Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Safaet Alam | -- | 1400 | 2023-04-06 05:37:16 | | | |
| 2 | Sirius Huang | Meta information modification | 1400 | 2023-04-07 04:36:18 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Ashrafi, S.; Alam, S.; Sultana, A.; Raj, A.; Emon, N.U.; Richi, F.T.; Sharmin, T.; Moon, M.; Park, M.N.; Kim, B. Papaverine and Its Mechanism of Action. Encyclopedia. Available online: https://encyclopedia.pub/entry/42831 (accessed on 06 March 2026).
Ashrafi S, Alam S, Sultana A, Raj A, Emon NU, Richi FT, et al. Papaverine and Its Mechanism of Action. Encyclopedia. Available at: https://encyclopedia.pub/entry/42831. Accessed March 06, 2026.
Ashrafi, Sania, Safaet Alam, Arifa Sultana, Asef Raj, Nazim Uddin Emon, Fahmida Tasnim Richi, Tasnuva Sharmin, Myunghan Moon, Moon Nyeo Park, Bonglee Kim. "Papaverine and Its Mechanism of Action" Encyclopedia, https://encyclopedia.pub/entry/42831 (accessed March 06, 2026).
Ashrafi, S., Alam, S., Sultana, A., Raj, A., Emon, N.U., Richi, F.T., Sharmin, T., Moon, M., Park, M.N., & Kim, B. (2023, April 06). Papaverine and Its Mechanism of Action. In Encyclopedia. https://encyclopedia.pub/entry/42831
Ashrafi, Sania, et al. "Papaverine and Its Mechanism of Action." Encyclopedia. Web. 06 April, 2023.
Copy Citation
Papaverine has been proven to be a high-value opioid alkaloid in the field of therapeutics either in solitude or in combination with other metabolites/molecules. Pharmacological research has revealed that papaverine demonstrates a variety of biological activities, including activity against erectile dysfunction, postoperative vasospasms, and pulmonary vasoconstriction, as well as antiviral, cardioprotective, anti-inflammatory, anticancer, neuroprotective, and gestational actions.
Papaver somniferum
opium
benzylisoquinoline
papaverine
alkaloid
antiviral
anticancer
1. Introduction
Since ancient times, medicinal plants have played a key role in traditional medicine systems. Phytochemicals are being mined more frequently to find novel leads in the drug discovery process or to find better alternatives to existing ones [1][2][3][4][5]. Around 75% of the global population, mostly from developing countries, depend primarily on traditional herbal medicines due to their affordability and environmentally beneficial qualities [6].
According to estimates, 20% of plant species produce 12,000 alkaloids combined, many of which have been used in both traditional and modern-day medicine for centuries. Among these, about 2500 of the substances are members of a structurally diverse class of metabolites known as benzylisoquinoline alkaloids (BIAs), which also includes the opiate drugs morphine and codeine. Additionally, the benzylisoquinoline alkaloid family is a prominent class of plant-derived chemicals that has shown a wide range of pharmacological activity, including antibacterial, antitussive, antispasmodic, and anticancer properties [7][8].
A prominent benzylisoquinoline alkaloid is papaverine, which can be obtained from Papaver somniferum L. (opium poppy). The opium alkaloids include papaverine, morphine, codeine, thebaine, noscapine, and narceine, as well as a small percentage of some other compounds. Various pieces of traditional research evidence have demonstrated opium alkaloids in Chinese and Indian herbal medicine to be effective at treating a variety of ailments, including chronic cough, rectum prolapse, diarrhea, dysentery, and gastrointestinal issues. In addition, papaverine has also been incorporated in therapeutic settings to treat erectile dysfunction, smooth muscle spasms, and spasms associated with gastrointestinal problems. Scientists have also found papaverine as a nonselective phosphodiesterase (PDE) inhibitor in mammals, boosting the amount of cAMP and cGMP available for cell signaling [9]. Hence, this secondary metabolite demands further exploration and requires pharmacological research investigations.
