Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Vishnu D. Rajput | -- | 2760 | 2023-02-17 08:17:29 | | | |
| 2 | Camila Xu | Meta information modification | 2760 | 2023-02-17 08:27:20 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Vinogradova, N.; Glukhov, A.; Chaplygin, V.; Kumar, P.; Mandzhieva, S.; Minkina, T.; Rajput, V.D. Heavy Metals in Medicinal Plants. Encyclopedia. Available online: https://encyclopedia.pub/entry/41334 (accessed on 07 February 2026).
Vinogradova N, Glukhov A, Chaplygin V, Kumar P, Mandzhieva S, Minkina T, et al. Heavy Metals in Medicinal Plants. Encyclopedia. Available at: https://encyclopedia.pub/entry/41334. Accessed February 07, 2026.
Vinogradova, Natalya, Alexander Glukhov, Victor Chaplygin, Pradeep Kumar, Saglara Mandzhieva, Tatiana Minkina, Vishnu D. Rajput. "Heavy Metals in Medicinal Plants" Encyclopedia, https://encyclopedia.pub/entry/41334 (accessed February 07, 2026).
Vinogradova, N., Glukhov, A., Chaplygin, V., Kumar, P., Mandzhieva, S., Minkina, T., & Rajput, V.D. (2023, February 17). Heavy Metals in Medicinal Plants. In Encyclopedia. https://encyclopedia.pub/entry/41334
Vinogradova, Natalya, et al. "Heavy Metals in Medicinal Plants." Encyclopedia. Web. 17 February, 2023.
Copy Citation
It is possible for heavy metals (HMs) to be present in pharmaceutical herb materials coming from anthropogenic activities like agriculture, industrial waste, and natural sources. In various ethnic groups, there is evidence that contaminants were purposefully added in the belief that they had some sort of therapeutic benefit. HM toxicity of medicinal plant products has been linked to a wide range of adverse health effects, causing dysfunction of the liver, kidney, and heart, and even death.
heavy metals (HMs)
herbal plants
atomic absorption spectroscopy
X-ray florescence
1. Heavy Metals, Medicinal Herbs, and Regulatory Documents
The common heavy metals (HMs) in the environment are lead, cadmium, and mercury, and their major sources are vehicles, industrial and thermal power plants, waste incinerators, and agricultural production [1][2][3][4]. Plants, especially trees, act as a barrier to the spread of HMs. A comparative analysis of approaches to regulation of HMs in medicinal plant materials and herbal medicinal products adopted in Russia, Europe, the United States, and Asian countries were carried out. For this purpose, the current editions of the Russian, European, American, and Asian pharmacopoeias, as well as international standards, have been studied (Table 1).
Table 1. Permissible concentrations of heavy metals according to regulatory documents in different countries.
| Food Items and Medicinal Plant Materials | Regulatory Document | Permissible Concentrations (mg/kg) | |||
|---|---|---|---|---|---|
| Pb | Cd | Hg | As | ||
| Medicinal plant materials and herbal medicinal products |
Russian pharmacopoeia [5] | 6.0 | 1.0 | 0.1 | 0.5 (Laminaria 90) |
| Herbal medicines, medicinal herbs |
European pharmacopoeia [6] | 5.0 | 1.0 | 0.1 | There are no general regulations (Laminaria 90) |
| Herbal medicines | United States pharmacopeia [7] | 5.0 | 0.5 | 1.0 (total) methyl mercury 0.2 |
Non-organic 2.0 |
| Medicinal plant materials and herbal medicinal products | Eurasian Economic Union pharmacopeia [8][9] | 6.0 | 1.0 | 0.1 | 0.5 |
| Medicinal plant materials (underground organs) | Pharmacopoeia of the People’s Republic of China [10] | 5.0 | 0.3 | 0.2 | 2.0 |
| Herbs consumed by humans | World Health Organization [11] | 10.0 | 0.3 | – | 1.0 |
| Traditional Chinese herbal medicines | ISO international standards [12] | 10.0 | 2.0 | 3.0 | 4.0 |
| Medicinal herbal preparations | Ayurvedic pharmacopoeia [13] | 10.0 | 0.3 | 1.0 | 3.0 |
| Medicinal herbal preparations | Thai pharmacopoeia [12] | 10.0 | 0.3 | – | 4.0 |
| Medicinal plant materials | Korean pharmacopoeia [12] | 5.0 | 0.3 | 0.2 | 3.0 |
| Traditional medicine products | Singapore Health Sciences Authority [14] | 20.0 | – | 0.5 | 5.0 |
According to the current State Pharmacopoeia of the Russian Federation (14th edition), the content of lead in medicinal plant materials and herbal medicinal products should not exceed 6.0 mg/kg; for cadmium—1.0 mg/kg; for mercury—0.1 mg/kg; and for arsenic—0.5 mg/kg [5]. The content limits of HMs in medicinal plant materials are similar to those for dry herbal dietary supplements and are less stringent than similar requirements for fruits, berries, and drinks (Table 1). A comparative analysis of the regulatory documentation in various countries has found that the requirements for the environmental safety of medicinal plant materials and herbal medicinal products differ significantly.
