Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | FNU RAVINDER KUMAR | -- | 2204 | 2023-04-10 16:10:49 | | | |
| 2 | Catherine Yang | + 1 word(s) | 2205 | 2023-04-11 03:24:23 | | | | |
| 3 | Catherine Yang | Meta information modification | 2205 | 2023-04-11 03:25:19 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Srivastava, V.; Nand, K.N.; Ahmad, A.; Kumar, R. Yeast-Based Virus-like Particles. Encyclopedia. Available online: https://encyclopedia.pub/entry/42904 (accessed on 14 January 2026).
Srivastava V, Nand KN, Ahmad A, Kumar R. Yeast-Based Virus-like Particles. Encyclopedia. Available at: https://encyclopedia.pub/entry/42904. Accessed January 14, 2026.
Srivastava, Vartika, Kripa N. Nand, Aijaz Ahmad, Ravinder Kumar. "Yeast-Based Virus-like Particles" Encyclopedia, https://encyclopedia.pub/entry/42904 (accessed January 14, 2026).
Srivastava, V., Nand, K.N., Ahmad, A., & Kumar, R. (2023, April 10). Yeast-Based Virus-like Particles. In Encyclopedia. https://encyclopedia.pub/entry/42904
Srivastava, Vartika, et al. "Yeast-Based Virus-like Particles." Encyclopedia. Web. 10 April, 2023.
Copy Citation
Virus-like particles (VLPs) are empty, nanoscale structures morphologically resembling viruses. Internal cavity, noninfectious, and particulate nature with a high density of repeating epitopes, make them an ideal platform for vaccine development and drug delivery.
yeast
virus-like particles (VLPs)
subunit
drug delivery
1. Introduction to Virus-Like Particles
Virus-like particles (VLPs), ghost viruses, or dummy viruses lacking genetic material are nanostructures first observed in sera samples from hepatitis patients in 1968 [1]. VLPs can exist naturally (in a virally infected host) and can be generated in the laboratory. The size of VLPs may vary from 20 nm to 200 nm or more [2]. The size of VLPs depends on the virus species and viral proteins used for developing these particles [2]. It is essential to mention that VLPs can be formed using the capsid, envelope, or core viral proteins [2]. These particles are usually formed naturally by folding viral proteins under appropriate conditions, including the optimum pH, salt concentration, temperature, and so on [3][4][5]. The VLPs form when monomeric proteins fold into a pentameric form, also called capsomers, which are then assembled to form VLPs [5]. VLPs can exist either non-enveloped, as seen in the case of HPV VLPs, or enveloped with a lipid membrane (eVLPs), such as SARS coronavirus VLPs [6][7]. The shape of VLPs also differs from icosahedral to rod-shaped [8]. These particles may be composed of a single protein or can be a fusion of two different proteins (chimeric VLPs) [9][10].
The feature that makes VLPs important in vaccine development is their high density of epitopes, particulate nature, and lack of genetic material that restricts their replication and makes them safe for the host [1][11][12][13]. In addition to their use in vaccine development, VLPs are also widely investigated to deliver drugs and other small molecules inside the host system. This property is attributed to the internal cavity in VLPs. Several studies also showed the feasibility of using VLPs in photo imaging [12][13]. Different applications of VLPs are shown in Figure 1.
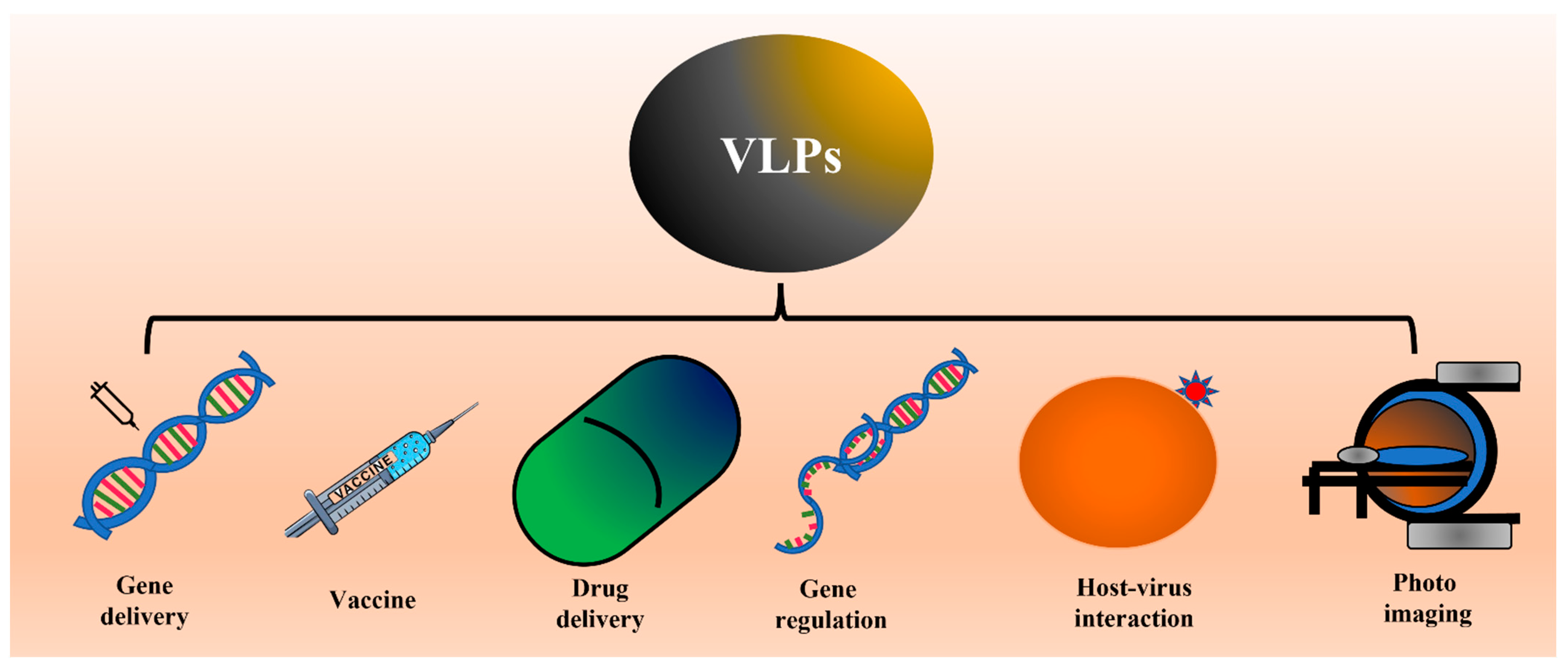
Figure 1. Schematic showing the different applications of VLPs. Several studies have already shown proof of concept for applications like vaccines, drug delivery, and photo imaging. The researchers propose the possible use of VLPs to study virus-host interaction or internalization.
The high epitope density and particulate nature of VLPs make them ideal systems for mounting immune responses. It is essential to mention that VLPs can mount both humoral and cellular immune responses [14][15][16][17][18][19]. Because of their natural resemblance to viruses, VLPs act as pathogen-associated structural patterns (PASP) and are easily recognized and taken up by host immune cells [14][15]. Additionally, their structural properties help activate immune cells like dendritic cells [20]. Due to their ability to mount both humoral and cellular immune responses, VLPs appear to be a better choice for vaccine delivery than purified proteins [21].
Due to their importance in vaccine development and drug delivery, efforts were made to express and purify VLPs from different biological systems and identify the most suitable hosts for producing VLPs commercially. To this end, VLPs are successfully expressed and purified from both the prokaryotic system (for example, Escherichia coli) [9] as well as from the eukaryotic system, including yeast (for example, S. cerevisiae) [22], insect cell lines [23][24], mammalian cell lines [14][25], and plants [26] (as shown in Figure 2).
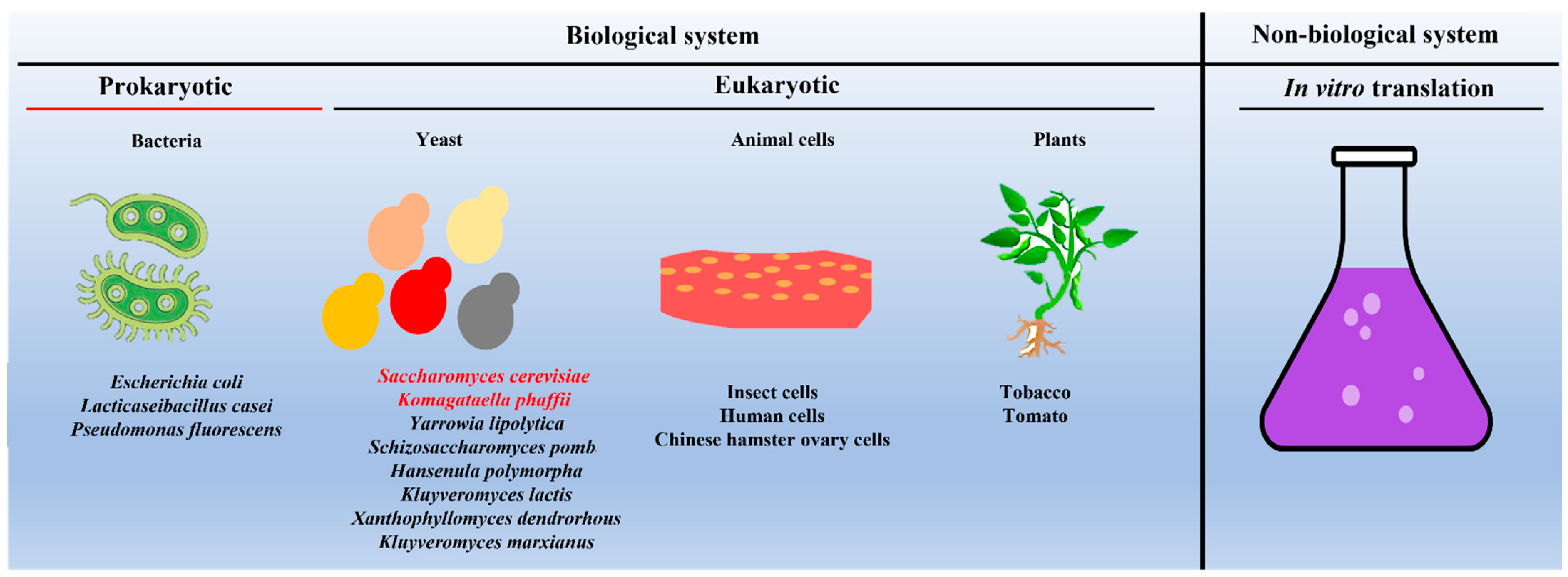
Figure 2. Schematic showing different systems used as hosts for expressing and purifying VLPs. Note: Saccharomyces cerevisiae and Komagataella phaffii (in red font) remain the most commonly used yeast species. In the case of in vitro production of VLPs, one can use the protein translational machinery of either bacteria (prokaryotes) or yeast (eukaryotes).
