Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by FNU RAVINDER KUMAR and Version 3 by Catherine Yang.
Virus-like particles (VLPs) are empty, nanoscale structures morphologically resembling viruses. Internal cavity, noninfectious, and particulate nature with a high density of repeating epitopes, make them an ideal platform for vaccine development and drug delivery.
- yeast
- virus-like particles (VLPs)
- subunit
- drug delivery
1. Introduction to Virus-Like Particles
Virus-like particles (VLPs), ghost viruses, or dummy viruses lacking genetic material are nanostructures first observed in sera samples from hepatitis patients in 1968 [1][26]. VLPs can exist naturally (in a virally infected host) and can be generated in the laboratory. The size of VLPs may vary from 20 nm to 200 nm or more [2][27]. The size of VLPs depends on the virus species and viral proteins used for developing these particles [2][27]. It is essential to mention that VLPs can be formed using the capsid, envelope, or core viral proteins [2][27]. These particles are usually formed naturally by folding viral proteins under appropriate conditions, including the optimum pH, salt concentration, temperature, and so on [3][4][5][28,29,30]. The VLPs form when monomeric proteins fold into a pentameric form, also called capsomers, which are then assembled to form VLPs [5][30]. VLPs can exist either non-enveloped, as seen in the case of HPV VLPs, or enveloped with a lipid membrane (eVLPs), such as SARS coronavirus VLPs [6][7][31,32]. The shape of VLPs also differs from icosahedral to rod-shaped [8][33]. These particles may be composed of a single protein or can be a fusion of two different proteins (chimeric VLPs) [9][10][34,35].
The feature that makes VLPs important in vaccine development is their high density of epitopes, particulate nature, and lack of genetic material that restricts their replication and makes them safe for the host [1][11][12][13][26,36,37,38]. In addition to their use in vaccine development, VLPs are also widely investigated to deliver drugs and other small molecules inside the host system. This property is attributed to the internal cavity in VLPs. Several studies also showed the feasibility of using VLPs in photo imaging [12][13][37,38]. Different applications of VLPs are shown in Figure 12.
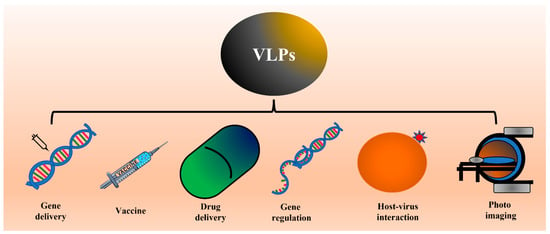
Figure 12. Schematic showing the different applications of VLPs. Several studies have already shown proof of concept for applications like vaccines, drug delivery, and photo imaging. ThWe researchers propose the possible use of VLPs to study virus-host interaction or internalization.
The high epitope density and particulate nature of VLPs make them ideal systems for mounting immune responses. It is essential to mention that VLPs can mount both humoral and cellular immune responses [14][15][16][17][18][19][39,40,41,42,43,44]. Because of their natural resemblance to viruses, VLPs act as pathogen-associated structural patterns (PASP) and are easily recognized and taken up by host immune cells [14][15][39,40]. Additionally, their structural properties help activate immune cells like dendritic cells [20][45]. Due to their ability to mount both humoral and cellular immune responses, VLPs appear to be a better choice for vaccine delivery than purified proteins [21][46].
Due to their importance in vaccine development and drug delivery, efforts were made to express and purify VLPs from different biological systems and identify the most suitable hosts for producing VLPs commercially. To this end, VLPs are successfully expressed and purified from both the prokaryotic system (for example, Escherichia coli) [9][34] as well as from the eukaryotic system, including yeast (for example, S. cerevisiae) [22][47], insect cell lines [23][24][48,49], mammalian cell lines [14][25][39,50], and plants [26][51] (as shown in Figure 23).
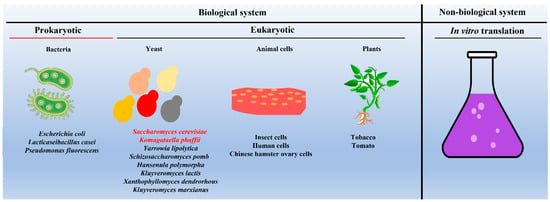
Figure 23. Schematic showing different systems used as hosts for expressing and purifying VLPs. Note: Saccharomyces cerevisiae and Komagataella phaffii (in red font) remain the most commonly used yeast species. In the case of in vitro production of VLPs, one can use the protein translational machinery of either bacteria (prokaryotes) or yeast (eukaryotes).