The Russian standard for lead is lower than that recommended by World Health Organization and the International Organization for Standardization (ISO) (10 mg/kg). Nevertheless, it is slightly higher than the corresponding standard set by the European, American, and Chinese pharmacopoeias (5 mg/kg). For cadmium, the standard established by the Russian pharmacopoeia is similar to the European one and is more than three times higher than those recommended by WHO and many Asian countries (0.3 mg/kg). Interestingly, in the recent edition (2020) of the Chinese pharmacopoeia, the maximum permissible concentration (MPC) for cadmium was changed from 0.3 mg/kg to 1.0 mg/kg [12][14].
This is an illustrative example of a trade-off between safety for human health and the need to use medicinal herbs that grow in a particular region.
The WHO, Russia, and a number of countries have similar requirements for mercury concentration; however, in the Chinese and Indian pharmacopoeias, the MPC is 10 times higher, while the American pharmacopoeia separately regulates the content of methylmercury. The Russian pharmacopoeia imposes the most stringent requirements for arsenic concentrations; the regulatory documents of other countries set standards that exceed the Russian ones by 2–8 times. When comparing the methods for analyzing medicinal plant materials for HMs, it has been revealed that they do not always correspond to each other, which may explain the difference in maximum permissible concentration (MPC) values. Thus, in foreign pharmacopoeias, the arsenic concentration in medicinal plant materials and herbal medicinal products is determined by decomposition in closed vessels, which eliminates the loss of the element at the stage of sample preparation.
There are a number of imperfections in the methods for determining HMs given in the current State Pharmacopoeia of the Russian Federation [5]. The incorrectness of using standard samples to analyze medicinal plant materials and herbal medicinal products, in which HMs are found the form of inorganic salts and are not associated with organic compounds, was noted [15]. According to the researchers, it is advisable to use standard samples of plant materials certified for the content of HMs, as the organic matrix has a significant influence on measurement results. In addition to the listed metals, the concentration of copper is regulated in China and Singapore, and the content of nickel is regulated in European countries. The introduction of MPCs for these metals, as well as zinc, iron, and manganese, possibly taking into account the regional characteristics of industrial activities, is also a pressing issue in Russian Federation. Another aspect to consider is the part of the plant used in pharmacy. The State Pharmacopoeia of the Russian Federation [5] imposes uniform requirements on the content of toxicants for all types of plant materials. In the Chinese pharmacopoeia [10], the standards for HM contents in underground organs are set separately, which is logical, because when growing on polluted soil, many species are able to limit the supply of xenobiotics to the aerial part, especially to the generative organs.
There is large number of publications that analyzes the regional features of HMs’ accumulation in medicinal herbs [15][16][17][18][19][20][21][22][23][24]. For example, the environmental purity of Cichorium intybus L. in the Trans-Ural region of the Republic of Bashkortostan (Russia) has been assessed according to the MPCs of chemical elements in feed for farm animals and feed additives (5 mg/kg Pb, 0.3 mg/kg Cd) [23]. The researchers have concluded that it is inappropriate to harvest the raw material under study as a medicinal plant material due to the excess of cadmium concentration by 3.0–6.5 times. However, by the current MPC for medicinal plant materials safety assessment, the results would have been different. Although the content of HMs in plants depends on the intensity of contamination in the harvesting area, a general conclusion about the unfavorable environmental situation is not enough to assess the possibility of growing medicinal herbs in a particular area. The safety of 51 samples of Tanacetum vulgare L. flowers, harvested in urban and agroecosystems of the Voronezh region, was established with respect to the content of HMs and arsenic [16]. The roots of Taraxacum officinale F.H. Wigg. plants growing in the Voronezh region near roads and railways were also found to be safe, while in the samples collected near a thermal power plant and a chemical enterprise, an excess of arsenic was recorded [16]. The aboveground parts of Artemisia frigida Willd. and Artemisia jacutica Drob. growing in the Republic of Buryatia, as well as A. frigida growing in Mongolia, had lead and cadmium concentrations within the normal range (except for the year in which forest fires occurred) [25]. It was found that Plantago major L. leaves harvested in the park area of the central part of the city of Kursk (Russia) were environmentally safe, while in the industrial area the concentration of lead in these medicinal plant materials was 20.5 times the MPC [26]. The content of HMs in medicinal plant materials growing in the Grodno region (in the Republic of Belarus) did not exceed the MPC [21]. This applied to both wild (Vaccinium vitis-idaea L., Vaccinium myrtillus L., Elytrigia repens (L.) Nevski, Artemisia absinthium L., Hypericum maculatum Crantz, Angelica sylvestris L.) and cultivated (Calendula officinalis L., Chamomilla recutita (L.) Rauschert, Aesculus hippocastanum L., Paeonia anomala L.) plants.
2. Impact of Heavy Metal on Herbal Plants
Soils have become a significant source of HM pollution and possess a high conversion of ion capability. Certain important HMs become crucial elements which are required in extremely low quantities for proper development of plant [27]. Such HMs play a lead role in physiochemical processes in plants. Plant roots absorbs HMs from soil by the phenomenon of diffusion [28]. These HMs dissolve into their complex structures around the surface of root tissues and are taken up through the apoplast and symplast mechanisms [29]. In contrast, Fe is a biological molecule that is rapidly reduced and oxidized in a wide range of biological reactions.