Apart from whole cells, several studies have shown that VLPs can be produced by in vitro protein translation systems [27]. The advantages and disadvantages of in vitro protein translation-based VLP generation are discussed by others [27][28][29]. In Table 1, the advantages and disadvantages of different systems used for the expression and purification of VLPs are compared.
Table 1. A comparison of the different model systems used as a host for the production of VLPs [28][30].
| Feature | Bacteria | Insect Cells | Mammalian Cells | Plant | Yeast |
|---|---|---|---|---|---|
| Production cost | Low | High | High | Moderate | Low |
| Growth media | Simple | Complex | Complex | Simple | Simple |
| Growth | Fast | Slow | Slow | Very slow | Fast |
| Growth duration | Very small | Small | Small | Long | Small |
| Indoor/Outdoor | Indoor | Indoor | Indoor | Outdoor/polyhouse | Indoor |
| Scale-up | Easy | Very difficult | Very difficult | Difficult | Easy |
| Secretion | No | Yes | Yes | NA | Yes |
| Enveloped/non-enveloped | Non-enveloped | Enveloped/Non-enveloped | Enveloped/Non-enveloped | NA | Non-enveloped/Enveloped possible |
| Speed of transformant screening | Very fast | Slow | Slow | Very slow | Fast |
| Effect of seasonal variations | No | No | No | Yes | No |
NA: clear information not available.
2. Yeast, Host to Produce VLPs on a Commercial Scale
As mentioned above, several biological systems have been evaluated over the years for a suitable host to produce VLPs on a commercial scale. An ideal host for the commercial purification of VLPs should be nonpathogenic, easy to handle, able to grow on economic media, able to express the protein of interest in the maximum amount, allow proper folding of a large amount of expressed protein, be genetically responsive, and be easily scaled up to industrial measures [30]. The secretion of the expressed protein(s) and VLPs (both enveloped and non-enveloped) into the medium will be another beneficial facet. Looking at these attributes, a yeast-based system appears as an ideal host for the commercial production of VLPs.
In the past, several yeasts were successfully used to express and purify clinically relevant proteins. Yeast species, S. cerevisiae and K. phaffii (formerly known as Pichia pastoris), fall under GARS (Generally Recognized as Safe), which is another significant advantage of using yeast-based systems for the development of VLPs [31]. Unlike the bacterial system, the yeast-based system does not suffer from endotoxin problems [32], and the solubility of expressed proteins and folding are much better in yeast compared to bacteria [33]. Furthermore, utilizing mammalian and insect cells for VLP production is expensive due to the high cost of media and poor scalability [34]. In contrast, yeast can quickly grow on simple media [35]. A yeast species, K. phaffii, can be grown to a high cell density on a commercial scale, which is not feasible with animal cells. The rapid growth of yeast cells (unlike animal cells, yeast cells grow faster with a doubling time of around 90–120 min, whereas animal cells have a doubling time of 16–18 h or more) is another advantage [36]. The growth of plants is slow and may take several weeks, months, or even years to reach the desired maturity. Another issue is the varying expression levels in different organs or tissues and more batch-to-batch variation. Other concerns include the possibility of escape into the natural environment [37]. The seasonal variation may severely impact plant growth, so the expression of the protein of interest remains an important consideration. Due to several advantages (as mentioned), yeast has been extensively used for expressing and purifying VLPs, especially S. cerevisiae (Table 2) and K. phaffii (Table 3).
Table 2. Studies in which S. cerevisiae yeast was used to generate VLPs.
| S. No | Protein Antigen | Virus | Protein Localization |
Promoter | References |
|---|---|---|---|---|---|
| 1 | VP2 | Human parvovirus 4 | IC | Hybrid GAL10-PYK1promoter | [38] |
| 2 | Capsid protein | Hepatitis E virus | IC | GAL promoter | [39] |
| 3 | Capsid protein | Porcine circovirus type 2 | EC | GPD, TEF2 | [40] |
| 4 | Nucleocapsid protein | Sendai virus | IC | GAL7 | [41] |
| 5 | VP2, VP1 | Human bocaviruses | IC | [42] | |
| 6 | Surface antigen | Hepatitis B virus | IC | GAL | [43][44][45] |
| 7 | p55(gag) | HIV-1 | EC | [46] | |
| 8 | VP1 | Human polyomaviruses | IC | GAL | [47] |
| 9 | L1 | HPV 16 | IC | GAL10 | [48][49] |
| 10 | Nucleocapsid protein | Tioman virus | IC | GAL10 | [50] |
| 11 | L-HDAg and surface antigen | Hepatitis delta virus | IC | GAD | [51] |
| 12 | Capsid protein | Porcine circovirus type 2 | IC | GAL10 | [52] |
| 13 | Capsid protein | Enterovirus 71 | IC | GAL10 | [53] |
| 14 | VP1 | Human and non-human polyomaviruses | IC | GAL | [54] |
| 15 | Capsid protein | Adeno-associated virus | IC | GAL1 | [55] |
| 16 | Nucleocapsid protein | Human parainfluenza virus 4 | IC | GAL7 | [56] |
| 17 * | Coat protein | Cacteriophage Qbeta virus | IC | GAL | [57] |
| 18 | Capsid protein | Nervous necrosis virus | IC | GAL | [58] |
| 19 | VP1,2 | Parvovirus B19 | IC | ADH2/GAPDH | [59][60] |
| 20 | VP1 | Bird polyomaviruses | IC | GAL | [61] |
| 21 | P1 | Enterovirus 71 and Coxsackievirus A16 | IC | GAL1 | [62] |
| 22 | Capsid protein | Porcine circovirus type 2 | IC | GAL1 | [63] |
| 23 | VP2,6,7 | Rotavirus | IC | PGK1, TEF1 | [64][65] |
| 24 | VP2 | Human parvovirus 4 | IC | GAL1-10 | [66] |
| 25 | Nucleocapsid protein | Human parainfluenza virus 2 | IC | GAL | [67] |
| 26 | Nucleocapsid protein | Menangle virus | IC | GAL7 | [68] |
| 27 | Gag | HIV-1 | IC | GAP | [69] |
| 28 | VP2 | Porcine parvovirus | IC | GAL1-10 | [70] |
| 29 | P1, CD3 | Coxsackievirus A16 | IC | GAL1 | [71] |
| 30 | Capsid protein | Porcine circovirus type 2 | IC | GAL | [72] |
| 31 | VP1,2 | Hepatitis B/Polyomavirus | IC | GAL7 | [73] |
| 32 | VP1 | Hamster polyomavirus | IC | [74] | |
| 33 | L1/L1 + L2 | Cottontail rabbit papillomavirus | IC | GAL1-10 | [75] |
| 34 | L1 | HPV 11 | IC | GAL | [76][77][78] |
| 35 | Coat protein | Potyvirus (Johnsongrass mosaic virus) | IC | ADC1 | [79] |
| 36 | P1, CD3 | Poliovirus type I | IC | [80] | |
| 37 | HIV-1 | IC | [81] | ||
| 38 | L1 | HPV 16 | IC | GAL | [82] |
| 39 | L1 | HPV 6,11 16 | IC | GAL | [83] |
| 40 | VP1 with Puumala hantavirus nucleocapsid protein segments | Hamster polyomavirus | IC | Hybrid GAL10-PYK1 | [84] |
| 41 | M protein | Hepatitis B virus | IC | GAL10/CYC1 | [85] |
| 42 | VP1,2 | Goose hemorrhagic polyomavirus | IC | GAL | [86] |
| 43 | CEA/VP1 | Hamster polyomavirus | IC | GAL | [87] |
| 44 | E7 oncoprotein of HPV16 | Hepatitis B virus | IC | [88] | |
| 45 | Capsid protein | Red-spotted grouper nervous necrosis virus | IC | GAL10 | [89] |
| 46 | L1 | HPV 58 | IC | GAL10 | [90] |
| 47 | C69R variant of surface antigen | Hepatitis B virus | IC | GAL10/CYC1 | [91] |
| 48 | VP1 | Human polyomavirus 2 | IC | GAL | [92] |
| 49 | VP2 | Parvovirus B19 | IC | GAL1 | [93] |
| 50 | L1 | HPV 11 | IC | GAL110-11 | [94] |
| 51 | VP1 with pre-S1 region of the Hepatitis B virus | Hamster polyomavirus | IC | GAL | [95] |
| 52 | Surface antigen | Hepatitis B virus | IC | GAL10 | [96] |
| 53 | L1 | HPV 16 | IC | [97] |
* Additionally, expressed in K. phaffii; IC: intracellular; EC: extracellular.
Table 3. Studies where K. phaffii yeast was used to generate VLPs.
