Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Safaet Alam and Version 2 by Sirius Huang.
Papaverine has been proven to be a high-value opioid alkaloid in the field of therapeutics either in solitude or in combination with other metabolites/molecules. Pharmacological research has revealed that papaverine demonstrates a variety of biological activities, including activity against erectile dysfunction, postoperative vasospasms, and pulmonary vasoconstriction, as well as antiviral, cardioprotective, anti-inflammatory, anticancer, neuroprotective, and gestational actions.
- Papaver somniferum
- opium
- benzylisoquinoline
- papaverine
- alkaloid
- antiviral
- anticancer
1. Introduction
Since ancient times, medicinal plants have played a key role in traditional medicine systems. Phytochemicals are being mined more frequently to find novel leads in the drug discovery process or to find better alternatives to existing ones [1][2][3][4][5][1,2,3,4,5]. Around 75% of the global population, mostly from developing countries, depend primarily on traditional herbal medicines due to their affordability and environmentally beneficial qualities [6].
According to estimates, 20% of plant species produce 12,000 alkaloids combined, many of which have been used in both traditional and modern-day medicine for centuries. Among these, about 2500 of the substances are members of a structurally diverse class of metabolites known as benzylisoquinoline alkaloids (BIAs), which also includes the opiate drugs morphine and codeine. Additionally, the benzylisoquinoline alkaloid family is a prominent class of plant-derived chemicals that has shown a wide range of pharmacological activity, including antibacterial, antitussive, antispasmodic, and anticancer properties [7][8][7,8].
A prominent benzylisoquinoline alkaloid is papaverine, which can be obtained from Papaver somniferum L. (opium poppy). The opium alkaloids include papaverine, morphine, codeine, thebaine, noscapine, and narceine, as well as a small percentage of some other compounds. Various pieces of traditional research evidence have demonstrated opium alkaloids in Chinese and Indian herbal medicine to be effective at treating a variety of ailments, including chronic cough, rectum prolapse, diarrhea, dysentery, and gastrointestinal issues. In addition, papaverine has also been incorporated in therapeutic settings to treat erectile dysfunction, smooth muscle spasms, and spasms associated with gastrointestinal problems. Scientists have also found papaverine as a nonselective phosphodiesterase (PDE) inhibitor in mammals, boosting the amount of cAMP and cGMP available for cell signaling [9]. Hence, this secondary metabolite demands further exploration and requires pharmacological research investigations.
2. Natural Source of Papaverine
Due to their phytochemical composition, members of the genus Papaver (family: Papaveraceae) are recognized for their therapeutic benefits. The most significant Papaver species that contributes phytochemicals for drug development is Papaver somniferum L. (opium poppy), which is highly produced in countries such as Afghanistan, Myanmar, Mexico, Laos, Turkey, Czechia, and Spain. Other commonly cultivated Papaver species include P. bracteatum Lindl. (Persian poppy), P. rhoeas L. (common poppy or corn poppy), P. dubium L., P. pseudo-orientale Medw., and P. orientale L., which are grown at high altitudes in north and northwest Iran, Russia, the Caucasia region, Europe, and America [10]. P. somniferum L. produces papaverine naturally in its unripe seed capsules. A total of 40 alkaloids have been found in the plant; however, morphine (10–15%), noscapine (4–5%), codeine (1–3%), papaverine (1–3%), and thebaine (1–3%) are the five primary alkaloids. The prevalence of papaverine in Indian species ranges from 0.5% to 3% [11].3. Chemistry of Papaverine
Benzylisoquinoline alkaloids hold a prominent place in alkaloid chemistry as they serve as in vivo precursors to many other naturally occurring isoquinolines. They are either 1,2,3,4-tetrahydro, as in coclaurine and N-nororientaline, or fully aromatic, as in papaverine, palaudine, and escholamine. Ring A in the benzylisoquinoline alkaloids may possess two or three oxygenated substituents, while ring C has only one or two substituents [12]. Papaverine, also known by its IUPAC nomenclature 1-[(3,4-dimethoxy phenyl) methyl]-6,7-dimethoxyisoquinoline, is one of the principal benzylisoquinoline alkaloids found in P. somniferum [13][14][13,14]. Naturally, it is produced as a byproduct of morphine, codeine, and narcotine synthesis. Its m/z ratio was determined to be 340.15417 [15]. It is a neutral solid that only slightly dissolves in water [16]. There are four methoxy groups in papaverine. Even if the molecule lacks a TV-methyl group, it still functions as a tertiary base. It is considered a pyridine derivative because it may be reduced to a secondary amine by adding four hydrogen atoms, with the heterocyclic ring fused to a benzene ring [12] (Figure 1).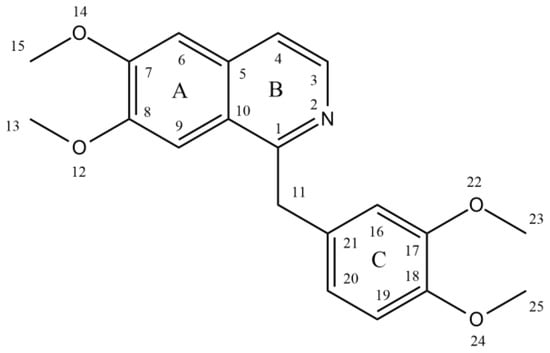
Figure 1.
Structure of papaverine.