It is also a crucial mediator for metabolic catalysts that participate in respiration, photosynthesis, and nutrient absorption [30]. At the cellular level, several unique metal transporters like IRT1(Fe, Zn, Mn, and Cd transport), ZIP (Zn transport), and NRAMP (Ni transport) are found on the biological membrane. Several HMs like Co, Cu, Fe, Mn, Ni, and Zn in small amounts are vital for plants. These HMs are necessary for the induction of morphological and metabolic process, the regulation of photosynthesis, the synthesis of chlorophyll, a high rate of the production of bioactive compounds, transpiration, protection of DNA, distraction of ROS, and enhancing nitrogen fixation in plants [31]. However, a high amount of HMs causes lipid peroxidation, ROS generation, and DNA damage. Several studies have found that higher concentrations of Zn have a negative impact on plant development and metabolism [32]. Additionally, according to Arora et al. [33], an increase in plant Fe2+ levels triggers the formation of free radicals that destroy protein molecules, DNA, and membranes (Figure 1).
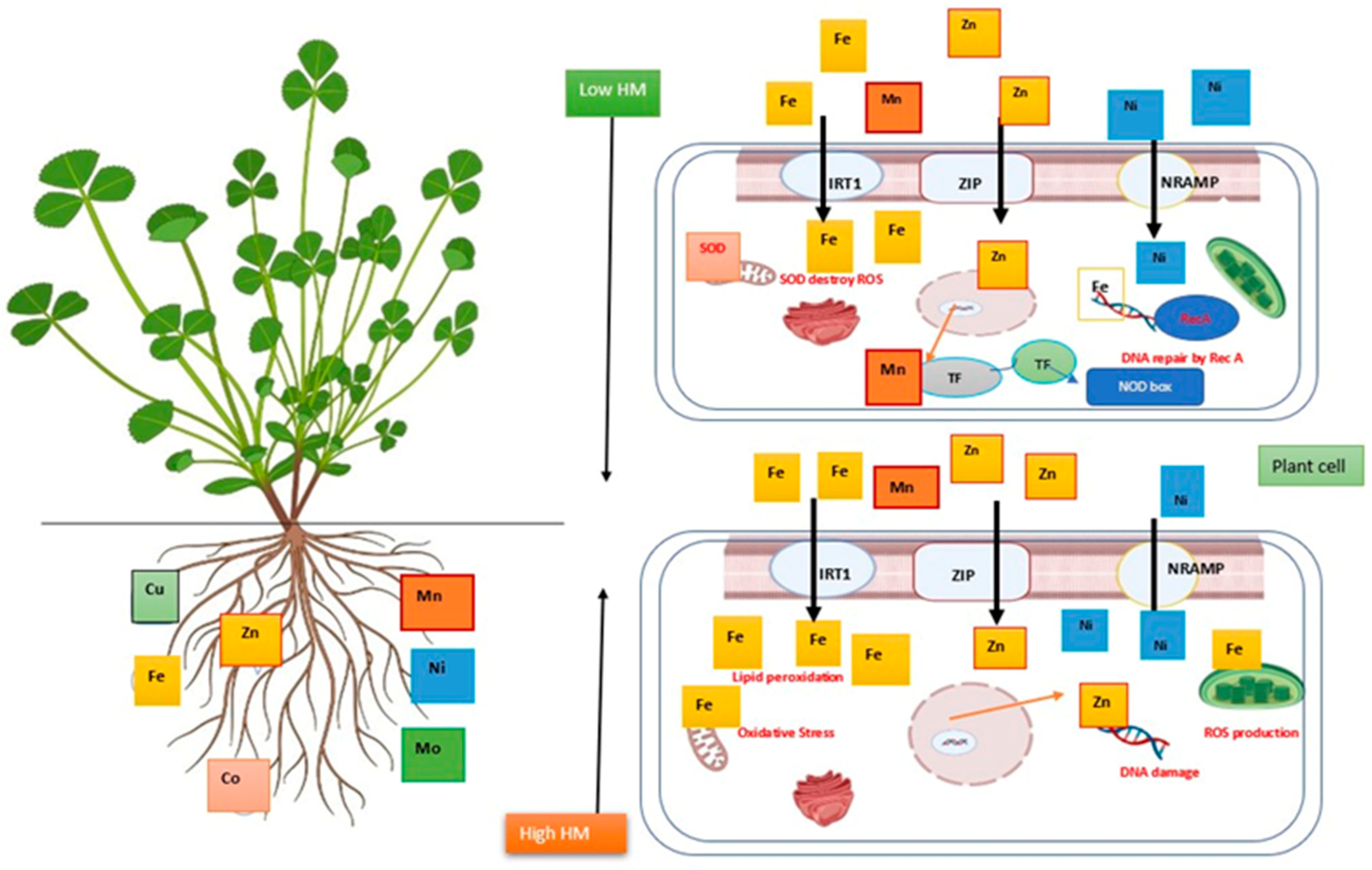
Figure 1. Impact of heavy metals’ contribution to the creation of proteins, nucleic acids, photosynthesis pigments, and cell membrane function and structure at low concentrations [34]. Mn enhances antioxidant capacity [35], while Fe increases N2-fixation and DNA repair [36]. At high concentrations, it causes conversion of numerous significant functional groups, lipid peroxidation (LPO), mitochondrial dysfunction, ROS production, and biochemical disruption via changing enzymatic activity [37][38].