| S. No | Protein Antigen | Virus | Protein Localization |
Promoter | References |
|---|---|---|---|---|---|
| 1 | Capsid protein | Red-spotted grouper nervous necrosis virus | IC | Pw42-2 | [98] |
| 2 | ZS and S | Zika virus | IC | AOX1 | [99] |
| 3 | 112-608aa of the ORF2 | Hepatitis E virus | EC | AOX1 | [100] |
| 4 | P1 and CD3 | Poliovirus type I | IC | AOX1 | [101][102] |
| 5 | Chimeric HPV-HIV L1P18 protein | HPV and HIV | IC | GAP | [103] |
| 6 | NY-ESO-1 cancer testis antigen | Norovirus | EC | AOX1 | [104] |
| 7 | Surface antigen | Hepatitis C virus | IC | AOX1 | [105][106] |
| 8 | P1 and 3CD | Enterovirus 71 | EC | AOX1 | [107][108] |
| 9 | E domain III | Dengue Virus | IC | AOX1 | [109][110] |
| 10 | P1 and 3CD | Coxsackievirus A16 | EC | GAP | [111] |
| 11 | VP1 | Norovirus | EC | AOX1 | [112] |
| 12 | E antigen | Dengue virus | IC | AOX1 | [113][114][115][116][117][118] |
| 13 | prME | Japanese encephalitis virus | EC | AOX1 | [119] |
| 14 | E antigen | Dengue virus | EC | GAP | [120] |
| 15 | Matrix protein | Nipah virus | IC | AOX1 | [121] |
| 16 | P1 and 3CD | Enterovirus D68 | IC | AOX1 | [122] |
| 17 | prM and E protein | Tick-borne encephalitis virus | EC | GAP | [123] |
| 18 | P1 and 3CD | Coxsackievirus A10 | IC | AOX1 | [124] |
| 19 | Surface antigen | Hepatitis B virus | IC | AOX1 | [125][126][127][128][129][130][131] |
| 20 | prM and E protein | Dengue virus | IC | GAP | [132] |
| 21 | L1 | HPV16 and 18 | IC | AOX1 | [133][134][135] |
| 22 | L1 | HPV 52 | AOX1 | [136] | |
| 23 | Capsid protein | Cowpea chlorotic mottle virus | EC | AOX1 | [137] |
| 24 | L1 | HPV 58 | IC | AOX1 | [138] |
| 25 | Envelope protein domain III (EDIII), hepatitis B surface antigen | Dengue virus | IC | AOX1 | [139] |
| 26 | VP2 | Infectious bursal disease virus | IC | AOX1 | [140] |
| 27 | P1 and 3CD | Coxsackievirus A16 | IC | AOX1 | [141] |
| 28 | Core protein | Hepatitis B virus | IC | AOX1 | [142][143] |
| 29 | E protein | Dengue virus | IC | AOX1 | [144] |
| 30 | L2 | Grapevine fanleaf virus | EC | AOX1 | [145] |
| 31 | Capsid protein (VP60) | Rabbit hemorrhagic disease virus | IC | AOX1 | [146] |
| 32 | HBc-influenza virus LAH domain | Hepatitis B/Influenza H3N2 virus | IC | AOX1 | [147] |
| 33 | CoreE1E2 Protein | Hepatitis C virus | EC | AOX1 | [148] |
| 34 | P1 and 3CD | Coxsackievirus A6 | IC | AOX1 | [149] |
| 35 | L1, L2 | HPV 16 | IC | AOX1 | [150] |
| 36 | prM/Env | Japanese encephalitis virus | IC | AOX1 | [151] |
| 37 | Den2E-HBsAg | Dengue/Hepatitis B virus | IC | AOX1 | [152] |
| 38 | prM and E protein | Dengue virus | IC | GAP | [153] |
| 39 | Polyprotein | Chikungunya virus | EC | AOX1 | [154] |
| 40 | L1 | HPV 16 | [155] | ||
| 41 | Major capsid protein | Iridovirus | EC | AOX1 | [156] |
| 42 | Surface antigen | Hepatitis B virus | [157] | ||
| 43 | VP1 | Rabbit hemorrhagic disease virus | IC | AOX1 | [158] |
| 44 | L1 | Bovine papillomavirus 1,2,4 | [159] | ||
| 45 | L1 | HPV 16 | IC | AOX1 | [160] |
| 46 | Capsid protein | Norovirus | IC | AOX1 | [161] |
| 47 | L1 | HPV 16 | EC | PGK1 | [162] |
| 48 | Core protein | Hepatitis B virus | [163] | ||
| 49 | VP1 | Calicivirus virus | EC | AOX1 | [164] |
| 50 | Core protein | Hepatitis C virus | IC | AOX1 | [165][166][167][168] |
IC: intracellular; EC: extracellular.
3. Aggregation of VLPs, a Matter of Concern
The production of VLPs on a commercial scale using the different hosts mentioned above is now becoming common practice. Several companies dealing in vaccines or drugs are producing VLPs commercially. Almost all VLP production suffers from the common issue of VLP aggregation when stored at a low-salt concentration and at 2–8 °C [169][170][171][172][173]. Dynamic Light Scattering (DLS), Transmission Electron Microscopes (TEM), and Atomic Force Microscopes (AFM) can detect the aggregation of VLPs, approaches commonly used for VLP characterization (reviewed by [3]). In addition, turbidity during elution or storage can also inform about possible VLP aggregation. The main problem with the aggregation of VLPs is that it affects immunogenicity and the level of an immune response. Aggregation of VLPs reduces their recovery using POROS resin used for the purification of VLPs on a commercial scale [87]. It was observed that the immune response raised by aggregated VLPs is lower than that raised by non-aggregated VLPs [169][174][175][176][177]. Additionally, aggregation can affect dose formulation. To obtain highly monodisperse VLPs, the manufacturer performs an ultracentrifuge or size exclusion to separate clean and aggregated VLPs. However, this leads to considerable wastage of VLPs and reduces the recovery of useful VLPs.
To prevent or minimize the aggregation of VLPs, different approaches have been used in the past. For example, adding L-arginine and glycine improves VLP recovery [177][178]. Sugars like sorbitol and trehalose are also shown to solubilize and improve VLP recovery by preventing their aggregation and by solubilizing protein monomers [179][180]. The use of polyethylene glycol (PEG) or glycerol also improves the solubilization of VLPs [181][182]. Apart from this, using surfactants or emulsifiers like Tween-80 is also helpful in preventing the aggregation of VLPs and improving the recovery of VLPs [183]. In several published studies, the author has added these compounds at one or another step in the purification of VLPs. Sometimes changes in the pH of the buffer and the buffer composition were also found helpful in preventing the aggregation of VLPs [184]. Additionally, to obtain VLPs of a uniform size, in vitro disassembly followed by reassembly is recommended [185]. Therefore, it requires substantial effort to find suitable conditions that improve the recovery of VLPs while simultaneously preventing their aggregation.
References
- Bayer, M.E.; Blumberg, B.S.; Werner, B. Particles associated with Australia antigen in the sera of patients with leukaemia, Down’s Syndrome and Hepatitis. Nature 1968, 218, 1057–1059.
- Grgacic, E.V.; Anderson, D.A. Virus-like particles: Passport to immune recognition. Methods 2006, 40, 60–65.
- Mejía-Méndez, J.L.; Vazquez-Duhalt, R.; Hernández, L.R.; Sánchez-Arreola, E.; Bach, H. Virus-like Particles: Fundamentals and Biomedical Applications. Int. J. Mol. Sci. 2022, 23, 8579.
- Lan, K.; Luo, M.H. Herpesviruses: Epidemiology, pathogenesis, and interventions. Virol. Sin. 2017, 32, 347–348.
- Le, D.T.; Müller, K.M. In Vitro Assembly of Virus-Like Particles and Their Applications. Life 2021, 11, 334.
- Jeong, H.; Seong, B.L. Exploiting virus-like particles as innovative vaccines against emerging viral infections. J. Microbiol. 2017, 55, 220–230.
- Mortola, E.; Roy, P. Efficient assembly and release of SARS coronavirus-like particles by a heterologous expression system. FEBS Lett. 2004, 576, 174–178.
- Pushko, P.; Pumpens, P.; Grens, E. Development of virus-like particle technology from small highly symmetric to large complex virus-like particle structures. Intervirology 2013, 56, 141–165.
- Latham, T.; Galarza, J.M. Formation of wild-type and chimeric influenza virus-like particles following simultaneous expression of only four structural proteins. J. Virol. 2001, 75, 6154–6165.
- Gedvilaite, A.; Kucinskaite-Kodze, I.; Lasickiene, R.; Timinskas, A.; Vaitiekaite, A.; Ziogiene, D.; Zvirbliene, A. Evaluation of Trichodysplasia Spinulosa-Associated Polyomavirus Capsid Protein as a New Carrier for Construction of Chimeric Virus-Like Particles Harboring Foreign Epitopes. Viruses 2015, 7, 4204–4229.
- Bai, B.; Hu, Q.; Hu, H.; Zhou, P.; Shi, Z.; Meng, J.; Lu, B.; Huang, Y.; Mao, P.; Wang, H. Virus-like particles of SARS-like coronavirus formed by membrane proteins from different origins demonstrate stimulating activity in human dendritic cells. PLoS ONE 2008, 3, e2685.
- Chung, Y.H.; Cai, H.; Steinmetz, N.F. Viral nanoparticles for drug delivery, imaging, immunotherapy, and theranostic applications. Adv. Drug Deliv. Rev. 2020, 156, 214–235.
- Steinmetz, N.F. Viral nanoparticles as platforms for next-generation therapeutics and imaging devices. Nanomedicine 2010, 6, 634–641.
- Mohsen, M.O.; Gomes, A.C.; Vogel, M.; Bachmann, M.F. Interaction of viral capsid-derived virus-like particles (VLPs) with the innate immune system. Vaccines 2018, 6, 37.
- Silva, A.L.; Peres, C.; Conniot, J.; Matos, A.I.; Moura, L.; Carreira, B.; Sainz, V.; Scomparin, A.; Satchi-Fainaro, R.; Préat, V.; et al. Nanoparticle impact on innate immune cell pattern-recognition receptors and inflammasomes activation. Semin. Immunol. 2017, 34, 3–24.
- Lee, Y.T.; Ko, E.J.; Lee, Y.; Kim, K.H.; Kim, M.C.; Lee, Y.N.; Kang, S.M. Intranasal vaccination with M2e5x virus-like particles induces humoral and cellular immune responses conferring cross-protection against heterosubtypic influenza viruses. PLoS ONE 2018, 13, e0190868.
- Wang, C.; Zheng, X.; Gai, W.; Wong, G.; Wang, H.; Jin, H.; Feng, N.; Zhao, Y.; Zhang, W.; Li, N.; et al. Novel chimeric virus-like particles vaccine displaying MERS-CoV receptor-binding domain induce specific humoral and cellular immune response in mice. Antiviral Res. 2017, 140, 55–61.
- Weber, J.; Cheinsong-Popov, R.; Callow, D.; Adams, S.; Patou, G.; Hodgkin, K.; Martin, S.; Gotch, F.; Kingsman, A. Immunogenicity of the yeast recombinant p17p24: Ty virus-like particles (p24-VLP) in healthy volunteers. Vaccine 1995, 13, 831–834.
- Yong, C.Y.; Yeap, S.K.; Goh, Z.H.; Ho, K.L.; Omar, A.R.; Tan, W.S. Induction of humoral and cell-mediated immune responses by hepatitis B virus epitope displayed on the virus-like particles of prawn nodavirus. Appl. Environ. Microbiol. 2015, 81, 882–889.
- Ye, L.; Lin, J.; Sun, Y.; Bennouna, S.; Lo, M.; Wu, Q.; Bu, Z.; Pulendran, B.; Compans, R.W.; Yang, C. Ebola virus-like particles produced in insect cells exhibit dendritic cell stimulating activity and induce neutralizing antibodies. Virology 2006, 351, 260–270.
- Available online: https://www.gavi.org/vaccineswork/what-are-protein-subunit-vaccines-and-how-could-they-be-used-against-covid-19 (accessed on 10 November 2022).