Apart from whole cells, several studies have shown that VLPs can be produced by in vitro protein translation systems [27][52]. The advantages and disadvantages of in vitro protein translation-based VLP generation are discussed by others [27][28][29][22,52,53]. In Table 1, the advantages and disadvantages of different systems used for the expression and purification of VLPs are compared.
Table 1. A comparison of the different model systems used as a host for the production of VLPs [28][30].
| Feature | Bacteria | Insect Cells | Mammalian Cells | Plant | Yeast |
|---|
Table 3.
Studies where K. phaffii
yeast was used to generate VLPs.
| S. No | Protein Antigen | Virus | Protein Localization |
Promoter | References |
|---|---|---|---|---|---|
| Production cost | Low | High | High | Moderate | Low |
| Growth media | Simple | Complex | Complex | Simple | Simple |
| Growth | Fast | Slow | Slow | ||
| 1 | |||||
| Very slow | |||||
| Fast | |||||
| Growth duration | Very small | Small | Small | Long | Small |
| Indoor/Outdoor | Indoor | Indoor | Indoor | Outdoor/polyhouse | Indoor |
| Scale-up | Easy | Very difficult | Very difficult | Difficult | Easy |
| Secretion | No | Yes | Yes | NA | Yes |
| Enveloped/non-enveloped | Non-enveloped | Enveloped/Non-enveloped | Enveloped/Non-enveloped | NA | Non-enveloped/Enveloped possible |
| Speed of transformant screening | Very fast | Slow | Slow | Very slow | Fast |
| Effect of seasonal variations | No | No | No | Yes | No |
NA: clear information not available.
2. Yeast, Host to Produce VLPs on a Commercial Scale
As mentioned above, several biological systems have been evaluated over the years for a suitable host to produce VLPs on a commercial scale. An ideal host for the commercial purification of VLPs should be nonpathogenic, easy to handle, able to grow on economic media, able to express the protein of interest in the maximum amount, allow proper folding of a large amount of expressed protein, be genetically responsive, and be easily scaled up to industrial measures [30][54]. The secretion of the expressed protein(s) and VLPs (both enveloped and non-enveloped) into the medium will be another beneficial facet. Looking at these attributes, a yeast-based system appears as an ideal host for the commercial production of VLPs. In the past, several yeasts were successfully used to express and purify clinically relevant proteins. Yeast species, S. cerevisiae and K. phaffii (formerly known as Pichia pastoris), fall under GARS (Generally Recognized as Safe), which is another significant advantage of using yeast-based systems for the development of VLPs [31][55]. Unlike the bacterial system, the yeast-based system does not suffer from endotoxin problems [32][56], and the solubility of expressed proteins and folding are much better in yeast compared to bacteria [33][57]. Furthermore, utilizing mammalian and insect cells for VLP production is expensive due to the high cost of media and poor scalability [34][58]. In contrast, yeast can quickly grow on simple media [35][59]. A yeast species, K. phaffii, can be grown to a high cell density on a commercial scale, which is not feasible with animal cells. The rapid growth of yeast cells (unlike animal cells, yeast cells grow faster with a doubling time of around 90–120 min, whereas animal cells have a doubling time of 16–18 h or more) is another advantage [36][60]. The growth of plants is slow and may take several weeks, months, or even years to reach the desired maturity. Another issue is the varying expression levels in different organs or tissues and more batch-to-batch variation. Other concerns include the possibility of escape into the natural environment [37][61]. The seasonal variation may severely impact plant growth, so the expression of the protein of interest remains an important consideration. Due to several advantages (as mentioned), yeast has been extensively used for expressing and purifying VLPs, especially S. cerevisiae (Table 2) and K. phaffii (Table 3).Table 2.
Studies in which S. cerevisiae
yeast was used to generate VLPs.