The major causes of HM pollution are anthropogenic activities like resource extraction, agricultural production, construction, industrial activities, inadequate garbage management processes, and excessive use of agrochemicals (Figure 2). HMs enter the environment through these activities and accumulate in living systems. These HMs are toxic in nature and cause several chronic illnesses like weakened immunity, cardiac instability, neonatal disorders, psychological disorders, and sensorimotor behavior issues [39]. Several HMs like Pb, As, Hg, and Cd are not required by plants or the human body, and they also cause a variety of health complications associated with the brain, liver, lungs, heart, kidneys, and nervous system, including hypertension, abdominal pain, rashes, intestinal ulcers, and other symptoms linked to various cancer types [40][41]. Although copper is a major element in several enzymatic reactions, consuming excess amounts of it can lead to internal organ injury but also induce skin infections, elevated lung tissue inflammation, abdominal discomfort, nausea, vomiting, and diarrhea [42].
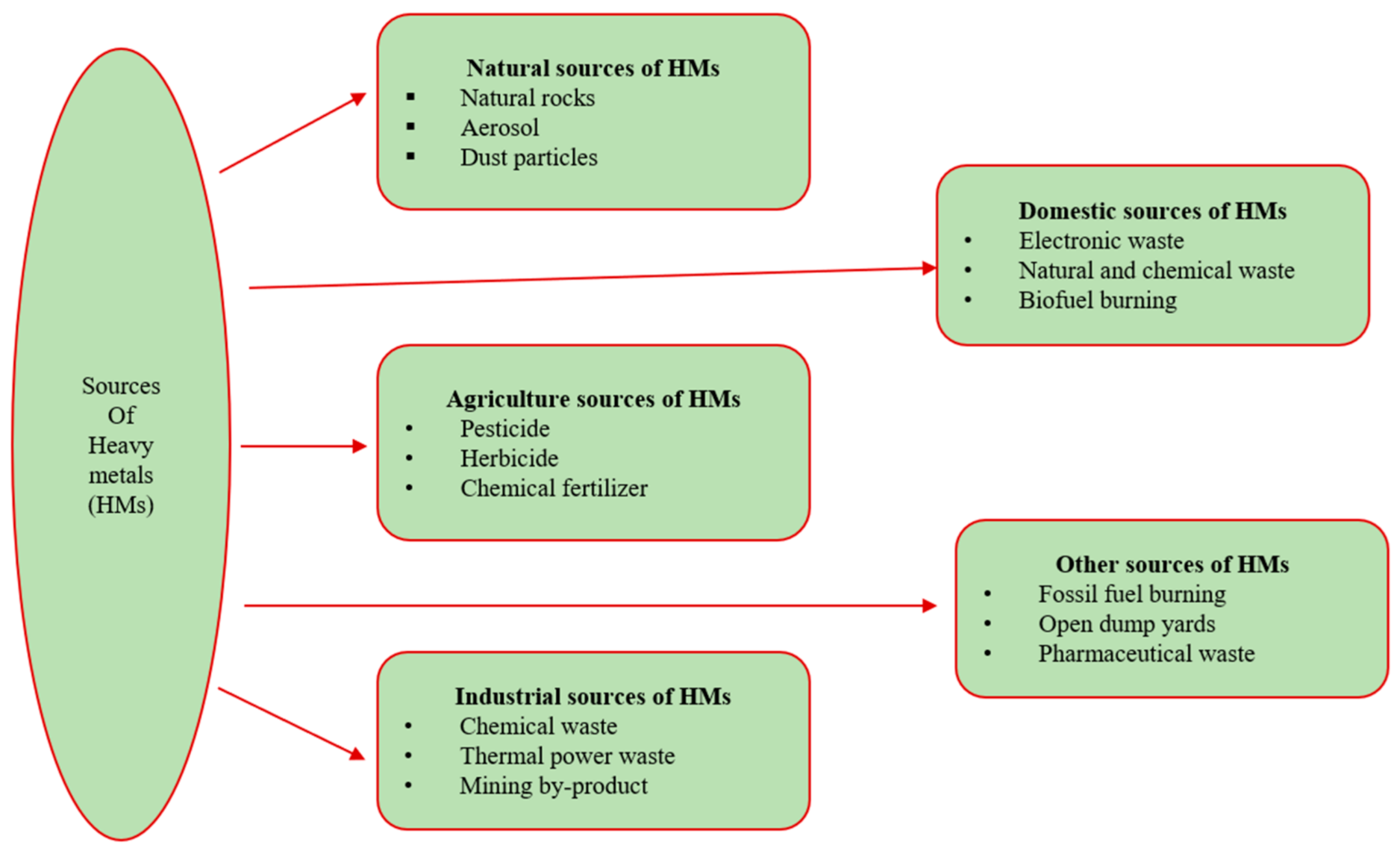
Figure 2. Various sources of heavy metals.
As they are known to cause harm, it is critical and urgent to conduct a thorough investigation into the dangers of metal contamination in medicinal herbs. As a result of the survey’s exact methodology, it is now clear that more screening and dosing frequency guidance for herbal medicines is required. This research explored pollutant levels and their risks, particularly when they are present in herbal remedies. The results serve as a groundwork for future research into preventative methods, uniform guidelines, and exogenous contaminants. Research studies conducted have led to suggestions that can rapidly lower or completely remove HM contents in active pharmaceutical ingredients.
3. Detection Method of the Heavy Metals in Medicinal Plants
There are several techniques like inductively coupled plasma mass spectrometry (ICPMS), atomic emission spectroscopy (AES), X-ray fluorescence (XRF), neutron activation analysis (NAA), anodic stripping voltammetry (ASV), thermolysis-coupled atomic absorption spectroscopy (TCAS), atomic absorption spectrometry (AAS), and graphite furnace atomic absorption spectrometry (GFAAS) which are used for the quantification of the HMs in herbal plant samples (Figure 3).