2. Natural Source of Papaverine
Due to their phytochemical composition, members of the genus Papaver (family: Papaveraceae) are recognized for their therapeutic benefits. The most significant Papaver species that contributes phytochemicals for drug development is Papaver somniferum L. (opium poppy), which is highly produced in countries such as Afghanistan, Myanmar, Mexico, Laos, Turkey, Czechia, and Spain. Other commonly cultivated Papaver species include P. bracteatum Lindl. (Persian poppy), P. rhoeas L. (common poppy or corn poppy), P. dubium L., P. pseudo-orientale Medw., and P. orientale L., which are grown at high altitudes in north and northwest Iran, Russia, the Caucasia region, Europe, and America [10]. P. somniferum L. produces papaverine naturally in its unripe seed capsules. A total of 40 alkaloids have been found in the plant; however, morphine (10–15%), noscapine (4–5%), codeine (1–3%), papaverine (1–3%), and thebaine (1–3%) are the five primary alkaloids. The prevalence of papaverine in Indian species ranges from 0.5% to 3% [11].
3. Chemistry of Papaverine
Benzylisoquinoline alkaloids hold a prominent place in alkaloid chemistry as they serve as in vivo precursors to many other naturally occurring isoquinolines. They are either 1,2,3,4-tetrahydro, as in coclaurine and N-nororientaline, or fully aromatic, as in papaverine, palaudine, and escholamine. Ring A in the benzylisoquinoline alkaloids may possess two or three oxygenated substituents, while ring C has only one or two substituents [12].
Papaverine, also known by its IUPAC nomenclature 1-[(3,4-dimethoxy phenyl) methyl]-6,7-dimethoxyisoquinoline, is one of the principal benzylisoquinoline alkaloids found in P. somniferum [13][14]. Naturally, it is produced as a byproduct of morphine, codeine, and narcotine synthesis. Its m/z ratio was determined to be 340.15417 [15]. It is a neutral solid that only slightly dissolves in water [16]. There are four methoxy groups in papaverine. Even if the molecule lacks a TV-methyl group, it still functions as a tertiary base. It is considered a pyridine derivative because it may be reduced to a secondary amine by adding four hydrogen atoms, with the heterocyclic ring fused to a benzene ring [12] (Figure 1).
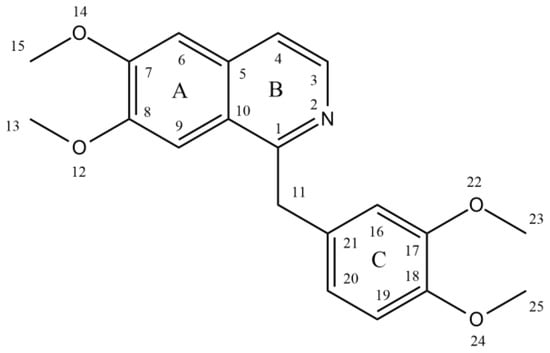
Figure 1. Structure of papaverine.
Guido Goldschmiedt first illustrated the structure of the papaverine between the years of 1885 and 1898. By forming methiodide and demonstrating the presence of four methoxy groups per mole, he established the existence of a tertiary nitrogen atom. Under different conditions, the base was oxidized with potassium permanganate to produce various related compounds (Figure 2).
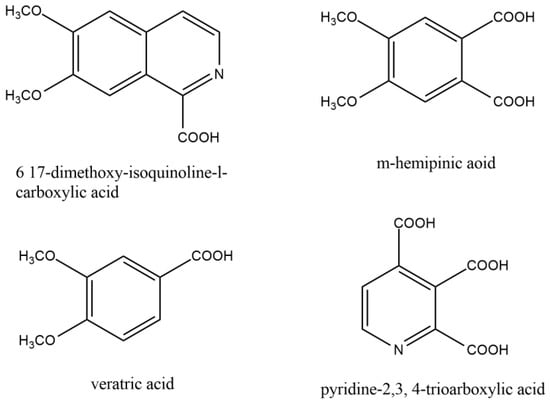
Figure 2. Similar compounds of papaverine helped to elucidate the structure of papaverine.
The current structure was recognized as papaverine based on the evidence mentioned above and other relevant data. Following the successful synthesis of papaverine in 1909, Pictet and Gams validated the molecular structure [14].