| S. No | Protein Antigen | Virus | Protein Localization |
Promoter | References | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | VP2 | Human parvovirus 4 | IC | Capsid proteinHybrid GAL10-PYK1promoter | [38] | [62] | |||||||
| Red-spotted grouper nervous necrosis virus | IC | Pw42-2 | [ | 98 | ] | [123] | 2 | Capsid protein | Hepatitis E virus | IC | GAL promoter | ||
| 2 | ZS and S | Zika virus | [ | 39 | ] | [63] | |||||||
| IC | AOX1 | [ | 99 | ] | [124] | 3 | Capsid protein | Porcine circovirus type 2 | EC | GPD, TEF2 | [40] | [64] | |
| 3 | 112-608aa of the ORF2 | Hepatitis E virus | EC | AOX1 | [100] | [125] | 4 | Nucleocapsid protein | Sendai virus | ||||
| 4 | P1 and CD3 | IC | GAL7 | Poliovirus type I | IC | AOX1 | [41] | [65] | |||||
| [ | 101 | ] | [ | 126 | [102] | ,127] | 5 | VP2, VP1 | Human bocaviruses | IC | |||
| 5 | [ | 42 | Chimeric HPV-HIV L1P18 protein | ] | [66] | ||||||||
| HPV and HIV | IC | GAP | [ | 103 | ] | [128] | 6 | Surface antigen | Hepatitis B virus | IC | GAL | ||
| 6 | NY-ESO-1 cancer testis antigen | Norovirus | [ | EC | 43] | AOX1 | [104] | [129 | [44][45] | [67,68,69] | |||
| ] | 7 | p55(gag) | HIV-1 | EC | [46] | [70] | |||||||
| 7 | Surface antigen | Hepatitis C virus | IC | AOX1 | [105] | 8 | VP1 | Human polyomaviruses | IC | GAL | [47] | [71] | |
| 9 | L1 | HPV 16 | IC | GAL10 | [48][49] | [72,73] | |||||||
| 10 | Nucleocapsid protein | Tioman virus | IC | GAL10 | [50] | [74] | |||||||
| 11 | L-HDAg and surface antigen | Hepatitis delta virus | IC | GAD | [51] | [75] | |||||||
| 12 | Capsid protein | Porcine circovirus type 2 | IC | GAL10 | [52] | [76] | |||||||
| ] | [ | 118 | ] | [ | 138,139,140 | 13 | Capsid protein | Enterovirus 71 | IC | GAL10 | [53] | [77] | |
| , | 14 | VP1 | Human and non-human polyomaviruses | IC | GAL | [54] | [78] | ||||||
| 141 | 15 | Capsid protein | Adeno-associated virus | IC | GAL1 | [55] | [79] | ||||||
| , | 16 | Nucleocapsid protein | Human parainfluenza virus 4 | IC | GAL7 | [56] | [80] | ||||||
| 142 | 17 * | Coat protein | Cacteriophage Qbeta virus | IC | GAL | [57] | [81] | ||||||
| , | 18 | Capsid protein | Nervous necrosis virus | IC | GAL | [58] | [82] | ||||||
| 143 | ] | ||||||||||||
| 13 | prME | Japanese encephalitis virus | EC | AOX1 | [119] | [144] | |||||||
| 14 | E antigen | Dengue virus | EC | GAP | [120] | [145] | |||||||
| 15 | Matrix protein | Nipah virus | IC | AOX1 | [121] | [146] | |||||||
| 16 | P1 and 3CD | Enterovirus D68 | IC | AOX1 | [122] | [147] | |||||||
| 17 | prM and E protein | Tick-borne encephalitis virus | EC | GAP | [123] | [148] | 19 | ||||||
| 18 | P1 and 3CD | Coxsackievirus A10 | IC | AOX1 | [124] | [149] | VP1,2 | Parvovirus B19 | IC | ADH2/GAPDH | [59][ | ||
| 19 | Surface antigen | 60 | Hepatitis B virus | ] | [ | IC83 | AOX1, | [ | 84] | ||||
| 125 | ] | [ | 126 | ] | [127][128][129][130][131] | [150,151,152, | 20 | VP1 | Bird polyomaviruses | IC | GAL | [61] | [85] |
| 153 | 21 | P1 | Enterovirus 71 and Coxsackievirus A16 | IC | GAL1 | [62] | [86] | ||||||
| 22 | Capsid protein | Porcine circovirus type 2 | IC | GAL1 | [63] | [87] | |||||||
| 23 | VP2,6,7 | Rotavirus | IC | PGK1, TEF1 | [64][65] | [88,89] | |||||||
| 24 | VP2 | Human parvovirus 4 | IC | GAL1-10 | [66] | [90] | |||||||
| 25 | Nucleocapsid protein | Human parainfluenza virus 2 | IC | GAL | [67] | [91] | |||||||
| 26 | Nucleocapsid protein | Menangle virus | IC | GAL7 | [68] | [92] | |||||||
| , | 154 | , | 155 | , | 156 | 27 | Gag | HIV-1 | IC | GAP | [69] | [93] | |
| 28 | VP2 | Porcine parvovirus | IC | GAL1-10 | [70] | [94] | |||||||
| 29 | P1, CD3 | Coxsackievirus A16 | IC | GAL1 | [71] | [95] | |||||||