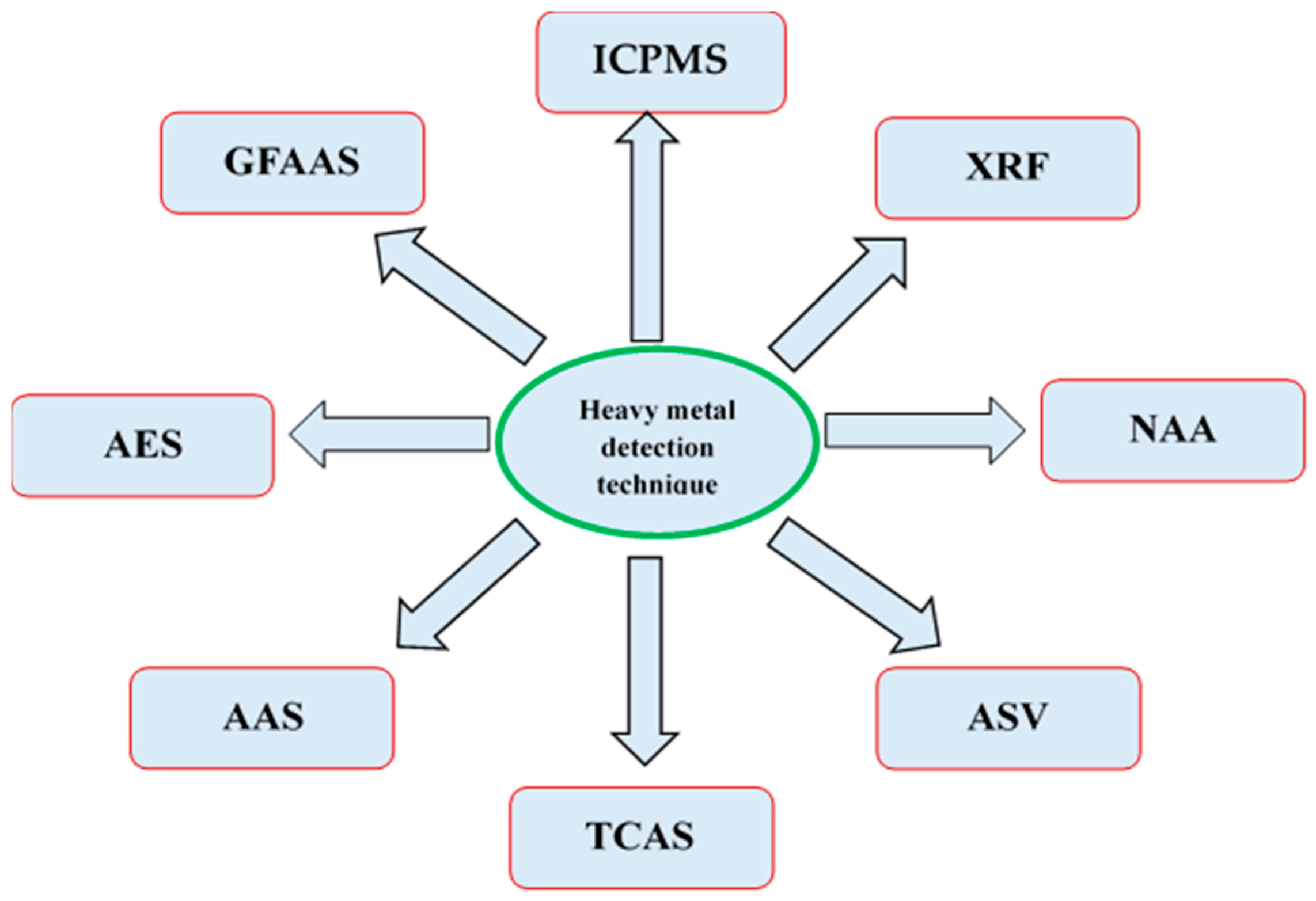
Figure 3. Techniques of heavy metal detection in herbal samples.
3.1. ICPMS
ICPMS detects the HMs on the basis of m/z ratio measurement. As an atomization source for atomic spectroscopy, it is significantly more difficult to use than a graphite furnace due to its high degree of atomization in argon plasma at 7000 K. This method possesses a maximum output capacity of multi-element detection ability in a broad range. ICPMS and AES can identify numerous metallic pollutants in an extra-specific way and can quantify an impurity with a significantly greater degree of sensitivity. The typical detection limits in a solvent are between 0.01 and 1 ppm. The mass of the study sample can be as low as 10 mg. ICPMS assessments are currently being performed and have a solid foundation. It is cost-effective while also producing superior outcomes [43].
3.2. AES
The majority of the time, this method is combined with optical emission spectroscopy. The material is made to become stimulated through the absorption of either a thermal or electrical charge, and then the emission that the agitated material gives off is investigated. Additionally, this method is connected to a solid; however, fluids are often the samples that are examined. It is able to analyze over 70 different elements at dosages as low as one part per million (ppm) [44].
3.3. XRF
When a specimen comes into contact with high-energy X-rays or gamma rays, it emits “secondary” (or fluorescent) X-rays with a unique spectrum. This technique is used in geochemical analyses, toxicology, as well as paleontology. It is also adopted in the analysis of elements and chemicals, specifically in the study of metallic materials, glass, ceramic materials, and construction materials [45].