4. Biosynthesis of Papaverine
Two units of tyrosine contribute as the precursors for the biosynthesis of papaverine, and the intermediate products include (S)- norcoclaurine, laudanine, norlaudanine, reticuline, norreticuline, tetrahydropapaverine, and dihydropapaverine. Recent investigations revealed that the primary pathway of papaverine biosynthesis in the opium poppy has been identified by systematic silencing of benzylisoquinoline alkaloid biosynthetic genes.
There are two suggested metabolic pathways for (S)-norcoclaurine. One involves only N-demethylated intermediates (the NH pathway), whereas the other involves (S)-reticuline and involves a number of N-methylated intermediates (the NCH3 pathway) [13][17][18]. The NH route advances via (S)- norreticuline [13][19][20] (Figure 3), whereas the NCH3 route involves (S)-reticuline [13][15][20][21] (Figure 4).
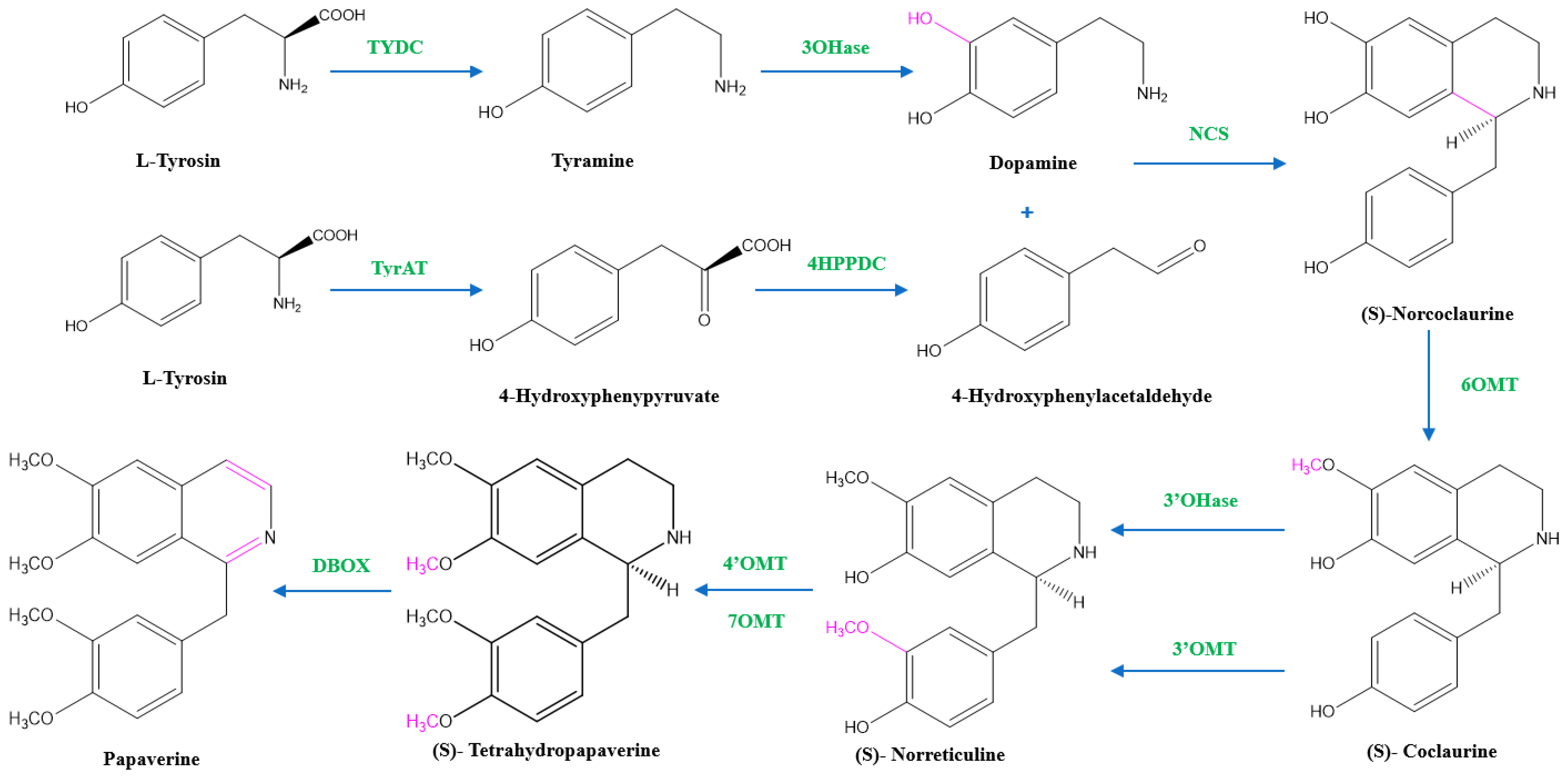
Figure 3. The NH pathway of papaverine synthesis. TYDC = tyrosine decarboxylase, TyrAT = L-tyrosine aminotransferase, 4HPPDC = 4-hydroxyphenylpyruvate decarboxylase, 3OHase = tyramine 3-hydroxylase, NCS = norcoclaurine synthase, 6OMT = norcoclaurine-6-O-methyltransferase, 3’OHase = 3’ hydroxylase, 3’OMT = 3′-O-methyltransferase, 4’OMT = 3′-hydroxy-N-methylcoclaurine 4′-O-methyltransferase, 7OMT = norreticuline 7-O-methyltransferase, DBOX = dihydrobenzophenanthridine oxidase.