- Sailaja, G.; Skountzou, I.; Quan, F.S.; Compans, R.W.; Kang, S.M. Human immunodeficiency virus-like particles activate multiple types of immune cells. Virology 2007, 362, 331–341.
- Le, D.T.; Radukic, M.T.; Mller, K.M. Adeno-associated virus capsid protein expression in Escherichia coli and chemically defined capsid assembly. Sci. Rep. 2019, 9, 18631.
- Joe, C.C.; Chatterjee, S.; Lovrecz, G.; Adams, T.E.; Thaysen-Andersen, M.; Walsh, R.; Locarnini, S.A.; Smooker, P.; Netter, H.J. Glycoengineered hepatitis B virus-like particles with enhanced immunogenicity. Vaccine 2020, 38, 3892–3901.
- Shiri, F.; Petersen, K.E.; Romanov, V.; Zou, Q.; Gale, B.K. Characterization and differential retention of Q beta bacteriophage virus-like particles using cyclical electrical field–flow fractionation and asymmetrical flow fieldflow fractionation. Anal. Bioanal. Chem. 2020, 412, 1563–1572.
- Zhai, L.; Yadav, R.; Kunda, N.K.; Anderson, D.; Bruckner, E.; Miller, E.K.; Basu, R.; Muttil, P.; Tumban, E. Oral immunization with bacteriophage MS2-L2 VLPs protects against oral and genital infection with multiple HPV types associated with head & neck cancers and cervical cancer. Antiviral Res. 2019, 166, 56–65.
- Glass, P.J.; White, L.J.; Ball, J.M.; Leparc-Goffart, I.; Hardy, M.E.; Estes, M.K. Norwalk virus open reading frame 3 encodes a minor structural protein. J. Virol. 2000, 74, 6581–6591.
- Nooraei, S.; Bahrulolum, H.; Hoseini, Z.S.; Katalani, C.; Hajizade, A.; Easton, A.J.; Ahmadian, G. Virus-like particles: Preparation, immunogenicity and their roles as nanovaccines and drug nanocarriers. J. Nanobiotechnol. 2021, 19, 59.
- Donaldson, B.; Al-Barwani, F.; Young, V.; Scullion, S.; Ward, V.; Young, S. Virus-Like Particles, a Versatile Subunit Vaccine Platform. In Subunit Vaccine Delivery; Part of the Advances in Delivery Science and Technology Book Series; Springer: New York, NY, USA, 2014; pp. 159–180.
- Kim, H.J.; Kim, H.J. Yeast as an expression system for producing virus-like particles: What factors do we need to consider? Lett. Appl. Microbiol. 2017, 64, 111–123.
- Kumar, R.; Kumar, P. Yeast-based vaccines: New perspective in vaccine development and application. FEMS Yeast Res. 2019, 19, foz007.
- Magalhães, P.O.; Lopes, A.M.; Mazzola, P.G.; Rangel-Yagui, C.; Penna, T.C.; Pessoa, A., Jr. Methods of endotoxin removal from biological preparations: A review. J. Pharm. Pharm. Sci. 2007, 10, 388–404.
- Baneyx, F.; Mujacic, M. Recombinant protein folding and misfolding in Escherichia coli. Nat. Biotechnol. 2004, 22, 1399–1408.
- Dalton, A.C.; Barton, W.A. Over-expression of secreted proteins from mammalian cell lines. Protein Sci. 2014, 23, 517–525.
- Bill, R.M. Recombinant protein subunit vaccine synthesis in microbes: A role for yeast? J. Pharm. Pharmacol. 2015, 67, 319–328.
- Karbalaei, M.; Rezaee, S.A.; Farsiani, H. Pichia pastoris: A highly successful expression system for optimal synthesis of heterologous proteins. J. Cell. Physiol. 2020, 235, 5867–5881.
- Desai, P.N.; Shrivastava, N.; Padh, H. Production of heterologous proteins in plants: Strategies for optimal expression. Biotechnol. Adv. 2010, 28, 427–435.
- Lazutka, J.; Simutis, K.; Matulis, P.; Petraitytė-Burneikienė, R.; Kučinskaitė-Kodzė, I.; Simanavičius, M.; Tamošiunas, P.L. Antigenicity study of the yeast-generated human parvovirus 4 (PARV4) virus-like particles. Virus Res. 2021, 292, 198236.
- Simanavicius, M.; Tamosiunas, P.L.; Petraityte-Burneikiene, R.; Johne, R.; Ulrich, R.G.; Zvirbliene, A.; Kucinskaite-Kodze, I. Generation in yeast and antigenic characterization of hepatitis E virus capsid protein virus-like particles. Appl. Microbiol. Biotechnol. 2018, 102, 185–198.
- Chen, P.; Zhang, L.; Chang, N.; Shi, P.; Gao, T.; Zhang, L.; Huang, J. Preparation of virus-like particles for porcine circovirus type 2 by Yeast Fab Assembly. Virus Genes 2018, 54, 246–255.
- Juozapaitis, M.; Slibinskas, R.; Staniulis, J.; Sakaguchi, T.; Sasnauskas, K. Generation of Sendai virus nucleocapsid-like particles in yeast. Virus Res. 2005, 108, 221–224.
- Tamošiūnas, P.L.; Petraitytė-Burneikienė, R.; Bulavaitė, A.; Marcinkevičiūtė, K.; Simutis, K.; Lasickienė, R.; Firantienė, R.; Ėmužytė, R.; Žvirblienė, A.; Sasnauskas, K. Yeast-generated virus-like particles as antigens for detection of human bocavirus 1-4 specific antibodies in human serum. Appl. Microbiol. Biotechnol. 2016, 100, 4935–4946.
- Burden, C.S.; Jin, J.; Podgornik, A.; Bracewell, D.G. A monolith purification process for virus-like particles from yeast homogenate. J. Chromatogr. B 2012, 880, 82–89.
- Kee, G.S.; Jin, J.; Balasundaram, B.; Bracewell, D.G.; Pujar, N.S.; Titchener-Hooker, N.J. Exploiting the intracellular compartmentalization characteristics of the S. cerevisiae host cell for enhancing primary purification of lipid-envelope virus-like particles. Biotechnol. Prog. 2010, 26, 26–33.
- Kee, G.S.; Pujar, N.S.; Titchener-Hooker, N.J. Study of detergent-mediated liberation of hepatitis B virus-like particles from S. cerevisiae homogenate: Identifying a framework for the design of future-generation lipoprotein vaccine processes. Biotechnol. Prog. 2008, 24, 623–631.
- Tsunetsugu-Yokota, Y.; Morikawa, Y.; Isogai, M.; Kawana-Tachikawa, A.; Odawara, T.; Nakamura, T.; Grassi, F.; Autran, B.; Iwamoto, A. Yeast-derived human immunodeficiency virus type 1 p55(gag) virus-like particles activate dendritic cells (DCs) and induce perforin expression in Gag-specific CD8+ T cells by cross-presentation of DCs. J. Virol. 2003, 77, 10250–10259.
- Norkiene, M.; Stonyte, J.; Ziogiene, D.; Mazeike, E.; Sasnauskas, K.; Gedvilaite, A. Production of recombinant VP1-derived virus-like particles from novel human polyomaviruses in yeast. BMC Biotechnol. 2015, 15, 68.
- Kim, H.J.; Cho, S.Y.; Park, M.H.; Kim, H.J. Comparison of the size distributions and immunogenicity of human papillomavirus type 16 L1 virus-like particles produced in insect and yeast cells. Arch. Pharm. Res. 2018, 41, 544–553.
- Kim, H.J.; Jin, Y.; Kim, H.J. The concentration of carbon source in the medium affects the quality of virus-like particles of human papillomavirus type 16 produced in Saccharomyces cerevisiae. PLoS ONE 2014, 9, e94467.
- Petraityte, R.; Tamosiunas, P.L.; Juozapaitis, M.; Zvirbliene, A.; Sasnauskas, K.; Shiell, B.; Russell, G.; Bingham, J.; Michalski, W.P. Generation of Tioman virus nucleocapsid-like particles in yeast Saccharomyces cerevisiae. Virus Res. 2009, 145, 92–96.
- Wu, H.L.; Chen, P.J.; Mu, J.J.; Chi, W.K.; Kao, T.L.; Hwang, L.H.; Chen, D.S. Assembly of hepatitis delta virus-like empty particles in yeast. Virology 1997, 236, 374–381.
- Nainys, J.; Lasickiene, R.; Petraityte-Burneikiene, R.; Dabrisius, J.; Lelesius, R.; Sereika, V.; Zvirbliene, A.; Sasnauskas, K.; Gedvilaite, A. Generation in yeast of recombinant virus-like particles of porcine circovirus type 2 capsid protein and their use for a serologic assay and development of monoclonal antibodies. BMC Biotechnol. 2014, 14, 100.
- Wang, X.; Xiao, X.; Zhao, M.; Liu, W.; Pang, L.; Sun, X.; Cen, S.; Yang, B.B.; Huang, Y.; Sheng, W.; et al. EV71 virus-like particles produced by co-expression of capsid proteins in yeast cells elicit humoral protective response against EV71 lethal challenge. BMC Res. Notes 2016, 9, 42.
- Sasnauskas, K.; Bulavaite, A.; Hale, A.; Jin, L.; Knowles, W.A.; Gedvilaite, A.; Dargeviciūte, A.; Bartkeviciūte, D.; Zvirbliene, A.; Staniulis, J.; et al. Generation of recombinant virus-like particles of human and non-human polyomaviruses in yeast Saccharomyces cerevisiae. Intervirology 2002, 45, 308–317.
- Backovic, A.; Cervelli, T.; Salvetti, A.; Zentilin, L.; Giacca, M.; Galli, A. Capsid protein expression and adeno-associated virus like particles assembly in Saccharomyces cerevisiae. Microb. Cell Fact. 2012, 11, 124.
- Bulavaitė, A.; Lasickienė, R.; Tamošiūnas, P.L.; Simanavičius, M.; Sasnauskas, K.; Žvirblienė, A. Synthesis of human parainfluenza virus 4 nucleocapsid-like particles in yeast and their use for detection of virus-specific antibodies in human serum. Appl. Microbiol. Biotechnol. 2017, 101, 2991–3004.
- Freivalds, J.; Dislers, A.; Ose, V.; Skrastina, D.; Cielens, I.; Pumpens, P.; Sasnauskas, K.; Kazaks, A. Assembly of bacteriophage Qbeta virus-like particles in yeast Saccharomyces cerevisiae and Pichia pastoris. J. Biotechnol. 2006, 123, 297–303.
