
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Andrew W. Taylor-Robinson | -- | 2218 | 2023-02-21 07:14:39 | | | |
| 2 | Rita Xu | Meta information modification | 2218 | 2023-02-21 07:22:39 | | |
Video Upload Options
Schistosomiasis, also known as bilharzia, is a major parasitic disease caused by blood flukes (trematode worms) of the genus Schistosoma that live in fresh waterways in tropical and subtropical zones. Over 200 million people are infected globally, 90% of whom live in sub-Saharan Africa. Nigeria has the highest burden of schistosomiasis in this region. Elimination programmes have focused on human infections, with limited attention on infections in livestock that could be transmissible to humans, i.e. zoonotic schistosomiasis. This is now recognized as a risk factor for increased transmission and recrudescence of infection of more than one schistosome species, as well as of potential hybrid variants. Members of farming communities who herd grazing cattle, goats and sheep in proximity to rivers containing Bulinus freshwater snails that are the intermediate host of asexual lifecycle stages are particularly at risk of becoming infected through daily contact with contaminated water.
1. Introduction
2. Life Cycle of Schistosoma haematobium
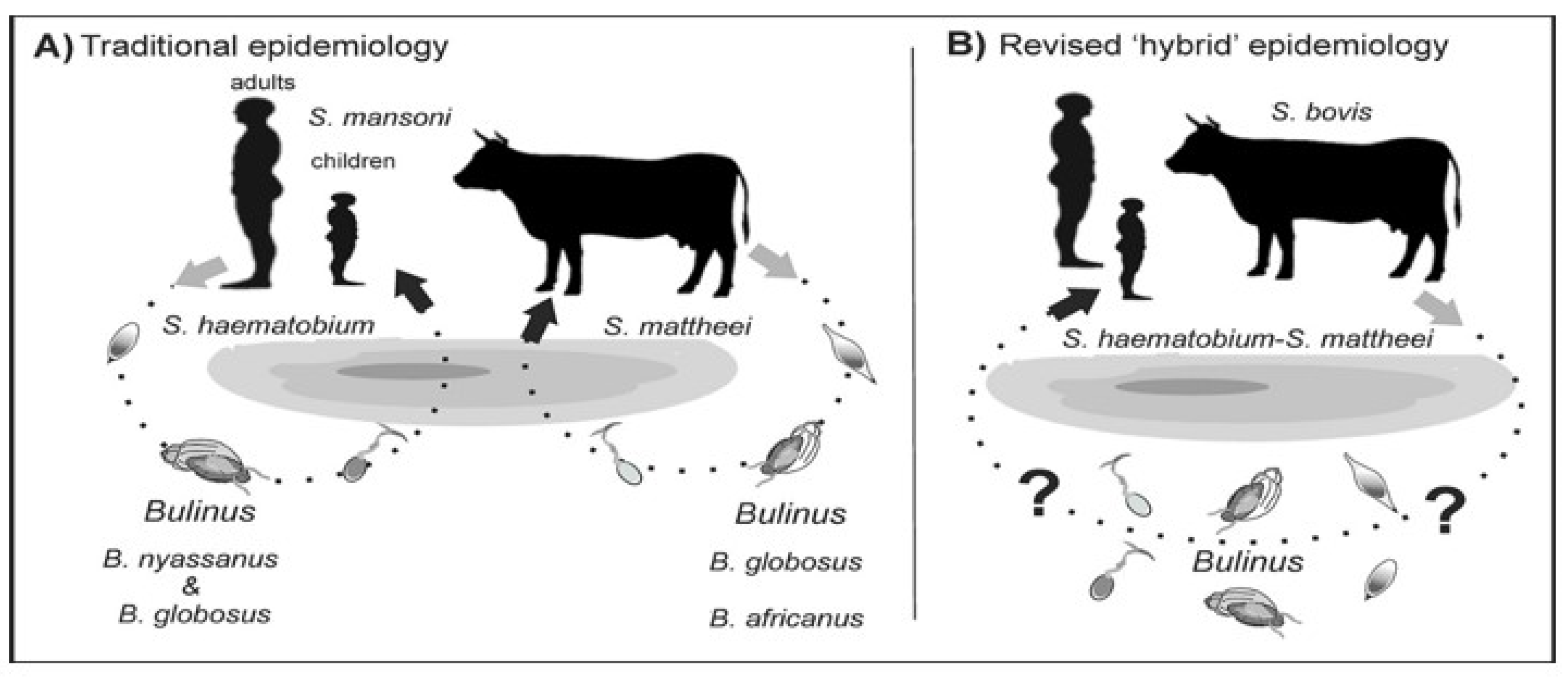
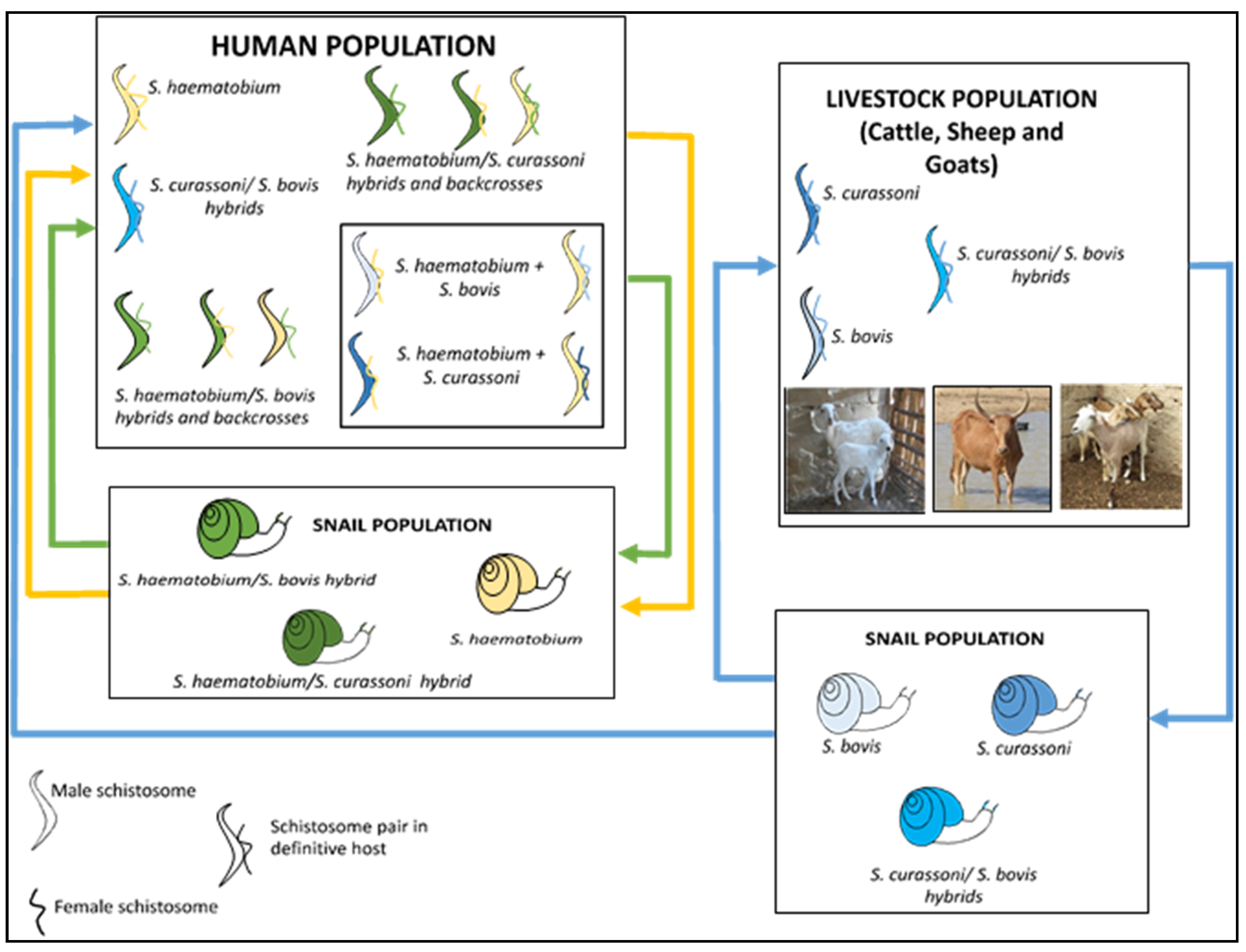
3. Zoonotic Schistosomiasis
4. Schistosomiasis Control Programme in Nigeria
5. Implication of Hybridization for Schistosomiasis Control Efforts
References
- Hotez, P.J.; Fenwick, A. Schistosomiasis in Africa: An emerging tragedy in our new global health decade. PLoS Negl. Trop Dis. 2009, 3, e485.