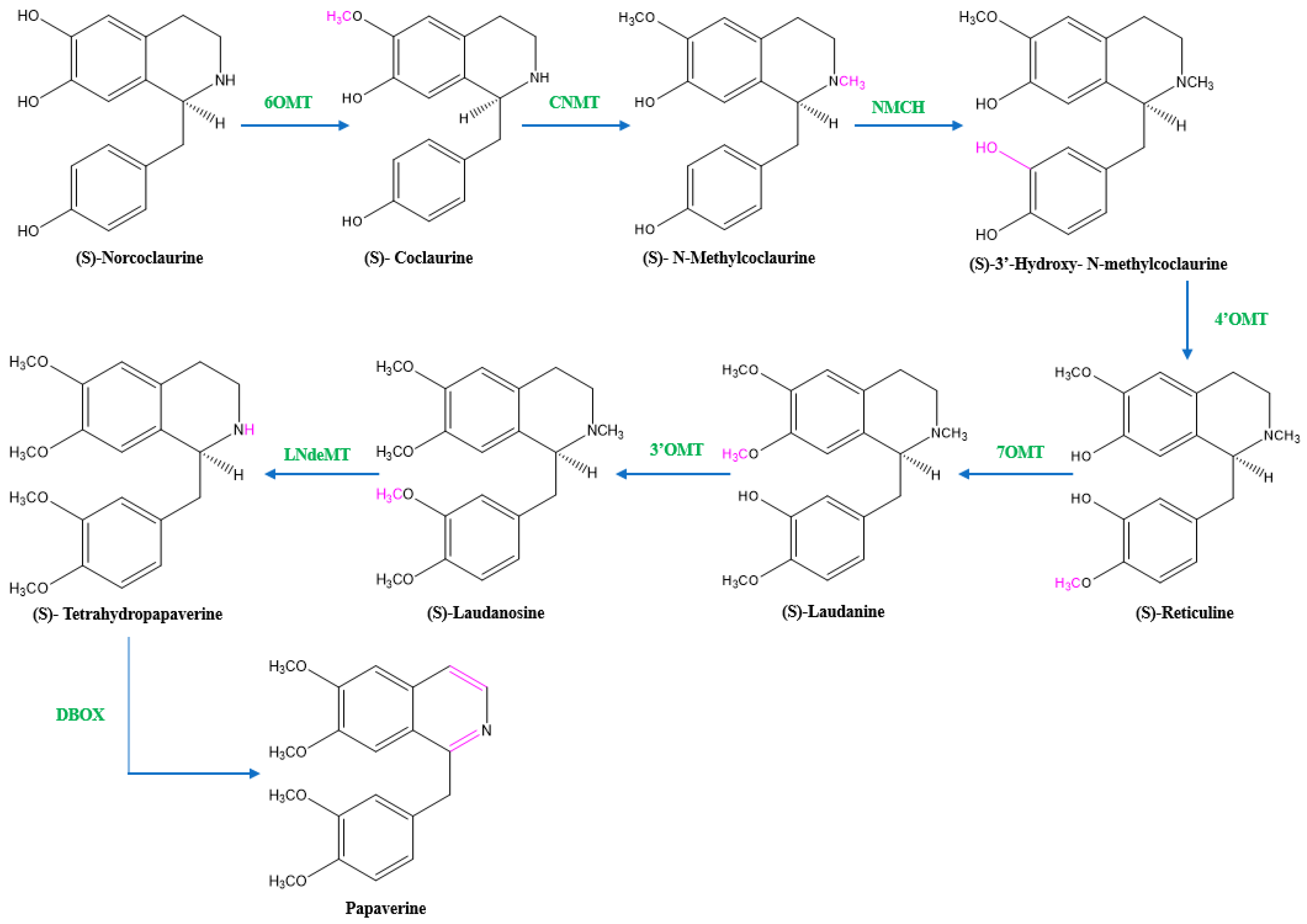
Figure 4. The NCH3 pathway of papaverine synthesis. 6OMT = norcoclaurine-6-O-methyltransferase, CNMT = coclaurine-N-methyltransferase, NMCH = (S)-N-methylcoclaurine 3′-hydroxylase, 4’OMT = 4′-O-methyltransferase, 7OMT = reticuline 7-O-methyltransferase, 3’OMT = 3′-O-methyltransferase, LNdeMT = laudanosine N-demethylase, DBOX = dihydrobenzophenanthridine oxidase.