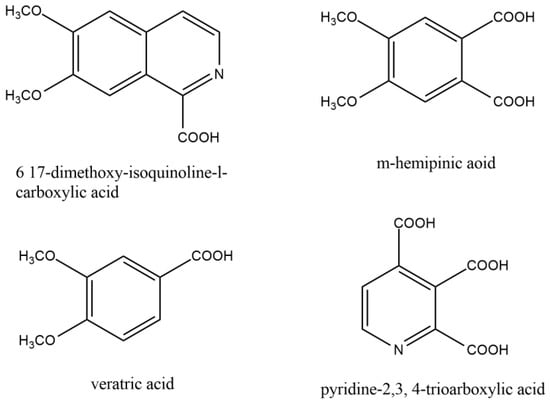
Figure 2.
Similar compounds of papaverine helped to elucidate the structure of papaverine.
4. Biosynthesis of Papaverine
Two units of tyrosine contribute as the precursors for the biosynthesis of papaverine, and the intermediate products include (S)- norcoclaurine, laudanine, norlaudanine, reticuline, norreticuline, tetrahydropapaverine, and dihydropapaverine. Recent investigations revealed that the primary pathway of papaverine biosynthesis in the opium poppy has been identified by systematic silencing of benzylisoquinoline alkaloid biosynthetic genes. There are two suggested metabolic pathways for (S)-norcoclaurine. One involves only N-demethylated intermediates (the NH pathway), whereas the other involves (S)-reticuline and involves a number of N-methylated intermediates (the NCH3 pathway) [13][17][18][13,17,18]. The NH route advances via (S)- norreticuline [13][19][20][13,19,20] (Figure 3), whereas the NCH3 route involves (S)-reticuline [13][15][20][21][13,15,20,21] (Figure 4).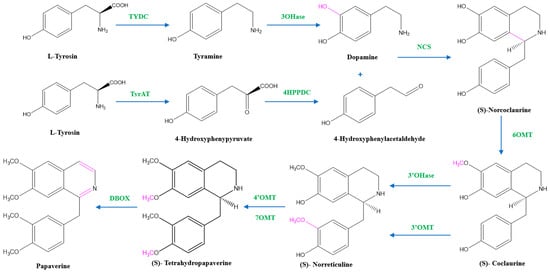
Figure 3. The NH pathway of papaverine synthesis. TYDC = tyrosine decarboxylase, TyrAT = L-tyrosine aminotransferase, 4HPPDC = 4-hydroxyphenylpyruvate decarboxylase, 3OHase = tyramine 3-hydroxylase, NCS = norcoclaurine synthase, 6OMT = norcoclaurine-6-O-methyltransferase, 3’OHase = 3’ hydroxylase, 3’OMT = 3′-O-methyltransferase, 4’OMT = 3′-hydroxy-N-methylcoclaurine 4′-O-methyltransferase, 7OMT = norreticuline 7-O-methyltransferase, DBOX = dihydrobenzophenanthridine oxidase.
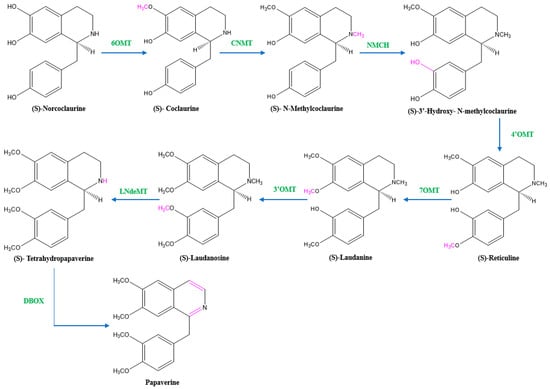
Figure 4. The NCH3 pathway of papaverine synthesis. 6OMT = norcoclaurine-6-O-methyltransferase, CNMT = coclaurine-N-methyltransferase, NMCH = (S)-N-methylcoclaurine 3′-hydroxylase, 4’OMT = 4′-O-methyltransferase, 7OMT = reticuline 7-O-methyltransferase, 3’OMT = 3′-O-methyltransferase, LNdeMT = laudanosine N-demethylase, DBOX = dihydrobenzophenanthridine oxidase.
5. Mechanism of Muscle Relaxing Action of Papaverine
Papaverine is recognized as the most effective smooth muscle relaxant, as it acts directly on smooth muscle by exerting a strong vasodilating effect. It has been observed to boost intracellular levels of cAMP and cGMP by blocking the cAMP and cGMP phosphodiesterase in smooth muscles (Figure 5) [23][24][25][26][27][23,24,25,26,27]. Inhibiting the release of calcium from the intracellular space and obstructing calcium ion channels in the cell membrane are two other ways that papaverine may work [28].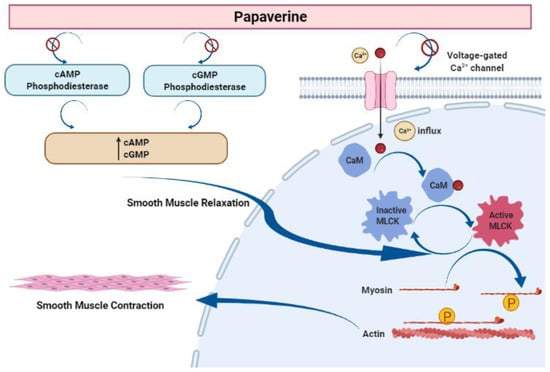
Figure 5. Mechanism of action of papaverine in smooth muscle relaxation. Smooth muscle contraction requires five steps: After the increase in intracellular Ca2+ concentration from the extracellular fluid, these ions bind to a protein called calmodulin (CaM). This complex activates a protein called myosin light-chain kinase (MLCK) (papaverine inhibits this step), which subsequently phosphorylates light chains of myosin heads, increasing the myosin ATPase activity. Finally, active myosin cross-bridges slide along actin and create muscle tension to contract the cell.