- Wi, G.R.; Hwang, J.Y.; Kwon, M.G.; Kim, H.J.; Kang, H.A.; Kim, H.J. Protective immunity against nervous necrosis virus in convict grouper Epinephelus septemfasciatus following vaccination with virus-like particles produced in yeast Saccharomyces cerevisiae. Vet. Microbiol. 2015, 177, 214–218.
- Penkert, R.R.; Young, N.S.; Surman, S.L.; Sealy, R.E.; Rosch, J.; Dormitzer, P.R.; Settembre, E.C.; Chandramouli, S.; Wong, S.; Hankins, J.S.; et al. Saccharomyces cerevisiae-derived virus-like particle parvovirus B19 vaccine elicits binding and neutralizing antibodies in a mouse model for sickle cell disease. Vaccine 2017, 35, 3615–3620.
- Chandramouli, S.; Medina-Selby, A.; Coit, D.; Schaefer, M.; Spencer, T.; Brito, L.A.; Zhang, P.; Otten, G.; Mandl, C.W.; Mason, P.W.; et al. Generation of a parvovirus B19 vaccine candidate. Vaccine 2013, 31, 3872–3878.
- Zielonka, A.; Gedvilaite, A.; Reetz, J.; Rösler, U.; Müller, H.; Johne, R. Serological cross-reactions between four polyomaviruses of birds using virus-like particles expressed in yeast. J. Gen. Virol. 2012, 93, 2658–2667.
- Zhao, H.; Li, H.Y.; Han, J.F.; Deng, Y.Q.; Zhu, S.Y.; Li, X.F.; Yang, H.Q.; Li, Y.X.; Zhang, Y.; Qin, E.D.; et al. Novel recombinant chimeric virus-like particle is immunogenic and protective against both enterovirus 71 and coxsackievirus A16 in mice. Sci. Rep. 2015, 5, 7878.
- Bucarey, S.A.; Noriega, J.; Reyes, P.; Tapia, C.; Sáenz, L.; Zuñiga, A.; Tobar, J.A. The optimized capsid gene of porcine circovirus type 2 expressed in yeast forms virus-like particles and elicits antibody responses in mice fed with recombinant yeast extracts. Vaccine 2009, 27, 5781–5790.
- Rodríguez-Limas, W.A.; Tyo, K.E.; Nielsen, J.; Ramírez, O.T.; Palomares, L.A. Molecular and process design for rotavirus-like particle production in Saccharomyces cerevisiae. Microb. Cell Fact. 2011, 10, 33.
- Rodríguez-Limas, W.A.; Pastor, A.R.; Esquivel-Soto, E.; Esquivel-Guadarrama, F.; Ramírez, O.T.; Palomares, L.A. Immunogenicity and protective efficacy of yeast extracts containing rotavirus-like particles: A potential veterinary vaccine. Vaccine 2014, 32, 2794–2798.
- Tamošiūnas, P.L.; Simutis, K.; Kodzė, I.; Firantienė, R.; Emužytė, R.; Petraitytė-Burneikienė, R.; Zvirblienė, A.; Sasnauskas, K. Production of human parvovirus 4 VP2 virus-like particles in yeast and their evaluation as an antigen for detection of virus-specific antibodies in human serum. Intervirology 2013, 56, 271–277.
- Bulavaitė, A.; Lasickienė, R.; Vaitiekaitė, A.; Sasnauskas, K.; Žvirblienė, A. Synthesis of human parainfluenza virus 2 nucleocapsid protein in yeast as nucleocapsid-like particles and investigation of its antigenic structure. Appl. Microbiol. Biotechnol. 2016, 100, 4523–4534.
- Juozapaitis, M.; Serva, A.; Kucinskaite, I.; Zvirbliene, A.; Slibinskas, R.; Staniulis, J.; Sasnauskas, K.; Shiell, B.J.; Bowden, T.R.; Michalski, W.P. Generation of menangle virus nucleocapsid-like particles in yeast Saccharomyces cerevisiae. J. Biotechnol. 2007, 130, 441–447.
- Tomo, N.; Goto, T.; Morikawa, Y. Trans-packaging of human immunodeficiency virus type 1 genome into Gag virus-like particles in Saccharomyces cerevisiae. Microb. Cell Fact. 2013, 12, 28.
- Tamošiūnas, P.L.; Petraitytė-Burneikienė, R.; Lasickienė, R.; Akatov, A.; Kundrotas, G.; Sereika, V.; Lelešius, R.; Žvirblienė, A.; Sasnauskas, K. Generation of recombinant porcine parvovirus virus-like particles in Saccharomyces cerevisiae and development of virus-specific monoclonal antibodies. J. Immunol. Res. 2014, 2014, 573531.
- Zhao, H.; Li, H.Y.; Han, J.F.; Deng, Y.Q.; Li, Y.X.; Zhu, S.Y.; He, Y.L.; Qin, E.D.; Chen, R.; Qin, C.F. Virus-like particles produced in Saccharomyces cerevisiae elicit protective immunity against Coxsackievirus A16 in mice. Appl. Microbiol. Biotechnol. 2013, 97, 10445–10452.
- Zaveckas, M.; Snipaitis, S.; Pesliakas, H.; Nainys, J.; Gedvilaite, A. Purification of recombinant virus-like particles of porcine circovirus type 2 capsid protein using ion-exchange monolith chromatography. J. Chromatogr. B 2015, 991, 21–28.
- Pleckaityte, M.; Bremer, C.M.; Gedvilaite, A.; Kucinskaite-Kodze, I.; Glebe, D.; Zvirbliene, A. Construction of polyomavirus-derived pseudotype virus-like particles displaying a functionally active neutralizing antibody against hepatitis B virus surface antigen. BMC Biotechnol. 2015, 15, 85.
- Sasnauskas, K.; Buzaite, O.; Vogel, F.; Jandrig, B.; Razanskas, R.; Staniulis, J.; Scherneck, S.; Krüger, D.H.; Ulrich, R. Yeast cells allow high-level expression and formation of polyomavirus-like particles. Biol. Chem. 1999, 380, 381–386.
- Jansen, K.U.; Rosolowsky, M.; Schultz, L.D.; Markus, H.Z.; Cook, J.C.; Donnelly, J.J.; Martinez, D.; Ellis, R.W.; Shaw, A.R. Vaccination with yeast-expressed cottontail rabbit papillomavirus (CRPV) virus-like particles protects rabbits from CRPV-induced papilloma formation. Vaccine 1995, 13, 1509–1514.
- Lowe, R.S.; Brown, D.R.; Bryan, J.T.; Cook, J.C.; George, H.A.; Hofmann, K.J.; Hurni, W.M.; Joyce, J.G.; Lehman, E.D.; Markus, H.Z.; et al. Human papillomavirus type 11 (HPV-11) neutralizing antibodies in the serum and genital mucosal secretions of African green monkeys immunized with HPV-11 virus-like particles expressed in yeast. J. Infect. Dis. 1997, 176, 1141–1145.
- Neeper, M.P.; Hofmann, K.J.; Jansen, K.U. Expression of the major capsid protein of human papillomavirus type 11 in Saccharomyces cerevisae. Gene 1996, 180, 1–6.
- Cook, J.C.; Joyce, J.G.; George, H.A.; Schultz, L.D.; Hurni, W.M.; Jansen, K.U.; Hepler, R.W.; Ip, C.; Lowe, R.S.; Keller, P.M.; et al. Purification of virus-like particles of recombinant human papillomavirus type 11 major capsid protein L1 from Saccharomyces cerevisiae. Protein Expr. Purif. 1999, 17, 477–484.
- Jagadish, M.N.; Ward, C.W.; Gough, K.H.; Tulloch, P.A.; Whittaker, L.A.; Shukla, D.D. Expression of potyvirus coat protein in Escherichia coli and yeast and its assembly into virus-like particles. J. Gen. Virol. 1991, 72, 1543–1550.
- Wang, X.W.; Sheng, W.; Zeng, Y. Formation and identification of virus-like particles of poliovirus type I. Chin. J. Exp. Clin. Virol. 2013, 27, 373–375. (In Chinese)
- Karpenko, L.I.; Lebedev, L.R.; Ignatyev, G.M.; Agafonov, A.P.; Poryvaeva, V.A.; Pronyaeva, T.R.; Ryabchikova, E.I.; Pokrovsky, A.G.; Ilyichev, A.A. Construction of artificial virus-like particles exposing HIV epitopes, and the study of their immunogenic properties. Vaccine 2003, 21, 386–392.
- Mukherjee, S.; Thorsteinsson, M.V.; Johnston, L.B.; DePhillips, P.A.; Zlotnick, A. A quantitative description of in vitro assembly of human papillomavirus 16 virus-like particles. J. Mol. Biol. 2008, 381, 229–237.
- Mach, H.; Volkin, D.B.; Troutman, R.D.; Wang, B.; Luo, Z.; Jansen, K.U.; Shi, L. Disassembly and reassembly of yeast-derived recombinant human papillomavirus virus-like particles (HPV VLPs). J. Pharm. Sci. 2006, 95, 2195–2206.
- Gedvilaite, A.; Zvirbliene, A.; Staniulis, J.; Sasnauskas, K.; Krüger, D.H.; Ulrich, R. Segments of puumala hantavirus nucleocapsid protein inserted into chimeric polyomavirus-derived virus-like particles induce a strong immune response in mice. Viral Immunol. 2004, 17, 51–68.
- Hadiji-Abbes, N.; Martin, M.; Benzina, W.; Karray-Hakim, H.; Gergely, C.; Gargouri, A.; Mokdad-Gargouri, R. Extraction and purification of hepatitis B virus-like M particles from a recombinant Saccharomyces cerevisiae strain using alumina powder. J. Virol. Methods 2013, 187, 132–137.
- Zielonka, A.; Gedvilaite, A.; Ulrich, R.; Lüschow, D.; Sasnauskas, K.; Müller, H.; Johne, R. Generation of virus-like particles consisting of the major capsid protein VP1 of goose hemorrhagic polyomavirus and their application in serological tests. Virus Res. 2006, 120, 128–137.
- Lawatscheck, R.; Aleksaite, E.; Schenk, J.A.; Micheel, B.; Jandrig, B.; Holland, G.; Sasnauskas, K.; Gedvilaite, A.; Ulrich, R.G. Chimeric polyomavirus-derived virus-like particles: The immunogenicity of an inserted peptide applied without adjuvant to mice depends on its insertion site and its flanking linker sequence. Viral Immunol. 2007, 20, 453–460.
- Pumpens, P.; Razanskas, R.; Pushko, P.; Renhof, R.; Gusars, I.; Skrastina, D.; Ose, V.; Borisova, G.; Sominskaya, I.; Petrovskis, I.; et al. Evaluation of HBs, HBc, and frCP virus-like particles for expression of human papillomavirus 16 E7 oncoprotein epitopes. Intervirology 2002, 45, 24–32.