3.4. NAA
The NAA method is used for determination of the heavy metals present in an herbal material by analyzing the energy and intensity of rays (mostly gamma rays) generated through immediate radiation or radionuclide decay. This method makes use of neutron bombardment [46]. NAA need not require material processing beforehand. Because it is an excitation research methodology, it involves bombarding atoms as well as neutrons produced by reactors, accelerators, or isotope neutron sources; as a result, it is ideal for the qualitative and quantitative measurement of the compositions and occurrences of various elements [46]. In addition to this, NAA is sensitive to a wide variety of elements, and this sensitivity enables it to precisely estimate the levels of trace elements present in a wide variety of samples, including MPs. The use of NAA is important for the rapid identification of the various components of MPs.
3.5. ASV
By using differential pulse anodic stripping, it is a straightforward procedure to simultaneously determine the contents of heavy metals in pharmaceutical herbs. The specimen is prepared through the dry ashing approach, in which 1 g of fine powdered plant matter is kept at 5000 °C for 2.5 h. A hanging mercury electrode and platinum wire are employed as the functioning and countering electrodes in this straightforward voltametric device. These potentials are assessed in relation to a reference electrode made of Ag/Ag Cl and KCl. Prior to the analysis, pure N2 is bubbled throughout the specimen for 400 s. The ASV approach is more sensitive. This technique yields accurate, repeatable outcomes. It has several drawbacks, like fouling, and it is time-consuming. Its use is constrained, as electrodes (E + 0.4 V) of As and Hg are readily oxidized [47].
3.6. TCAS
According to this procedure, plant materials or herbal products are heated. When the herbal sample is heated to a high temperature, the atomic absorption (AA) detectors are used to analyze HM vapors which have been thermally liberated from molecular HMs of the herbal sample. The procedure does not require any pre-processing of herbal samples. This procedure requires less time than other ones (four minutes every cycle). There is no requirement for calibrating, allowing for continuous testing to be performed. It has a few limitations, such as it only being able to identify Hg. HM level assessments cannot to be performed simultaneously [48].
3.7. AAS
AAS is a common technique of spectrum analysis that is used for both quantitative and qualitative analyses of the HMs present in MPs. Specific analysis of a wide spectrum of elements like Cd, Ni, Pb, and Zn in either solid or liquid specimens is possible with the help of such a technique [49]. In AAS, components such as a beam of light, atomizer, splitter, and detection system are implemented [50]. Although significant strides have been made in the advancement of technology for the identification of heavy metals, AAS is still widely used.
3.8. GFAAS
In GFAAS, an herbal sample is first introduced directly to the graphite tubes in the equipment known as a graphite furnaces. Here only a small portion of the nebulized vapor actually makes it to the flame after the atomized sample has quickly passed through a straightforward surface. Therefore, in order to boost the analytic sensitivity, advanced models employing graphite furnaces for electrical vaporization were utilized. The residual atomic samples of herbs are again employed by GFAAS, which detects the presence of lead and cadmium and the concentrations of copper, arsenic, and mercury in the herbal materials after the solvent and matrix materials have been removed using heat [51].
References
- Liu, X.; Ju, Y.; Mandzhieva, S.; Pinskii, D.; Minkina, T.; Rajput, V.D.; Roane, T.; Huang, S.; Li, Y.; Ma, L.Q.; et al. Sporadic Pb accumulation by plants: Influence of soil biogeochemistry, microbial community and physiological mechanisms. J. Hazard. Mater 2023, 444, 130391.
- Chaplygin, V.; Dudnikova, T.; Chernikova, N.; Fedorenko, A.; Mandzhieva, S.; Fedorenko, G.; Sushkova, S.; Nevidomskaya, D.; Minkina, T.; Sathishkumar, P.; et al. Phragmites australis cav. As a bioindicator of hydromorphic soils pollution with heavy metals and polyaromatic hydrocarbons. Chemosphere 2022, 308, 136409.
- Bezuglova, O.S.; Gorbov, S.N.; Tischenko, S.A.; Aleksikova, A.S.; Tagiverdiev, S.S.; Sherstnev, A.K.; Dubinina, M.N. Accumulation and migration of heavy metals in soils of the Rostov region, south of Russia. J. Soils Sediments 2016, 16, 1203–1213.
- Erofeeva, E.A. Hormesis in plants: Its common occurrence across stresses. Curr. Opin. Toxicol. 2022, 30, 100333.
- State Pharmacopoeia of the Russian Federation, XIV ed.; Ministry of Health of the Russian Federation: Moscow, Russia, 2018; Volume II, p. 1449.
- European Directorate for the Quality of Medicines & HealthCare (EDQM); Council of Europe: Strasbourg, France, 2019; Volume 7.
- USP44–NF39; 561 Articles of Botanical Origin. United States Pharmacopeia: Rockville, MD, USA, 2020; p. 15.
- European Pharmacopoeia, 10th ed.; Council of Europe: Strasbourg, France, 2019; Volume 1, p. 4370.
- Pharmacopoeia of the Eurasian Economic Union; Ministry of Health of the Russian Federation: Moscow, Russia, 2021; 568p.
- Pharmacopoeia of the People’s Republic of China; China Food and Drug Administration: Beijing, China, 2015; Volume 1, 2266p.
- World Health Organization (WHO). Quality Control Methods for Medicinal Plant Materials; World Health Organization: Geneva, Switzerland, 2005.
