTs nanofluids using different surfactants: A comparison study. J. Mol. Liq. 2019, 19, 30396–30397.
- Cacua, K.; Buitrago-Sierra, R.; Pabon, E.; Gallego, A.; Zapata, C.; Herrera, B. Nanofluids stability effect on a thermosyphon thermal performance. Int. J. Therm. Sci. 2020, 153, 106347.
- Ouikhalfan, M.; Labihi, A.; Belaqziz, M.; Chehouani, H.; Benhamou, B.; Sarı, A.; Belfkira, A. Stability and thermal conductivity enhancement of aqueous nanofluid based on surfactant-modified TiO2. J. Dispers. Sci. Technol. 2020, 41, 374–382.
- Siddiqui, F.; Tso, C.; Chan, K.; Fu, S.; Chao, C. On trade-off for dispersion stability and thermal transport of Cu-Al2O3 hybrid nanofluid for various mixing ratios. Int. J. Heat Mass Transf. 2019, 132, 1200–1216.
- Etedali, S.; Afrand, M.; Abdollahi, A. Effect of different surfactants on the pool boiling heat transfer of SiO2/ deionized water nanofluid on a copper surface. Int. J. Therm. Sci. 2019, 145, 105977.
- Giwa, S.; Sharifpur, M.; Goodarzi, M.; Alsulami, H.; Meyer, J. Influence of base fluid, temperature, and concentration on the thermophysical properties of hybrid nanofluids of alumina–ferrofluid: Experimental data, modeling through enhanced ANN, ANFIS, and curve fitting. J. Therm. Anal. Calorim. 2020, 6, 4149–4167.
- Kazemi, I.; Sefid, M.; Afrand, M. Improving the thermal conductivity of water by adding mono & hybrid nanoadditives containing graphene and silica: A comparative experimental study. Int. Commun. Heat Mass Transf. 2020, 116, 104648.
- Gallego, A.; Cacua, K.; Herrera, B.; Cabaleiro, D.; Piñeiro, M.; Lugo, L. Experimental evaluation of the effect in the stability and thermophysical properties of water-Al2O3 based nanofluids using SDBS as dispersant agent. Adv. Powder Technol. 2020, 31, 560–570.
- Shah, S.N.A.; Shahabuddin, S.; Sabri, M.F.M.; Salleh, M.F.M.; Ali, M.A.; Hayat, N.; Sidik, N.; Smykano, M.; Saidur, R. Experimental investigation on stability, thermal conductivity and rheological properties of rGO/ethylene glycol based nanofluids. Int. J. Heat Mass Transf. 2020, 150, 118981.
- Ilyas, S.; Ridha, S.; Kareem, F. Dispersion stability and surface tension of SDS Stabilized saline nanofluids with graphene nanoplatelets. Colloids Surf. A Physicochem. Eng. Asp. 2020, 592, 124584.
- Wu, S.; Ortiz, C. Experimental investigation of the effect of magnetic field on vapour absorption with LiBr-H2O nanofluid. Energy 2020, 193, 116640.
- Xiao, X.; Jia, H.; Wen, D.; Zhao, X. Thermal performance analysis of a solar energy storage unit encapsulated with HITEC salt/copper foam/nanoparticles composite. Energy 2020, 192, 116593.
- Lee, J.; Kang, Y. CO2 absorption enhancement by Al2O3 nanoparticles in NaCl aqueous solution. Energy 2020, 53, 206–211.
- Vajjha, R.; Das, D. Experimental determination of thermal conductivity of three nanofluids and development of new correlations. Int. J. Heat Mass Transf. 2009, 52, 4675–4682.
- Zhou, Y.; Zheng, S. Multi-level uncertainty optimisation on phase change materials integrated renewable systems with hybrid ventilations and active cooling. Energy 2020, 202, 117747.
- Bhattad, B.; Rao, B.N.; Atgur, V.; Veza, I.; Zamri, M.F.M.A.; Fattah I.M.R. Thermal performance evaluation of plate-type heat exchanger with alumina-titania hybrid suspensions. Fluids 2023, 8 (4), 120, 10.3390/fluids8040120.