The first step in papaverine biosynthesis is the condensation of two L-tyrosine derivatives, 4-hydroxyphenylacetaldehyde (4HPAA) and dopamine, which is accomplished through decarboxylation, meta-hydroxylation, and transamination to produce the precursor to all other benzylisoquinoline alkaloids, (S)-norcoclaurine. Tyrosine decarboxylase (TYDC) and tyramine 3-hydroxylase (3OHase) transform L-tyrosine into tyramine and dopamine, respectively. L-tyrosine can be transaminated by L-tyrosine aminotransferase (TyrAT) in the production of 4HPAA, and then decarboxylated by an enzyme identified as 4-hydroxyphenylpyruvate decarboxylase (4HPPDC). Norcoclaurine synthase (NCS) is the enzyme that catalyzes the condensation of (S)-norcoclaurine from 4HPAA and dopamine.
Norcoclaurine-6-O-methyltransferase (6OMT) first transforms (S)-norcoclaurine into (S)-coclaurine. In the NH pathway, (S)-coclaurine first undergoes 3′ hydroxylation by 3′ hydroxylase (3′OHase) and then is converted to (S)-norreticuline by 3′-O-methyltransferase (3′OMT). On the other hand, in the NCH3 pathway, (S)-coclaurine is taken up by coclaurine N-methyltransferase (CNMT) to yield (S)-N-methylcoclaurine. (S)-N-methylcoclaurine is hydroxylated to 3′-hydroxy-N-methylcoclaurine by (S)-N-methylcoclaurine 3′-hydroxylase (NMCH), which is then transformed into (S)-reticuline by 3′-hydroxy-N-Methylcoclaurine 4′-O-methyltransferase (4′OMT). It is interesting to note that only NMCH has been reported to exhibit strict stereoisomer and substrate specificity, accepting only (S)-N-methylcoclaurine and rejecting either the corresponding (R)-N-methylcoclaurine or N-desmethyl compounds. As a distinction, the O- and N-methyltransferases often accept a wide range of (R)- and (S)-tetrahydroisoquinolines [19]. The enzyme reticuline 7-O-methyltransferase (7OMT) can further methylate reticuline to produce laudanine, which can then be fully O-methylated to laudanosine by 3′-O-methyltransferase.
The final steps in papaverine biosynthesis comprise the oxidation of the fully O-methylated and N-desmethyl molecule tetrahydropapaverine by dihydrobenzophenanthridine oxidase (DBOX).
Advanced quantum chemical density functional theory (DFT) calculations, as well as diffuse reflectance (Ds), experimental electronic absorption (EAs), matrix-associated laser desorption ionization (MALDI) coupled with Orbitrap imaging mass spectrometry (MS), fluorescence spectroscopy (Fs), and circular dichroic (CD) have been used for theoretical and experimental elucidation of the papaverine biosynthetic pathway via (S)-reticuline (the NCH3 pathway) [19][20][22].