- Hotez, P.J.; Kamath, A. Neglected tropical diseases in sub-Saharan Africa: Review of their prevalence, distribution, and disease burden. PLoS Negl. Trop. Dis. 2009, 3, e412.
- World Health Organization. Schistosomiasis. 8 January 2022. Available online: https://www.who.int/news-room/fact-sheets/detail/schistosomiasis (accessed on 30 January 2023).
- World Health Organization. Ending the Neglect to Attain the Sustainable Development Goals: A Road Map for Neglected Tropical Diseases 2021–2030; World Health Organization: Geneva, Switzerland, 2020.
- Stothard, J.R.; Kayuni, S.A.; Al-Harbi, M.H.; Musaya, J.; Webster, B.L. Future schistosome hybridizations: Will all Schistosoma haematobium hybrids please stand-up! PLoS Negl. Trop. Dis. 2020, 14, e0008201.
- Engels, D.; Chitsulo, L.; Montresor, A.; Savioli, L. The global epidemiological situation of schistosomiasis and new approaches to control and research. Acta Trop. 2002, 82, 139–146.
- Centers for Disease Control and Prevention. DPDx—Laboratory Identification of Parasites of Public Health Concern. Schistosomiasis. Available online: https://www.cdc.gov/dpdx/schistosomiasis/index.html (accessed on 30 January 2023).
- Ekpo, U.F.; Hürlimann, E.; Schur, N.; Oluwole, A.S.; Abe, E.M.; Mafe, M.A.; Nebe, O.J.; Isiyaku, S.; Olamiju, F.; Kadiri, M.; et al. Mapping and prediction of schistosomiasis in Nigeria using compiled survey data and Bayesian geospatial modelling. Geospat. Health 2013, 7, 355–366.
- World Health Organization. Helminth Control in School-Age Children: A Guide for Managers of Control Programmes, 2nd ed.; World Health Organization: Geneva, Switzerland, 2011; Available online: https://apps.who.int/iris/bitstream/handle/10665/44671/9789241548267_eng.pdf (accessed on 30 January 2023).
- Rollinson, D. A wake-up call for urinary schistosomiasis: Reconciling research effort with public health importance. Parasitology 2009, 136, 1593–1610.
- Webster, B.L.; Southgate, V.R. Mating interactions of Schistosoma haematobium and S. intercalatum with their hybrid offspring. Parasitology 2003, 126, 327–338.
- Léger, E.; Garba, A.; Hamidou, A.A.; Webster, B.L.; Pennance, T.; Rollinson, D.; Webster, J.P. Introgressed animal schistosomes Schistosoma curassoni and S. bovis naturally infecting humans. Emerg. Infect. Dis. 2016, 22, 2212–2214.
- Huyse, T.; Webster, B.L.; Geldof, S.; Stothard, J.R.; Diaw, O.T.; Polman, K.; Rollinson, D. Bidirectional introgressive hybridisation between cattle and human schistosome species. PLoS Pathog. 2009, 5, e1000571.
- Soentjens, P.; Cnops, L.; Huyse, T.; Yansouni, C.; Vos, D.D.; Bottieau, E.; Clerinx, J.; Esbroeck, M.V. Diagnosis and clinical management of Schistosoma haematobium–Schistosoma bovis hybrid infection in a cluster of travelers returning from Mali. Clin. Infect. Dis. 2016, 63, 1626–1629.