- Choi, Y.R.; Kim, H.J.; Lee, J.Y.; Kang, H.A.; Kim, H.J. Chromatographically purified capsid proteins of red-spotted grouper nervous necrosis virus expressed in Saccharomyces cerevisiae form virus-like particles. Protein Expr. Purif. 2013, 89, 162–168.
- Kwag, H.L.; Kim, H.J.; Chang, D.Y.; Kim, H.J. The production and immunogenicity of human papillomavirus type 58 virus-like particles produced in Saccharomyces cerevisiae. J. Microbiol. 2012, 50, 813–820.
- Hadiji-Abbes, N.; Mihoubi, W.; Martin, M.; Karakasyan-Dia, C.; Frikha, F.; Gergely, C.; Jouenne, T.; Gargouri, A.; Mokdad-Gargouri, R. Characterization of C69R variant HBsAg: Effect on binding to anti-HBs and the structure of virus-like particles. Arch. Virol. 2015, 160, 2427–2433.
- Stolt, A.; Sasnauskas, K.; Koskela, P.; Lehtinen, M.; Dillner, J. Seroepidemiology of the human polyomaviruses. J. Gen. Virol. 2003, 84, 1499–1504.
- Lowin, T.; Raab, U.; Schroeder, J.; Franssila, R.; Modrow, S. Parvovirus B19 VP2-proteins produced in Saccharomyces cerevisiae: Comparison with VP2-particles produced by baculovirus-derived vectors. J. Vet. Med. B Infect. Dis. Vet. Public Health 2005, 52, 348–352.
- Joyce, J.G.; Tung, J.S.; Przysiecki, C.T.; Cook, J.C.; Lehman, E.D.; Sands, J.A.; Jansen, K.U.; Keller, P.M. The L1 major capsid protein of human papillomavirus type 11 recombinant virus-like particles interacts with heparin and cell-surface glycosaminoglycans on human keratinocytes. J. Biol. Chem. 1999, 274, 5810–5822.
- Gedvilaite, A.; Frömmel, C.; Sasnauskas, K.; Micheel, B.; Ozel, M.; Behrsing, O.; Staniulis, J.; Jandrig, B.; Scherneck, S.; Ulrich, R. Formation of immunogenic virus-like particles by inserting epitopes into surface-exposed regions of hamster polyomavirus major capsid protein. Virology 2000, 273, 21–35.
- Fu, J.; VanDusen, W.J.; Kolodin, D.G.; O’Keefe, D.O.; Herber, W.K.; George, H.A. Continuous culture study of the expression of hepatitis B surface antigen and its self-assembly into virus-like particles in Saccharomyces cerevisiae. Biotechnol. Bioeng. 1996, 49, 578–586.
- Towne, V.; Zhao, Q.; Brown, M.; Finnefrock, A.C. Pairwise antibody footprinting using surface plasmon resonance technology to characterize human papillomavirus type 16 virus-like particles with direct anti-HPV antibody immobilization. J. Immunol. Methods 2013, 388, 1–7.
- Barsøe, S.; Toffan, A.; Pascoli, F.; Stratmann, A.; Pretto, T.; Marsella, A.; Er-Rafik, M.; Vendramin, N.; Olesen, N.J.; Sepúlveda, D.; et al. Long-Term Protection and Serologic Response of European Sea Bass Vaccinated with a Betanodavirus Virus-Like Particle Produced in Pichia pastoris. Vaccines 2021, 9, 447.
- Shanmugam, R.K.; Ramasamy, V.; Shukla, R.; Arora, U.; Swaminathan, S.; Khanna, N. Pichia pastoris-expressed Zika virus envelope domain III on a virus-like particle platform: Design, production and immunological evaluation. Pathog. Dis. 2019, 77, ftz026.
- Gupta, J.; Kaul, S.; Srivastava, A.; Kaushik, N.; Ghosh, S.; Sharma, C.; Batra, G.; Banerjee, M.; Shalimar Nayak, B.; Ranjith-Kumar, C.T.; et al. Expression, Purification and Characterization of the Hepatitis E Virus Like-Particles in the Pichia pastoris. Front. Microbiol. 2020, 11, 141.
- Sherry, L.; Grehan, K.; Snowden, J.S.; Knight, M.L.; Adeyemi, O.O.; Rowlands, D.J.; Stonehouse, N.J. Comparative Molecular Biology Approaches for the Production of Poliovirus Virus-Like Particles Using Pichia pastoris. mSphere 2020, 5, e00838-19.
- Sherry, L.; Grehan, K.; Swanson, J.J.; Bahar, M.W.; Porta, C.; Fry, E.E.; Stuart, D.I.; Rowlands, D.J.; Stonehouse, N.J. Production and Characterisation of Stabilised PV-3 Virus-like Particles Using Pichia pastoris. Viruses 2022, 14, 2159.
- Eto, Y.; Saubi, N.; Ferrer, P.; Joseph-Munné, J. Expression of Chimeric HPV-HIV Protein L1P18 in Pichia pastoris; Purification and Characterization of the Virus-like Particles. Pharmaceutics 2021, 13, 1967.
- Parker, S.A.; Maloy, M.H.; Tome-Amat, J.; Bardliving, C.L.; Batt, C.A.; Lanz, K.J.; Olesberg, J.T.; Arnold, M.A. Optimization of norovirus virus-like particle production in Pichia pastoris using a real-time near-infrared bioprocess monitor. Biotechnol. Prog. 2016, 32, 518–526.
- Pechelyulko, A.; Andreeva-Kovalevskaya, Z.; Dmitriev, D.; Lavrov, V.; Massino, Y.; Nagel, A.; Segal, O.; Sokolova, O.S.; Solonin, A.; Tarakanova, Y.; et al. A simple method to purify recombinant HCV core protein expressed in Pichia pastoris for obtaining virus-like particles and producing monoclonal antibodies. Protein Expr. Purif. 2021, 183, 105864.
- Acosta-Rivero, N.; Aguilar, J.C.; Musacchio, A.; Falcón, V.; Viña, A.; de la Rosa, M.C.; Morales, J. Characterization of the HCV core virus-like particles produced in the methylotrophic yeast Pichia pastoris. Biochem. Biophys. Res. Commun. 2001, 287, 122–125.
- Yang, Z.; Gao, F.; Wang, X.; Shi, L.; Zhou, Z.; Jiang, Y.; Ma, X.; Zhang, C.; Zhou, C.; Zeng, X.; et al. Development and characterization of an enterovirus 71 (EV71) virus-like particles (VLPs) vaccine produced in Pichia pastoris. Hum. Vaccin. Immunother. 2020, 16, 1602–1610.
- Zhang, C.; Ku, Z.; Liu, Q.; Wang, X.; Chen, T.; Ye, X.; Li, D.; Jin, X.; Huang, Z. High-yield production of recombinant virus-like particles of enterovirus 71 in Pichia pastoris and their protective efficacy against oral viral challenge in mice. Vaccine 2015, 33, 2335–2341.
- Shukla, R.; Rajpoot, R.K.; Arora, U.; Poddar, A.; Swaminathan, S.; Khanna, N. Pichia pastoris-Expressed Bivalent Virus-Like Particulate Vaccine Induces Domain III-Focused Bivalent Neutralizing Antibodies without Antibody-Dependent Enhancement in Vivo. Front. Microbiol. 2018, 8, 2644.
- Arora, U.; Tyagi, P.; Swaminathan, S.; Khanna, N. Virus-like particles displaying envelope domain III of dengue virus type 2 induce virus-specific antibody response in mice. Vaccine 2013, 31, 873–878.
- Feng, Q.; He, Y.; Lu, J. Virus-Like Particles Produced in Pichia Pastoris Induce Protective Immune Responses against Coxsackievirus A16 in Mice. Med. Sci. Monit. 2016, 22, 3370–3382.
- Tomé-Amat, J.; Fleischer, L.; Parker, S.A.; Bardliving, C.L.; Batt, C.A. Secreted production of assembled Norovirus virus-like particles from Pichia pastoris. Microb. Cell Fact. 2014, 13, 134.
- Poddar, A.; Ramasamy, V.; Shukla, R.; Rajpoot, R.K.; Arora, U.; Jain, S.K.; Swaminathan, S.; Khanna, N. Virus-like particles derived from Pichia pastoris-expressed dengue virus type 1 glycoprotein elicit homotypic virus-neutralizing envelope domain III-directed antibodies. BMC Biotechnol. 2016, 16, 50.
- Tripathi, L.; Mani, S.; Raut, R.; Poddar, A.; Tyagi, P.; Arora, U.; de Silva, A.; Swaminathan, S.; Khanna, N. Pichia pastoris-expressed dengue 3 envelope-based virus-like particles elicit predominantly domain III-focused high titer neutralizing antibodies. Front. Microbiol. 2015, 6, 1005.
- Tan, B.H.; Fu, J.L.; Sugrue, R.J. Characterization of the dengue virus envelope glycoprotein expressed in Pichia pastoris. Methods Mol. Biol. 2007, 379, 163–176.
- Mani, S.; Tripathi, L.; Raut, R.; Tyagi, P.; Arora, U.; Barman, T.; Sood, R.; Galav, A.; Wahala, W.; de Silva, A.; et al. Pichia pastoris-expressed dengue 2 envelope forms virus-like particles without pre-membrane protein and induces high titer neutralizing antibodies. PLoS ONE 2013, 8, e64595.
- Rajpoot, R.K.; Shukla, R.; Arora, U.; Swaminathan, S.; Khanna, N. Dengue envelope-based ‘four-in-one’ virus-like particles produced using Pichia pastoris induce enhancement-lacking, domain III-directed tetravalent neutralising antibodies in mice. Sci. Rep. 2018, 8, 8643.
- Khetarpal, N.; Shukla, R.; Rajpoot, R.K.; Poddar, A.; Pal, M.; Swaminathan, S.; Arora, U.; Khanna, N. Recombinant Dengue Virus 4 Envelope Glycoprotein Virus-Like Particles Derived from Pichia pastoris are Capable of Eliciting Homotypic Domain III-Directed Neutralizing Antibodies. Am. J. Trop. Med. Hyg. 2017, 96, 126–134.