5. Mechanism of Muscle Relaxing Action of Papaverine
Papaverine is recognized as the most effective smooth muscle relaxant, as it acts directly on smooth muscle by exerting a strong vasodilating effect. It has been observed to boost intracellular levels of cAMP and cGMP by blocking the cAMP and cGMP phosphodiesterase in smooth muscles (Figure 5) [23][24][25][26][27]. Inhibiting the release of calcium from the intracellular space and obstructing calcium ion channels in the cell membrane are two other ways that papaverine may work [28].
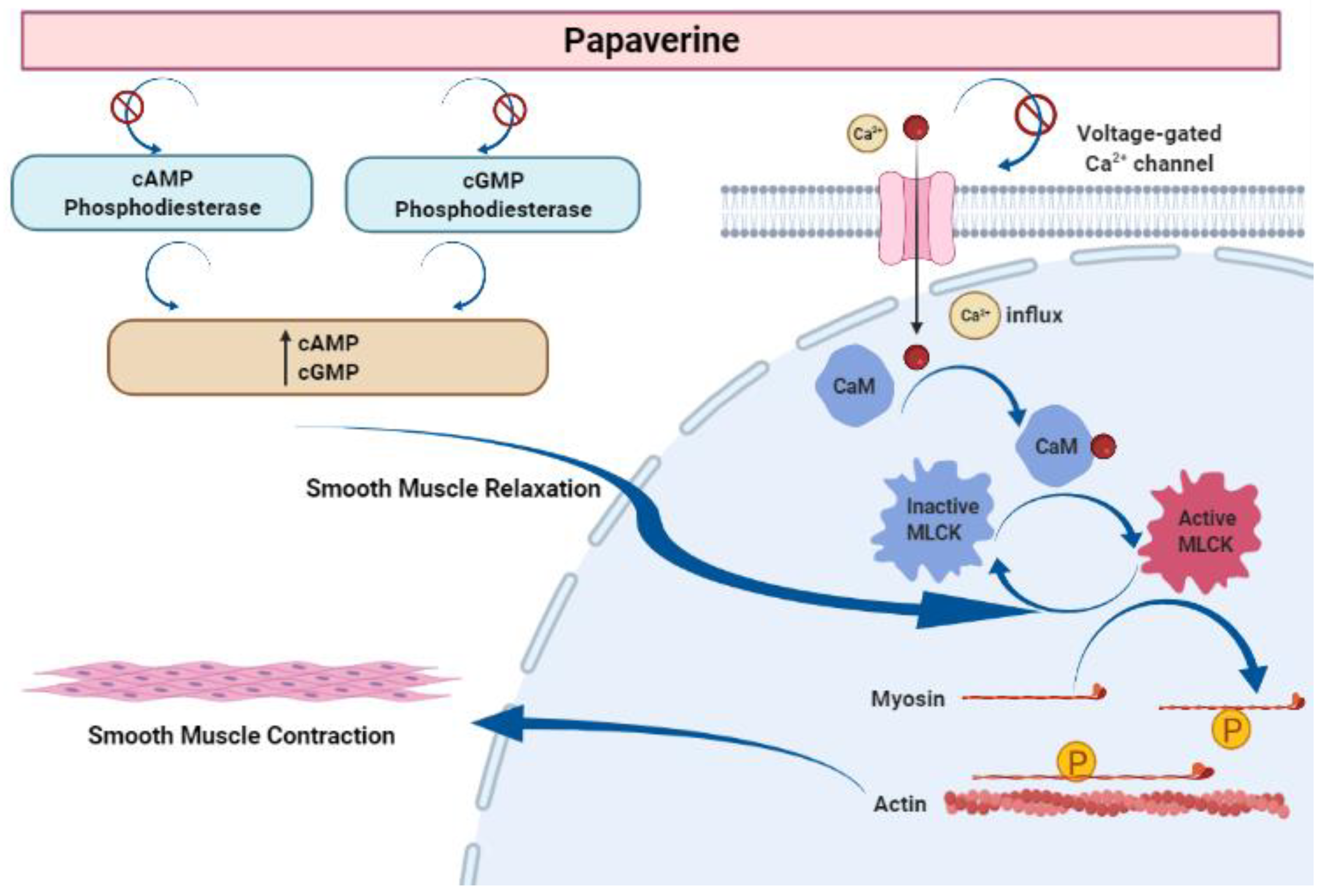
Figure 5. Mechanism of action of papaverine in smooth muscle relaxation. Smooth muscle contraction requires five steps: After the increase in intracellular Ca2+ concentration from the extracellular fluid, these ions bind to a protein called calmodulin (CaM). This complex activates a protein called myosin light-chain kinase (MLCK) (papaverine inhibits this step), which subsequently phosphorylates light chains of myosin heads, increasing the myosin ATPase activity. Finally, active myosin cross-bridges slide along actin and create muscle tension to contract the cell.