- Chen, Y.G.; Huang, J.H.; Luo, R.; Ge, H.Z.; Wołowicz, A.; Wawrzkiewicz, M.; Gładysz-Płaska, A.; Li, B.; Yu, Q.X.; Kołodyńska, D.; et al. Impacts of heavy metals and medicinal crops on ecological systems, environmental pollution, cultivation, and production processes in China. Ecotoxicol. Environ. Safet. 2021, 219, 17.
- Debnath, M.; Paul, N.; Bhattacharya, S.; Biswas, M.; Haldar, P.K. Formulation and assessment of microbial and heavy metal contents of Vidangadilouham: A classical Ayurvedic formulation. Int. J. Herb. Med. 2020, 8, 101–102.
- Vyas, P.; Vohora, D. Pharmaceutical regulations for complementary medicine. In Pharmaceutical Medicine and Translational Clinical Research; Vohora, D., Singh, G., Eds.; Academic Press: Cambridge, MA, USA, 2018; Chapter 13; pp. 233–264.
- Shchukin, V.M.; Kuzmina, N.E.; Erina, A.A.; Yashkir, V.A.; Merkulov, V.A. Comparative analysis of the heavy metal, Aluminum, and Arsenic contents in brown algae of various origins. Pharm. Chem. J. 2018, 52, 627–634.
- D’yakova, N.A.; Samylina, I.A.; Slivkin, A.I.; Gaponov, S.P.; Myndra, A.A. Estimated heavy-metal and Arsenic contents in medicinal plant raw materials of the Voronezh region. Pharm. Chem. J. 2018, 52, 220–223.
- Chizzola, R.; Michitsch, H.; Franz, C. Monitoring of metallic micronutrients and heavy metals in herbs, spices and medicinal plants from Austria. Eur. Food Res. Technol. 2003, 216, 407–411.
- Siromlya, T.I. Influence of traffic pollution on ecological state of Plantago major L. Contemp. Probl. Ecol. 2011, 4, 499–507.
- Parveen, R.; Abbasi, A.M.; Shaheen, N.; Shah, M.H. Accumulation of selected metals in the fruits of medicinal plants grown in urban environment of Islamabad, Pakistan. Arab. J. Chem. 2017, 13, 308–317.
- Li, J.; Wang, Y.; Yang, H.; Tang, Y. Five heavy metals accumulation and health risk in a traditional Chinese medicine cortex Moutan collected from different sites in China. Hum. Ecol. Risk Assess. 2018, 24, 2288–2298.
- Kuzovkova, A.A.; Drebenkova, I.V.; Velentei, Y.N.; Pleshkova, A.A.; Bychok, G.E.; Chernik, D.V.; Maskalevich, N.V. Heavy metal contamination of wild and cultivated medicinal plants in the Republic of Belarus. Occup. Med. Human Ecol. 2020, 4, 112–117.
- Vinogradova, N.A.; Glukhov, A.Z. Ecological and phytochemical features of Crataegus fallacina Klokov under conditions of technogenic pollution. Contemp. Probl. Ecol. 2021, 14, 90–97.
- Buskunova, G.G.; Khasanova, R.F.; Semenova, I.N.; Ilbulova, G.R. The heavy metals in the system “soil as a wild-growing medicinal plant” on the example of Tanacetum vulgare L. Ecol. Ind. Russ. 2020, 24, 37–41.
- Zhang, Z.; Song, J.; Zhang, H.; Zheng, Z.; Li, T.; Wu, S.; He, B.; Mao, B.; Yu, Y.; Fang, H. Analysis method development and health risk assessment of pesticide and heavy metal residues in Dendrobium Candidum. RSC Adv. 2022, 1, 6869–6875.
- Dylenova, E.P.; Zhigzhitzhapova, S.V.; Randalova, T.E.; Radnaeva, L.D.; Shiretorova, V.G.; Pavlov, I.A. Biophile elements and heavy metals in Artemisia frigida willd. and Artemisia jacutica drob. Khimiya Rastitel’nogo Syr’ya 2019, 4, 199–205.
- Babkina, L.A.; Lukyanchikov, D.S.; Lukyanchikova, O.V. Features of the accumulation of heavy metals by plantain leaves. Samara Sci. Bull. 2018, 7, 19–24.
- Alloway, B.J. Heavy metals and metalloids as micronutrients for plants and animals. In Heavy Metals in Soils; Springer: Dordrecht, The Netherlands, 2013; pp. 195–209.
- Peralta-Videa, J.R.; Lopez, M.L.; Narayan, M.; Saupe, G.; Gardea-Torresdey, J. The biochemistry of environmental heavy metal uptake by plants: Implications for the food chain. Int. J. Biochem. Cell Biol. 2009, 41, 1665–1677.
- Hossain, M.A.; Piyatida, P.; da Silva, J.A.T.; Fujita, M. Molecular mechanism of heavy metal toxicity and tolerance in plants: Central role of glutathione in detoxification of reactive oxygen species and methylglyoxal and in heavy metal chelation. J. Bot. 2012, 37.
- Hell, R.; Stephan, U.W. Iron uptake, trafficking and homeostasis in plants. Planta 2003, 216, 541–551.
- Blaylock, M.J.; Huang, J.W. Phytoextraction of metals. In Phytoremediation of Toxic Metals: Using Plants to Clean Up the Environment; Wiley: New York, NY, USA, 2000; pp. 53–70.