- Savassi, B.A.E.S.; Mouahid, G.; Lasica, C.; Mahaman, S.-D.K.; Garcia, A.; Courtin, D.; Allienne, J.-F.; Ibikounlé, M.; Moné, H. Cattle as natural host for Schistosoma haematobium (Bilharz, 1852) Weinland, 1858 x Schistosoma bovis Sonsino, 1876 interactions, with new cercarial emergence and genetic patterns. Parasitol. Res. 2020, 119, 2189–2205.
- Savassi, B.A.E.S.; Dobigny, G.; Etougbétché, J.R.; Avocegan, T.T.; Quinsou, F.T.; Gauthier, P.; Ibikounlé, M.; Moné, H.; Mouahid, G. Mastomys natalensis (Smith, 1834) as a natural host for Schistosoma haematobium (Bilharz, 1852) Weinland, 1858 x Schistosoma bovis Sonsino, 1876 introgressive hybrids. Parasitol. Res. 2021, 120, 1755–1770.
- Tchuenté, L.; Southgate, V.; Njiokou, F.; Njiné, T.; Kouemeni, L.; Jourdane, J. The evolution of schistosomiasis at Loum, Cameroon: Replacement of Schistosoma intercalatum by S. haematobium through introgressive hybridisation. Trans. R. Soc. Trop. Med. Hyg. 1997, 91, 664–665.
- Webster, B.L.; Tchuenté, L.T.; Jourdane, J.; Southgate, V.R. The interaction of Schistosoma haematobium and S. guineensis in Cameroon. J. Helminthol. 2005, 79, 193–197.
- Grimes, J.E.; Croll, D.; Harrison, W.E.; Utzinger, J.; Freeman, M.C.; Templeton, M.R. The roles of water, sanitation and hygiene in reducing schistosomiasis: A review. Parasit Vectors 2015, 8, 156.
- Nelwan, M.L. Schistosomiasis: Life cycle, diagnosis, and control. Curr. Ther. Res. Clin. Exp. 2019, 91, 5–9.
- Mafiana, C.F.; Ekpo, U.F.; Ojo, D.A. Urinary schistosomiasis in preschool children in settlements around Oyan Reservoir in Ogun State, Nigeria: Implications for control. Trop. Med. Int. Health 2003, 8, 78–82.
- Curwen, R.S.; Wilson, R.A. Invasion of skin by schistosome cercariae: Some neglected facts. Trends Parasitol. 2003, 19, 63–66.
- He, Y.X.; Salafsky, B.; Ramaswany, K. Comparison of skin invasion among three major species of Schistosoma. Trends Parasitol. 2005, 21, 201–203.
- Nation, C.S.; Da’dara, A.A.; Marchant, J.K.; Skelly, P.J. Schistosome migration in the definitive host. PLoS Negl. Trop. Dis. 2020, 14, e0007951.
- McManus, D.P.; Dunne, D.W.; Sacko, M.; Utzinger, J.; Vennervald, B.J.; Zhou, X.-N. Schistosomiasis. Nat. Rev. Dis. Prim. 2018, 4, 13.
- Centers for Disease Control and Prevention. Parasites—Schistosomiasis. In Biology; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2019. Available online: https://www.cdc.gov/parasites/schistosomiasis/biology.html (accessed on 30 January 2023).
- Chomel, B.B. Control and prevention of emerging parasitic zoonoses. Int. J. Parasitol. 2008, 38, 1211–1217.
- Huyse, T.; Van Den Broeck, F.; Hellemans, B.; Volckaert, F.A.M.; Polman, K. Hybridisation between the two major African schistosome species of humans. Int. J. Parasitol. 2013, 43, 687–689.
- Standley, C.J.; Mugisha, L.; Dobson, A.P.; Stothard, J.R. Zoonotic schistosomiasis in non-human primates: Past, present and future activities at the human–wildlife interface in Africa. J. Helminthol. 2012, 86, 131–140.
- Léger, E.; Webster, J.P. Hybridizations within the Genus Schistosoma: Implications for evolution, epidemiology and control. Parasitology 2017, 144, 65–80.