| [ | 79 | ] | [ | 103 | ] | ||||||||
| 36 | P1, CD3 | Poliovirus type I | IC | [80] | [104] | ||||||||
| 37 | HIV-1 | IC | [81] | [105] | |||||||||
| 38 | L1 | HPV 16 | IC | GAL | [82] | [106] | |||||||
| 39 | L1 | HPV 6,11 16 | IC | GAL | [83] | [107] | |||||||
| 40 | VP1 with Puumala hantavirus nucleocapsid protein segments | Hamster polyomavirus | IC | Hybrid GAL10-PYK1 | [84] | [108] | |||||||
| 41 | M protein | Hepatitis B virus | IC | GAL10/CYC1 | [85] | [109] | |||||||
| 42 | VP1,2 | Goose hemorrhagic polyomavirus | IC | GAL | [86] | [110] | |||||||
| 43 | CEA/VP1 | Hamster polyomavirus | IC | GAL | [87] | [111] | |||||||
| [ | 106 | ] | [ | 130 | ,131] | ||||||||
| 8 | P1 and 3CD | Enterovirus 71 | EC | AOX1 | [107][108] | [132,133] | |||||||
| 9 | E domain III | Dengue Virus | IC | AOX1 | [109][110] | [134,135] | |||||||
| 10 | P1 and 3CD | Coxsackievirus A16 | EC | GAP | [111] | [136] | |||||||
| 11 | VP1 | Norovirus | EC | AOX1 | [112] | [137] | |||||||
| 12 | E antigen | Dengue virus | IC | AOX1 | [113][114][115][116][117 | ] | |||||||
| 20 | prM and E protein | Dengue virus | IC | GAP | [132] | [157] | |||||||
| 21 | L1 | HPV16 and 18 | IC | AOX1 | [133][134][135] | [158,159,160] | |||||||
| 22 | L1 | HPV 52 | AOX1 | [136] | [161] | ||||||||
| 23 | Capsid protein | Cowpea chlorotic mottle virus | EC | AOX1 | [137] | [162] | |||||||
| 24 | L1 | HPV 58 | IC | AOX1 | [138] | [163] | |||||||
| 25 | Envelope protein domain III (EDIII), hepatitis B surface antigen | Dengue virus | IC | AOX1 | [139] | [164] | |||||||
| 26 | VP2 | Infectious bursal disease virus | IC | AOX1 | [140] | [165] | |||||||
| 27 | P1 and 3CD | Coxsackievirus A16 | IC | AOX1 | [141] | [166] | |||||||
| 28 | Core protein | Hepatitis B virus | IC | AOX1 | [142][143] | [167,168] | |||||||
| 29 | E protein | Dengue virus | IC | AOX1 | [144] | [169] | 30 | Capsid protein | |||||
| 30 | Porcine circovirus type 2 | IC | GAL | [ | 72] | [96] | |||||||
| L2 | Grapevine fanleaf virus | EC | AOX1 | [ | 145] | [170] | 31 | VP1,2 | Hepatitis B/Polyomavirus | IC | GAL7 | [73] | [97] |
| 31 | Capsid protein (VP60) | Rabbit hemorrhagic disease virus | IC | AOX1 | [146] | [171] | 32 | VP1 | Hamster polyomavirus | ||||
| 32 | HBc-influenza virus LAH domain | IC | Hepatitis B/Influenza H3N2 virus | IC | AOX1 | [74] | [98] | ||||||
| [ | 147 | 33 | L1/L1 + L2 | Cottontail rabbit papillomavirus | IC | GAL1-10 | [75] | [99] | |||||
| ] | [ | 172 | ] | ||||||||||
| 33 | CoreE1E2 Protein | Hepatitis C virus | EC | AOX1 | [148] | [173] | 34 | L1 | HPV 11 | IC | |||
| 34 | P1 and 3CD | Coxsackievirus A6 | GAL | [ | 76][77][78] | [ | IC100 | AOX1, | [ | 101,102] | |||
| 149 | ] | [ | 174 | ] | 35 | Coat protein | Potyvirus (Johnsongrass mosaic virus) | IC | |||||
| 35 | L1, L2 | HPV 16 | IC | ADC1 | AOX1 | [150] | [175] | ||||||
| 36 | prM/Env | Japanese encephalitis virus | IC | AOX1 | [151] | [176] | |||||||
| 37 | Den2E-HBsAg | Dengue/Hepatitis B virus | IC | AOX1 | [152] | [177] | |||||||
| 38 | prM and E protein | Dengue virus | IC | GAP | [153] | [178] | |||||||
| 39 | Polyprotein | Chikungunya virus | EC | AOX1 | [154] | [179] | |||||||
| 40 | L1 | HPV 16 | [155] | [180] | |||||||||
| 41 | Major capsid protein | Iridovirus | EC | AOX1 | [156] | [181] | |||||||
| 42 | Surface antigen | Hepatitis B virus | [157] | [182] | |||||||||
| 43 | VP1 | Rabbit hemorrhagic disease virus | IC | AOX1 | [158] | [183] | 44 | E7 oncoprotein of HPV16 | Hepatitis B virus | IC | [88] | [112] | |
| 44 | L1 | Bovine papillomavirus 1,2,4 | [159] | [184] | 45 | Capsid protein | Red-spotted grouper nervous necrosis virus | IC | GAL10 | [89] | [113] | ||
| 45 | L1 | HPV 16 | IC | AOX1 | [160] | [185] | 46 | L1 | HPV 58 | IC | GAL10 | [90 | |