- Zhao, P.; Jiang, Y.; Wang, J.; Fan, H.; Cao, R. Secreted expression of Japanese encephalitis virus prME in Pichia pastoris and immunogenicity evaluation of the virus-like particles in mice. Chin. J. Biotechnol. 2017, 33, 863–874. (In Chinese)
- Liu, W.; Jiang, H.; Zhou, J.; Yang, X.; Tang, Y.; Fang, D.; Jiang, L. Recombinant dengue virus-like particles from Pichia pastoris: Efficient production and immunological properties. Virus Genes 2010, 40, 53–59.
- Joseph, N.M.; Ho, K.L.; Tey, B.T.; Tan, C.S.; Shafee, N.; Tan, W.S. Production of the virus-like particles of nipah virus matrix protein in Pichia pastoris as diagnostic reagents. Biotechnol. Prog. 2016, 32, 1038–1045.
- Zhang, C.; Zhang, X.; Zhang, W.; Dai, W.; Xie, J.; Ye, L.; Wang, H.; Chen, H.; Liu, Q.; Gong, S.; et al. Enterovirus D68 virus-like particles expressed in Pichia pastoris potently induce neutralizing antibody responses and confer protection against lethal viral infection in mice. Emerg. Microbes Infect. 2018, 7, 3.
- Yun, S.M.; Jeong, Y.E.; Wang, E.; Lee, Y.J.; Han, M.G.; Park, C.; Lee, W.J.; Choi, W. Cloning and Expression of Recombinant Tick-Borne Encephalitis Virus-like Particles in Pichia pastoris. Osong Public Health Res. Perspect. 2014, 5, 274–278.
- Zhou, Y.; Zhang, C.; Liu, Q.; Gong, S.; Geng, L.; Huang, Z. A virus-like particle vaccine protects mice against coxsackievirus A10 lethal infection. Antiviral Res. 2018, 152, 124–130.
- Zahid, M.; Rinas, U. Guidelines for Small-Scale Production and Purification of Hepatitis B Surface Antigen Virus-Like Particles from Recombinant Pichia pastoris. Methods Mol. Biol. 2019, 1923, 309–322.
- Lünsdorf, H.; Gurramkonda, C.; Adnan, A.; Khanna, N.; Rinas, U. Virus-like particle production with yeast: Ultrastructural and immunocytochemical insights into Pichia pastoris producing high levels of the hepatitis B surface antigen. Microb. Cell Fact. 2011, 10, 48.
- Hosseini, S.N.; Sarvari, T.; Bashiri, G.; Khatami, M.; Shojaosadati, S.A. Assessing virus like particles formation and r-HBsAg aggregation during large scale production of recombinant hepatitis B surface antigen from Pichia pastoris. Int. J. Biol. Macromol. 2019, 139, 697–711.
- Gurramkonda, C.; Zahid, M.; Nemani, S.K.; Adnan, A.; Gudi, S.K.; Khanna, N.; Ebensen, T.; Lünsdorf, H.; Guzmán, C.A.; Rinas, U. Purification of hepatitis B surface antigen virus-like particles from recombinant Pichia pastoris and in vivo analysis of their immunogenic properties. J. Chromatogr. B 2013, 940, 104–111.
- Liu, R.; Lin, Q.; Sun, Y.; Lu, X.; Qiu, Y.; Li, Y.; Guo, X. Expression, purification, and characterization of hepatitis B virus surface antigens (HBsAg) in yeast Pichia Pastoris. Appl. Biochem. Biotechnol. 2009, 158, 432–444.
- Gurramkonda, C.; Adnan, A.; Gäbel, T.; Lünsdorf, H.; Ross, A.; Nemani, S.K.; Swaminathan, S.; Khanna, N.; Rinas, U. Simple high-cell density fed-batch technique for high-level recombinant protein production with Pichia pastoris: Application to intracellular production of Hepatitis B surface antigen. Microb. Cell Fact. 2009, 8, 13.
- Watelet, B.; Quibriac, M.; Rolland, D.; Gervasi, G.; Gauthier, M.; Jolivet, M.; Letourneur, O. Characterization and diagnostic potential of hepatitis B virus nucleocapsid expressed in E. coli and P. pastoris. J. Virol. Methods 2002, 99, 99–114.
- Tang, Y.X.; Jiang, L.F.; Zhou, J.M.; Yin, Y.; Yang, X.M.; Liu, W.Q.; Fang, D.Y. Induction of virus-neutralizing antibodies and T cell responses by dengue virus type 1 virus-like particles prepared from Pichia pastoris. Chin. Med. J. 2012, 125, 1986–1992.
- Gupta, G.; Glueck, R.; Rishi, N. Physicochemical characterization and immunological properties of Pichia pastoris based HPV16L1 and 18L1 virus like particles. Biologicals 2017, 46, 11–22.
- Hanumantha, R.N.; Baji, B.P.; Rajendra, L.; Sriraman, R.; Pang, Y.Y.; Schiller, J.T.; Srinivasan, V.A. Expression of codon optimized major capsid protein (L1) of human papillomavirus type 16 and 18 in Pichia pastoris; purification and characterization of the virus-like particles. Vaccine 2011, 29, 7326–7334.
- Gupta, G.; Giannino, V.; Rishi, N.; Glueck, R. Immunogenicity of next-generation HPV vaccines in non-human primates: Measles-vectored HPV vaccine versus Pichia pastoris recombinant protein vaccine. Vaccine 2016, 34, 4724–4731.
- Dewi, K.S.; Chairunnisa, S.; Swasthikawati, S.; Yuliawati Agustiyanti, D.F.; Mustopa, A.Z.; Kusharyoto, W.; Ningrum, R.A. Production of codon-optimized Human papillomavirus type 52 L1 virus-like particles in Pichia pastoris BG10 expression system. Prep. Biochem. Biotechnol. 2022, 53, 148–156.
- Zhu, J.; Yang, K.; Liu, A.; Lu, X.; Yang, L.; Zhao, Q. Highly secretory expression of recombinant cowpea chlorotic mottle virus capsid proteins in Pichia pastoris and in-vitro encapsulation of ruthenium nanoparticles for catalysis. Protein Expr. Purif. 2020, 174, 105679.
- Jiang, Z.; Tong, G.; Cai, B.; Xu, Y.; Lou, J. Purification and immunogenicity study of human papillomavirus 58 virus-like particles expressed in Pichia pastoris. Protein Expr. Purif. 2011, 80, 203–210.
- Ramasamy, V.; Arora, U.; Shukla, R.; Poddar, A.; Shanmugam, R.K.; White, L.J.; Mattocks, M.M.; Raut, R.; Perween, A.; Tyagi, P.; et al. A tetravalent virus-like particle vaccine designed to display domain III of dengue envelope proteins induces multi-serotype neutralizing antibodies in mice and macaques which confer protection against antibody dependent enhancement in AG129 mice. PLoS Negl. Trop. Dis. 2018, 12, e0006191.
- Wang, M.; Pan, Q.; Lu, Z.; Li, K.; Gao, H.; Qi, X.; Gao, Y.; Wang, X. An optimized, highly efficient, self-assembled, subvirus-like particle of infectious bursal disease virus (IBDV). Vaccine 2016, 34, 3508–3514.
- Zhang, C.; Liu, Q.; Ku, Z.; Hu, Y.; Ye, X.; Zhang, Y.; Huang, Z. Coxsackievirus A16-like particles produced in Pichia pastoris elicit high-titer neutralizing antibodies and confer protection against lethal viral challenge in mice. Antiviral Res. 2016, 129, 47–51.
- Stephen, S.L.; Beales, L.; Peyret, H.; Roe, A.; Stonehouse, N.J.; Rowlands, D.J. Recombinant Expression of Tandem-HBc Virus-Like Particles (VLPs). Methods Mol. Biol. 2018, 1776, 97–123.
- Freivalds, J.; Dislers, A.; Ose, V.; Pumpens, P.; Tars, K.; Kazaks, A. Highly efficient production of phosphorylated hepatitis B core particles in yeast Pichia pastoris. Protein Expr. Purif. 2011, 75, 218–224.
- Sugrue, R.J.; Fu, J.; Howe, J.; Chan, Y.C. Expression of the dengue virus structural proteins in Pichia pastoris leads to the generation of virus-like particles. J. Gen. Virol. 1997, 78, 1861–1866.
- Yazdani, R.; Shams-Bakhsh, M.; Hassani-Mehraban, A.; Arab, S.S.; Thelen, N.; Thiry, M.; Crommen, J.; Fillet, M.; Jacobs, N.; Brans, A.; et al. Production and characterization of virus-like particles of grapevine fanleaf virus presenting L2 epitope of human papillomavirus minor capsid protein. BMC Biotechnol. 2019, 19, 81.
- Yan, W.W.; Cui, Z.Z.; Wang, Y.K. Expression of capsid gene of Chinese isolate of rabbit hemorrhagic disease virus in Pichia pastoris. Chin. J. Biotechnol. 2005, 21, 135–138. (In Chinese)
- Kazaks, A.; Lu, I.N.; Farinelle, S.; Ramirez, A.; Crescente, V.; Blaha, B.; Ogonah, O.; Mukhopadhyay, T.; de Obanos, M.P.; Krimer, A.; et al. Production and purification of chimeric HBc virus-like particles carrying influenza virus LAH domain as vaccine candidates. BMC Biotechnol. 2017, 17, 79.
- Fazlalipour, M.; Keyvani, H.; Monavari, S.H.; Mollaie, H.R. Expression, Purification and Immunogenic Description of a Hepatitis C Virus Recombinant CoreE1E2 Protein Expressed by Yeast Pichia pastoris. Jundishapur J. Microbiol. 2015, 8, e17157.
- Zhou, Y.; Shen, C.; Zhang, C.; Zhang, W.; Wang, L.; Lan, K.; Liu, Q.; Huang, Z. Yeast-produced recombinant virus-like particles of coxsackievirus A6 elicited protective antibodies in mice. Antiviral Res. 2016, 132, 165–169.
- Sanchooli, A.; Aghaiypour, K.; Kiasari, B.A.; Samarbaf-Zadeh, A.; Ghadiri, A.; Makvandi, M. VLP Production from Recombinant L1/L2 HPV-16 Protein Expressed in Pichia pastoris. Protein Pept. Lett. 2018, 25, 783–790.
- Yang, L.; Xiao, A.; Wang, H.; Zhang, X.; Zhang, Y.; Li, Y.; Wei, Y.; Liu, W.; Chen, C. A VLP-Based Vaccine Candidate Protects Mice against Japanese Encephalitis Virus Infection. Vaccines 2022, 10, 197.
- Bisht, H.; Chugh, D.A.; Raje, M.; Swaminathan, S.S.; Khanna, N. Recombinant dengue virus type 2 envelope/hepatitis B surface antigen hybrid protein expressed in Pichia pastoris can function as a bivalent immunogen. J. Biotechnol. 2002, 99, 97–110.