- Fan, P.C.; Lin, L.H. Hybridization of Schistosoma mansoni and Schistosoma japonicum in mice. Southeast Asian J. Trop. Med. Public Health 2005, 36, 89–96.
- Boissier, J.; Moné, H.; Mitta, G.; Bargues, M.D.; Molyneux, D.; Mas-Coma, S. Schistosomiasis reaches Europe. Lancet Infect. Dis. 2015, 15, 757–758.
- Steinauer, M.L.; Hanelt, B.; Mwangi, I.N.; Maina, G.M.; Agola, L.E.; Kinuthia, J.M.; Mutuku, M.W.; Mungai, B.N.; Wilson, W.D.; Mkoji, G.M.; et al. Introgressive hybridization of human and rodent schistosome parasites in western Kenya. Mol. Ecol. 2008, 17, 5062–5074.
- Borlase, A.; Webster, J.P.; Rudge, J.W. Opportunities and challenges for modelling epidemiological and evolutionary dynamics in a multihost, multiparasite system: Zoonotic hybrid schistosomiasis in West Africa. Evol. Appl. 2017, 11, 501–515.
- The Carter Center. Schistosomiasis Control Program. 2021. Available online: https://www.cartercenter.org/resources/pdfs/factsheets/schistosomiasis-facts.pdf (accessed on 30 January 2023).
- Mogaji, H.O.; Dedeke, G.A.; Bada, B.S.; Bankole, S.; Adeniji, A.; Fagbenro, M.T.; Omitola, O.O.; Oluwole, A.S.; Odoemene, N.S.; Abe, E.M.; et al. Distribution of ascariasis, trichuriasis and hookworm infections in Ogun State, Southwestern Nigeria. PLoS ONE 2020, 15, e0233423.
- World Health Organization Regional Office for Africa. Expanded Special Project for Elimination of Neglected Tropical Diseases (ESPEN). Optimizing Schistosomiasis MDA Implementation in Countries. Data Analysis Tool, June–July 2019. Available online: https://espen.afro.who.int/system/files/content/resources/Schistosomiasis%20Data%20analysis%20tool%20-%20Presentation%20%2820190724_English%29.pdf (accessed on 30 January 2023).
- WHO. Guideline on Control and Elimination of Human Schistosomiasis; World Health Organization: Geneva, Switzerland, 2022; Available online: https://apps.who.int/iris/rest/bitstreams/1410449/retrieve (accessed on 30 January 2023).
- World Health Organization; Regional Office for Africa. Expanded Special Project for Elimination of Neglected Tropical Diseases (ESPEN). Nigeria Overview. Available online: https://espen.afro.who.int/countries/nigeria (accessed on 30 January 2023).
- World Health Organization. Schistosomiasis and soil-transmitted helminthiases: Progress report, 2021. Wkly. Epidemiol. Rec. 2022, 97, 621–632. Available online: https://www.who.int/publications/i/item/who-wer9748-621-632 (accessed on 30 January 2023).
- Krentel, A.; Gyapong, M.; Mallya, S.; Boadu, N.Y.; Amuyunzu-Nyamongo, M.; Stephens, M.; McFarland, D.A. Review of the factors influencing the motivation of community drug distributors towards the control and elimination of neglected tropical diseases (NTDs). PLoS Negl. Trop. Dis. 2017, 11, e0006065.
- El-Setouhy, M.; Abd Elaziz, K.M.; Helmy, H.; Farid, H.A.; Kamal, H.A.; Ramzy, R.M.R.; Shannon, W.D.; Weil, G.J. The effect of compliance on the impact of mass drug administration for elimination of lymphatic filariasis in Egypt. Am. J. Trop. Med. Hyg. 2007, 77, 1069–1073.
- Olamiju, O.J.; Olamiju, F.O.; Adeniran, A.A.; Mba, I.C.; Ukwunna, C.C.; Okoronkwo, C.; Ekpo, U.F. Public awareness and knowledge of neglected tropical diseases (NTDs) control activities in Abuja, Nigeria. PLoS Negl. Trop. Dis. 2014, 8, e3209.