| 46 | Capsid protein | Norovirus | IC | ] | [114] | ||||||||
| AOX1 | [ | 161 | ] | [ | 186] | 47 | C69R variant of surface antigen | Hepatitis B virus | |||||
| 47 | L1 | IC | HPV 16 | ECGAL10/CYC1 | [91] | [115] | |||||||
| PGK1 | [ | 162 | ] | [ | 187] | 48 | VP1 | Human polyomavirus 2 | IC | GAL | [92 | ||
| 48 | Core protein | Hepatitis B virus | ] | [116] | |||||||||
| [ | 163 | ] | [ | 188 | ] | 49 | VP2 | Parvovirus B19 | IC | GAL1 | [93] | [117 | |
| 49 | ] | ||||||||||||
| VP1 | Calicivirus virus | EC | AOX1 | [ | 164] | [189] | 50 | L1 | HPV 11 | IC | GAL110-11 | [94] | [118] |
| 50 | Core protein | Hepatitis C virus | IC | AOX1 | [165][166][167][168] | [190,191,192,193] | 51 | VP1 with pre-S1 region of the Hepatitis B virus | Hamster polyomavirus | IC | GAL | [95] | [119] |
| 52 | Surface antigen | Hepatitis B virus | IC | GAL10 | [96] | [120] | |||||||
| 53 | L1 | HPV 16 | IC | [97] | [121] |
* Additionally, expressed in K. phaffii; IC: intracellular; EC: extracellular.
IC: intracellular; EC: extracellular.
3. Aggregation of VLPs, a Matter of Concern
The production of VLPs on a commercial scale using the different hosts mentioned above is now becoming common practice. Several companies dealing in vaccines or drugs are producing VLPs commercially. Almost all VLP production suffers from the common issue of VLP aggregation when stored at a low-salt concentration and at 2–8 °C [169][170][171][172][173][213,214,215,216,217]. Dynamic Light Scattering (DLS), Transmission Electron Microscopes (TEM), and Atomic Force Microscopes (AFM) can detect the aggregation of VLPs, approaches commonly used for VLP characterization (reviewed by [3][28]). In addition, turbidity during elution or storage can also inform about possible VLP aggregation. The main problem with the aggregation of VLPs is that it affects immunogenicity and the level of an immune response. Aggregation of VLPs reduces their recovery using POROS resin used for the purification of VLPs on a commercial scale [87][111]. It was observed that the immune response raised by aggregated VLPs is lower than that raised by non-aggregated VLPs [169][174][175][176][177][213,218,219,220,221]. Additionally, aggregation can affect dose formulation. To obtain highly monodisperse VLPs, the manufacturer performs an ultracentrifuge or size exclusion to separate clean and aggregated VLPs. However, this leads to considerable wastage of VLPs and reduces the recovery of useful VLPs.
To prevent or minimize the aggregation of VLPs, different approaches have been used in the past. For example, adding L-arginine and glycine improves VLP recovery [177][178][221,222]. Sugars like sorbitol and trehalose are also shown to solubilize and improve VLP recovery by preventing their aggregation and by solubilizing protein monomers [179][180][210,212]. The use of polyethylene glycol (PEG) or glycerol also improves the solubilization of VLPs [181][182][223,224]. Apart from this, using surfactants or emulsifiers like Tween-80 is also helpful in preventing the aggregation of VLPs and improving the recovery of VLPs [183][225]. In several published studies, the author has added these compounds at one or another step in the purification of VLPs. Sometimes changes in the pH of the buffer and the buffer composition were also found helpful in preventing the aggregation of VLPs [184][226]. Additionally, to obtain VLPs of a uniform size, in vitro disassembly followed by reassembly is recommended [185][227]. Therefore, it requires substantial effort to find suitable conditions that improve the recovery of VLPs while simultaneously preventing their aggregation.