- Rout, G.R.; Das, P. Effect of metal toxicity on plant growth and metabolism: I. Zinc. In Sustainable agriculture; Lichtfouse, E., Navarrete, M., Debaeke, P., Véronique, S., Alberola, C., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 873–884.
- Arora, A.; Sairam, R.K.; Srivastava, G.C. Oxidative stress and antioxidative system in plants. Curr. Sci. 2002, 82, 1227–1238.
- Oves, M.; Khan, S.; Qari, H.; Felemban, N.; Almeelbi, T. Heavy Metals: Biological importance and detoxification strategies. J. Bioremed. Biodegrad. 2016, 7, 334.
- Shenker, M.; Plessner, O.E.; Tel-Or, E. Manganese nutrition effects on tomato growth, chlorophyll concentration, and superoxide dismutase activity. J. Plant Physiol. 2004, 161, 197–202.
- Moller, I.M.; Jensen, P.E.; Hansson, A. Oxidative modifications to cellular components in plants. Annu. Rev. Plant Biol. 2007, 58, 459–481.
- Anjum, N.A.; Duarte, A.C.; Pereira, E.; Ahmad, I. Plant-beneficial elements status assessment in soil-plant system in the vicinity of a chemical industry complex: Shedding light on forage grass safety issues. Environ. Sci. Pollut. Res. Int. 2015, 22, 2239–2246.
- de Oliveira Jucoski, G.; Cambraia, J.; Ribeiro, C.; de Oliveira, J.A.; de Paula, S.O.; Oliva, M.A. Impact of iron toxicity on oxidative metabolism in young Eugenia uniflora L. plants. Acta Physiol. Plant 2013, 35, 1645–1657.
- Dehno, M.A.; Harami, S.R.M.; Noora, M.R. Environmental geochemistry of heavy metals in coral reefs and sediments of Chabahar Bay. Results Eng. 2022, 13, 100346.
- Bharti, R.; Sharma, R. Effect of heavy metals: An overview. Mater. Today Proc. 2022, 51, 880–885.
- Rahman, M.M.; Hossain, M.K.F.B.; Afrin, S.; Saito, T.; Kurasaki, M. Effects of Metals on Human Health and Ecosystem; Springer: Berlin/Heidelberg, Germany, 2022; pp. 1–39.
- Pirhadi, M.; Shariatifar, N.; Bahmani, M.; Manouchehri, A. Heavy metals in wheat grain and its impact on human health: A mini-review. J. Chem. Health Risks 2022, 12, 421–426.
- Wilschefski, S.C.; Baxter, M.R. Inductively coupled plasma mass spectrometry: Introduction to analytical aspects. Clin. Biochem. Rev. 2019, 40, 15.
- Rehan, I.; Gondal, M.A.; Aldakheel, R.K.; Almessiere, M.A.; Rehan, K.; Khan, S.; Sultana, S.; Khan, M.Z. Determination of nutritional and toxic metals in black tea leaves using calibration free LIBS and ICP: AES technique. Arab. J. Sci. Eng. 2022, 47, 7531–7539.
- Nguyen, H.M.; Huynh, N.T.K.; Nguyen, N.T.Y.; Ha, L.T.; Pham, T.T. Evaluating the content of some metal elements in soil and their effects on the total phenolic and flavonoid contents of some medicinal plants using X-ray fluorescence (XRF) Method. Res. Sq. 2022, 1, 1–24.
- Garg, A.N.; Singh, R.; Maharia, R.S.; Dutta, R.K.; Datta, A. Quantification of minor, trace and toxic elements in stems of Santalum album (L.), Mangiferra indica (L.) and Tinospora cordifolia by instrumental neutron activation analysis. J. Plant Sci. Phytopathol. 2022, 6, 8–14.
- Khamcharoen, W.; Duchda, P.; Songsrirote, K.; Ratanawimarnwong, N.; Limchoowong, N.; Jittangprasert, P.; Mantim, T.; Siangproh, W. An application of miniaturized electrochemical sensing for determination of arsenic in herbal medicines. Anal. Methods 2022, 14, 3087–3093.
- Papadopoulos, A.; Assimomytis, N.; Varvaresou, A. Sample preparation of cosmetic products for the determination of heavy Metals. Cosmetics 2022, 9, 21.
- Lawi, D.J.; Abdulwhaab, W.S.; Abojassim, A.A. Health risk study of heavy metals from consumption of drugs (solid and liquid) samples derived from medicinal plants in Iraq. Biol. Trace Elem. Res. 2022, 9, 1–13.
- Hyder, Z.; Rizwani, G.H.; Ahmed, I.; Shareef, H.; Azhar, I.; Aqeel, E. Determination of Heavy metals content, Lead (Pb), Mercury (Hg), Cadmium (Cd), Nickel (Ni), and Copper (Cu) with risk assessment to human consumption as a food and medicine in herbal species through Atomic Absorption Spectroscopy. Res. Sq. 2022, 1, 1–16.
- Alinia-Ahandani, E.; Nazem, H.; Malekirad, A.A.; Fazilati, M. The safety evaluation of toxic elements in medicinal plants: A Systematic Review. J. Hum. Environ. Health Promot. 2022, 8, 62–68.
More
Information
Subjects:
Environmental Sciences
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.1K
Entry Collection:
Environmental Sciences
Revisions:
2 times
(View History)
Update Date:
17 Feb 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