References
- Arora, L.; Hosn, M.A. Spinal Cord Perfusion Protection for Thoraco-Abdominal Aortic Aneurysm Surgery. Curr. Opin. Anaesthesiol. 2019, 32, 72–79.
- Grdina, D.J.; Murley, J.S.; Kataoka, Y. Radioprotectants: Current Status and New Directions. Oncology 2002, 63, 2–10.
- Weiss, J.F.; Landauer, M.R. Protection against Ionizing Radiation by Antioxidant Nutrients and Phytochemicals. Toxicology 2003, 189, 1–20.
- Alam, S.; Emon, N.; Shahriar, S.; Richi, F.; Haque, M.; Islam, M.; Sakib, S.; Ganguly, A. Pharmacological and Computer-Aided Studies Provide New Insights into Millettia Peguensis Ali (Fabaceae). Saudi Pharm. J. 2020, 28, 1777–1790.
- Ashrafi, S.; Alam, S.; Emon, N.; Ahsan, M. Isolation, Characterization and Pharmacological Investigations of a New Phenolic Compound along with Four Others Firstly Reported Phytochemicals from Glycosmis. Molecules 2022, 27, 5972.
- Amin, M.N.; Emdadul, M.; Mukul, H.; Millat, M.S.; Saif, M.; Rashed, U. Phytochemical Nature and Pharmacological Evaluation of Chloroform Extract of Pandanus Fascicularis L. (Fruits): An in Vivo Study Open Access. Artic. J. Bioanal. Biomed. 2017, 9, 4.
- Pyne, M.E.; Narcross, L.; Fossati, E.; Bourgeois, L.; Burton, E.; Gold, N.D.; Martin, V.J.J. Reconstituting Plant Secondary Metabolism in Saccharomyces Cerevisiae for Production of High-Value Benzylisoquinoline Alkaloids. Methods Enzymol. 2016, 575, 195–224.
- Ziegler, J.; Facchini, P.J. Alkaloid Biosynthesis: Metabolism and Trafficking. Annu. Rev. Plant Biol. 2008, 59, 735–769.
- Benej, M.; Hong, X.; Vibhute, S.; Scott, S.; Wu, J.; Graves, E.; Le, Q.T.; Koong, A.C.; Giaccia, A.J.; Yu, B.; et al. Papaverine and Its Derivatives Radiosensitize Solid Tumors by Inhibiting Mitochondrial Metabolism. Proc. Natl. Acad. Sci. USA 2018, 115, 10756–10761.
- Butnariu, M.; Quispe, C.; Herrera-Bravo, J.J.; Pentea, M.; Sarac, I.; Küşümler, A.; Özçelik, B.; Painuli, S.; Semwal, P.; Imran, M.; et al. Papaver Plants: Current Insights on Phytochemical and Nutritional Composition Along with Biotechnological Applications. Oxid. Med. Cell. Longev. 2022, 2022, 2041769.
- Peter, K. V Handbook of Herbs and Spices; Elsevier: Amsterdam, The Netherlands, 2012; ISBN 0857095684.
- Shamma, M. The Isoquinoline Alkaloids Chemistry and Pharmacology; Elsevier: Amsterdam, The Netherlands, 2012.
- Desgagné-Penix, I.; Facchini, P.J. Systematic Silencing of Benzylisoquinoline Alkaloid Biosynthetic Genes Reveals the Major Route to Papaverine in Opium Poppy. Plant J. 2012, 72, 331–344.
- Yan, J.; Mi, J.Q.; He, J.T.; Guo, Z.Q.; Zhao, M.P.; Chang, W.B. Development of an Indirect Competitive ELISA for the Determination of Papaverine. Talanta 2005, 66, 1005–1011.