- Liu, Y.; Zhou, J.; Yu, Z.; Fang, D.; Fu, C.; Zhu, X.; He, Z.; Yan, H.; Jiang, L. Tetravalent recombinant dengue virus-like particles as potential vaccine candidates: Immunological properties. BMC Microbiol. 2014, 14, 233.
- Saraswat, S.; Athmaram, T.N.; Parida, M.; Agarwal, A.; Saha, A.; Dash, P.K. Expression and Characterization of Yeast Derived Chikungunya Virus Like Particles (CHIK-VLPs) and Its Evaluation as a Potential Vaccine Candidate. PLoS Negl. Trop. Dis. 2016, 10, e0004782.
- Coimbra, E.C.; Gomes, F.B.; Campos, J.F.; D’arc, M.; Carvalho, J.C.; Mariz, F.C.; Jesus, A.L.; Stocco, R.C.; Beçak, W.; Freitas, A.C. Production of L1 protein from different types of HPV in Pichia pastoris using an integrative vector. Braz. J. Med. Biol. Res. 2011, 44, 1209–1214.
- Zhou, Y.; Fan, Y.; LaPatra, S.E.; Ma, J.; Xu, J.; Meng, Y.; Jiang, N.; Zeng, L. Protective immunity of a Pichia pastoris expressed recombinant iridovirus major capsid protein in the Chinese giant salamander, Andrias davidianus. Vaccine 2015, 33, 5662–5669.
- Hosseini, S.N.; Javidanbardan, A.; Alizadeh Salim, B.S.; Khatami, M. Large-scale purification of recombinant hepatitis B surface antigen from Pichia pastoris with non-affinity chromatographic methods as a substitute to immunoaffinity chromatography. Prep. Biochem. Biotechnol. 2018, 48, 683–692.
- Fernández, E.; Toledo, J.R.; Méndez, L.; González, N.; Parra, F.; Martín-Alonso, J.M.; Limonta, M.; Sánchez, K.; Cabrales, A.; Estrada, M.P.; et al. Conformational and thermal stability improvements for the large-scale production of yeast-derived rabbit hemorrhagic disease virus-like particles as multipurpose vaccine. PLoS ONE 2013, 8, e56417.
- Jesus, A.L.; Mariz, F.C.; Souza, H.M.; Cordeiro, M.N.; Coimbra, E.C.; Leitão, M.C.; Nascimento, L.M.; Stocco, R.C.; Beçak, W.; Freitas, A.C. Expression of the bovine papillomavirus type 1, 2 and 4 L1 genes in the yeast Pichia pastoris. Genet. Mol. Res. 2012, 11, 2598–2607.
- Bazan, S.B.; de Alencar Muniz Chaves, A.; Aires, K.A.; Cianciarullo, A.M.; Garcea, R.L.; Ho, P.L. Expression and characterization of HPV-16 L1 capsid protein in Pichia pastoris. Arch. Virol. 2009, 154, 1609–1617.
- Xia, M.; Farkas, T.; Jiang, X. Norovirus capsid protein expressed in yeast forms virus-like particles and stimulates systemic and mucosal immunity in mice following an oral administration of raw yeast extracts. J. Med. Virol. 2007, 79, 74–83.
- Mariz, F.C.; Coimbra, E.C.; Jesus, A.L.; Nascimento, L.M.; Torres, F.A.; Freitas, A.C. Development of an IP-Free Biotechnology Platform for Constitutive Production of HPV16 L1 Capsid Protein Using the Pichia pastoris PGK1 Promoter. BioMed Res. Int. 2015, 2015, 594120.
- Rolland, D.; Gauthier, M.; Dugua, J.M.; Fournier, C.; Delpech, L.; Watelet, B.; Letourneur, O.; Arnaud, M.; Jolivet, M. Purification of recombinant HBc antigen expressed in Escherichia coli and Pichia pastoris: Comparison of size-exclusion chromatography and ultracentrifugation. J. Chromatogr. B Biomed. Sci. Appl. 2001, 753, 51–65.
- Fernández, E.; Toledo, J.R.; Mansur, M.; Sánchez, O.; Gil, D.F.; González-González, Y.; Lamazares, E.; Fernández, Y.; Parra, F.; Farnós, O. Secretion and assembly of calicivirus-like particles in high-cell-density yeast fermentations: Strategies based on a recombinant non-specific BPTI-Kunitz-type protease inhibitor. Appl. Microbiol. Biotechnol. 2015, 99, 3875–3886.
- Acosta-Rivero, N.; Rodriguez, A.; Musacchio, A.; Falcón, V.; Suarez, V.M.; Martinez, G.; Guerra, I.; Paz-Lago, D.; Morera, Y.; de la Rosa, M.C.; et al. In vitro assembly into virus-like particles is an intrinsic quality of Pichia pastoris derived HCV core protein. Biochem. Biophys. Res. Commun. 2004, 325, 68–74.
- Acosta-Rivero, N.; Falcón, V.; Alvarez, C.; Musacchio, A.; Chinea, G.; Cristina de la Rosa, M.; Rodriguez, A.; Dueñas-Carrera, S.; Tsutsumi, V.; Shibayama, M.; et al. Structured HCV nucleocapsids composed of P21 core protein assemble primary in the nucleus of Pichia pastoris yeast. Biochem. Biophys. Res. Commun. 2003, 310, 48–53.
- Acosta-Rivero, N.; Alvarez-Obregón, J.C.; Musacchio, A.; Falcón, V.; Dueñas-Carrera, S.; Marante, J.; Menéndez, I.; Morales, J. In vitro self-assembled HCV core virus-like particles induce a strong antibody immune response in sheep. Biochem. Biophys. Res. Commun. 2002, 290, 300–304.
- Majeau, N.; Gagné, V.; Bolduc, M.; Leclerc, D. Signal peptide peptidase promotes the formation of hepatitis C virus non-enveloped particles and is captured on the viral membrane during assembly. J. Gen. Virol. 2005, 86, 3055–3064.
- Chen, Y.; Zhang, Y.; Quan, C.; Luo, J.; Yang, Y.; Yu, M.; Kong, Y.; Ma, G.; Su, Z. Aggregation and antigenicity of virus like particle in salt solution–A case study with hepatitis B surface antigen. Vaccine 2015, 33, 4300–4306.
- Lang, R.; Winter, G.; Vogt, L.; Zurcher, A.; Dorigo, B.; Schimmele, B. Rational design of a stable, freeze-dried virus-like particle-based vaccine formulation. Drug Dev. Ind. Pharm. 2009, 35, 83–97.
- Jezek, J.; Chen, D.; Watson, L.; Crawford, J.; Perkins, S.; Tyagi, A.; Jones-Braun, L. A heat-stable hepatitis B vaccine formulation. Hum. Vaccines 2009, 5, 529–535.
- Kissmann, J.; Ausar, S.F.; Foubert, T.R.; Brock, J.; Switzer, M.H.; Detzi, E.J.; Vedvick, T.S.; Middaugh, C.R. Physical stabilization of Norwalk virus-like particles. J. Pharm. Sci. 2008, 97, 4208–4218.
- Tsoka, S.; Holwill, I.; Hoare, M. Virus-like particle analysis in yeast homogenate using a laser light-scattering assay. Biotechnol. Bioeng. 1999, 63, 290–297.
- Shi, L.; Sanyal, G.; Ni, A.; Luo, Z.; Doshna, S.; Wang, B.; Graham, T.L.; Wang, N.; Volkin, D.B. Stabilization of human papillomavirus virus-like particles by non-ionic surfactants. J. Pharm. Sci. 2005, 94, 1538–1551.
- Ding, Y.; Chuan, Y.P.; He, L.; Middelberg, A.P. Modeling the competition between aggregation and self-assembly during virus-like particle processing. Biotechnol. Bioeng. 2010, 107, 550–560.
- Huang, Y.; Bi, J.; Zhou, W.; Li, Y.; Wang, Y.; Ma, G.; Su, Z. Improving recovery of recombinant hepatitis B virus surface antigen by ion-exchange chromatographic supports with low ligand density. Process Biochem. 2006, 41, 2320–2326.
- Meingast, C.; Heldt, C.L. Arginine-enveloped virus inactivation and potential mechanisms. Biotechnol. Prog. 2020, 36, e2931.
- Kissmann, J.; Joshi, S.B.; Haynes, J.R.; Dokken, L.; Richardson, C.; Middaugh, C.R. H1N1 influenza virus-like particles: Physical degradation pathways and identification of stabilizers. J. Pharm. Sci. 2011, 100, 634–645.
- Sakuragi, S.; Goto, T.; Sano, K.; Morikawa, Y. HIV type 1 Gag virus-like particle budding from spheroplasts of Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 2002, 99, 7956–7961.
- Fuenmayor, J.; Gòdia, F.; Cervera, L. Production of virus-like particles for vaccines. N. Biotechnol. 2017, 39, 174–180.
- Hashemi, K.; Ghahramani Seno, M.M.; Ahmadian, M.R.; Malaekeh-Nikouei, B.; Bassami, M.R.; Dehghani, H.; Afkhami-Goli, A. Optimizing the synthesis and purification of MS2 virus like particles. Sci. Rep. 2021, 11, 19851.
- Galula, J.U.; Chang, G.J.; Chao, D.Y. Production and Purification of Dengue Virus-like Particles from COS-1 Cells. Bio. Protoc. 2019, 9, e3280.
- Pease, L.F., 3rd; Lipin, D.I.; Tsai, D.H.; Zachariah, M.R.; Lua, L.H.; Tarlov, M.J.; Middelberg, A.P. Quantitative characterization of virus-like particles by asymmetrical flow field flow fractionation, electrospray differential mobility analysis, and transmission electron microscopy. Biotechnol Bioeng. 2009, 102, 845–855.
- Lua, L.H.L.; Connors, N.K.; Sainsbury, F.; Chuan, Y.P.; Wibowo, N.; Middelberg, A.P.J. Bioengineering Virus-like Particles as Vaccines. Biotechnol. Bioeng. 2014, 111, 425–440.
- Zhao, Q.; Modis, Y.; High, K.; Towne, V.; Meng, Y.; Wang, Y.; Alexandroff, J.; Brown, M.; Carragher, B.; Potter, C.S.; et al. Disassembly and reassembly of human papillomavirus virus-like particles produces more virion-like antibody reactivity. Virol. J. 2012, 9, 52.
More
Information
Subjects:
Mycology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.7K
Entry Collection:
Biopharmaceuticals Technology
Revisions:
3 times
(View History)
Update Date:
11 Apr 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