- Assaré, R.K.; N’Tamon, R.N.; Bellai, L.G.; Koffi, J.A.; Mathieu, T.-B.I.; Ouattara, M.; Hürlimann, E.; Coulibaly, J.T.; Diabaté, S.; N’Goran, E.K.; et al. Characteristics of persistent hotspots of Schistosoma mansoni in western Côte d’Ivoire. Parasit Vectors 2020, 13, 337.
- Mogaji, H.O.; Odoh, I.M.; Iyeh, C.I.; Adeniran, A.A.; Oyedeji, S.I.; Okoh, H.I.; Bayegun, A.A.; Omitola, O.O.; Umunnakwe, C.U.; Olamiju, F.O.; et al. Attendee’s awareness about preventive chemotherapy neglected tropical diseases (PC-NTD) control during the first world neglected tropical diseases day in Ekiti State, Nigeria. PLoS Negl. Trop. Dis. 2021, 15, e0009315.
- King, K.C.; Stelkens, R.B.; Webster, J.P.; Smith, D.F.; Brockhurst, M.A. Hybridization in parasites: Consequences for adaptive evolution, pathogenesis, and public health in a changing world. PLoS Pathog. 2015, 11, e1005098.
- Moné, H.; Minguez, S.; Ibikounlé, M.; Allienne, J.-F.; Massougbodji, A.; Mouahid, G. Natural interactions between S. haematobium and S. guineensis in the Republic of Benin. Sci. World J. 2012, 2012, 793420.
- Webster, B.L.; Diaw, O.T.; Seye, M.M.; Webster, J.P.; Rollinson, D. Introgressive hybridization of Schistosoma haematobium group species in Senegal: Species barrier break down between ruminant and human schistosomes. PLoS Negl. Trop. Dis. 2013, 7, e2110.
- Webster, J.P.; Molyneux, D.H.; Hotez, P.J.; Fenwick, A. The contribution of mass drug administration to global health: Past, present and future. Philos. Trans./R Soc. Lond. B—Biol. Sci. 2014, 369, 20130434.
- Lamberton, P.H.L.; Hogan, S.C.; Kabatereine, N.B.; Fenwick, A.; Webster, J.P. In vitro praziquantel test capable of detecting reduced in vivo efficacy in Schistosoma mansoni human infections. Am. J. Trop. Med. Hyg. 2010, 83, 1340–1347.
- Valentim, C.L.L.; Cioli, D.; Chevalier, F.D.; Cao, X.; Taylor, A.B.; Holloway, S.P.; Pica-Mattoccia, L.; Guidi, A.; Basso, A.; Tsai, I.J.; et al. Genetic and molecular basis of drug resistance and species-specific drug action in schistosome parasites. Science 2013, 342, 1385–1389.
- Webster, B.L.; Diaw, O.T.; Seye, M.M.; Faye, D.S.; Stothard, J.R.; Sousa-Figueiredo, J.C.; Rollinson, D. Praziquantel treatment of school children from single and mixed infection foci of intestinal and urogenital schistosomiasis along the Senegal River Basin: Monitoring treatment success and re-infection patterns. Acta Trop. 2013, 128, 292–302.
- Grigg, M.E.; Bonnefoy, S.; Hehl, A.B.; Suzuki, Y.; Boothroyd, J.C. Success and virulence in Toxoplasma as the result of sexual recombination between two distinct ancestries. Science 2001, 294, 161–165.
- Schelkle, B.; Faria, P.J.; Johnson, M.B.; van Oosterhout, C.; Cable, J. Mixed infections and hybridisation in monogenean parasites. PLoS ONE 2012, 7, e39506.
- Hanelt, B.; Mwangi, I.N.; Kinuthia, J.M.; Maina, G.M.; Agola, L.E.; Mutuku, M.W.; Steinauer, M.L.; Agwanda, B.R.; Kigo, L.; Mungai, B.N.; et al. Schistosomes of small mammals from the Lake Victoria Basin, Kenya: New species, familiar species, and implications for schistosomiasis control. Parasitology 2010, 137, 1109–1118.