- Han, X.; Lamshöft, M.; Grobe, N.; Ren, X.; Fist, A.; Kutchan, T.; Spiteller, M.; Zenk, M. The Biosynthesis of Papaverine Proceeds via (S)-Reticuline. Phytochemistry 2010, 71, 1305–1312.
- Gaber, A.; Alsanie, W.F.; Kumar, D.N.; Refat, M.S.; Saied, E.M. Novel Papaverine Metal Complexes with Potential Anticancer Activities. Molecules 2020, 25, 5447.
- Srivastava, A.; Agrawal, L.; Raj, R.; Jaidi, M.; Raj, S.K.; Gupta, S.; Dixit, R.; Singh, P.C.; Tripathi, T.; Sidhu, O.P.; et al. Ageratum Enation Virus Infection Induces Programmed Cell Death and Alters Metabolite Biosynthesis in Papaver Somniferum. Front. Plant Sci. 2017, 8, 1172.
- Agarwal, P.; Pathak, S.; Kumar, R.S.; Dhar, Y.V.; Pandey, A.; Shukla, S.; Trivedi, P.K. 3′O-Methyltransferase, Ps3′OMT, from Opium Poppy: Involvement in Papaverine Biosynthesis. Plant Cell Rep. 2019, 38, 1235–1248.
- Beaudoin, G.A.W.; Facchini, P.J. Benzylisoquinoline Alkaloid Biosynthesis in Opium Poppy. Planta 2014, 240, 19–32.
- Ivanova, B.; Spiteller, M. On the Biosynthetic Pathway of Papaverine via (S)-Reticuline—Theoretical vs. Experimental Study. Nat. Prod. Commun. 2012, 7, 581–586.
- Pathak, S.; Lakhwani, D.; Gupta, P.; Mishra, B.K.; Shukla, S.; Asif, M.H.; Trivedi, P.K. Comparative Transcriptome Analysis Using High Papaverine Mutant of Papaver Somniferum Reveals Pathway and Uncharacterized Steps of Papaverine Biosynthesis. PLoS ONE 2013, 8, e65622.
- Pienkny, S.; Brandt, W.; Schmidt, J.; Kramell, R.; Ziegler, J. Functional Characterization of a Novel Benzylisoquinoline O-methyltransferase Suggests Its Involvement in Papaverine Biosynthesis in Opium Poppy (Papaver somniferum L.). Plant J. 2009, 60, 56–67.
- Abusnina, A.; Lugnier, C. Therapeutic Potentials of Natural Compounds Acting on Cyclic Nucleotide Phosphodiesterase Families. Cell. Signal. 2017, 39, 55–65.
- Debnath, B.; Singh, W.; Das, M.; Goswami, S.; Singh, M.; Maiti, D.; Manna, K. Role of Plant Alkaloids on Human Health: A Review of Biological Activities. Mater. Today Chem. 2018, 9, 56–72.
- Dong, H.H.; Guang, Y.B.; Tae, K.Y.; Byung, S.S.; Yong, G.K.; Chul, J.K. The Effect of Papaverine on Ion Channels in Rat Basilar Smooth Muscle Cells. Neurol. Res. 2007, 29, 544–550.
- Gomes, D.; Joubert, A.; Visagie, M. In Vitro Effects of Papaverine on Cell Proliferation, Reactive Oxygen Species, and Cell Cycle Progression in Cancer Cells. Molecules 2021, 26, 6388.
- Shimizu, K.; Yoshihara, E.; Takahashi, M.; Gotoh, K.; Orita, S.; Urakawa, N.; Nakajyo, S. Mechanism of Relaxant Response to Papaverine on the Smooth Muscle of Non-Pregnant Rat Uterus. J. Smooth Muscle Res. 2000, 36, 83–91.
- Liu, J.K.; Couldwell, W.T. Intra-Arterial Papaverine Infusions for the Treatment of Cerebral Vasospasm Induced by Aneurysmal Subarachnoid Hemorrhage. Neurocrit. Care 2005, 2, 124–132.
More
Information
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
4.3K
Entry Collection:
Organic Synthesis
Revisions:
2 times
(View History)
Update Date:
07 Apr 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





