Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Evroula Hapeshi | -- | 7011 | 2023-02-16 10:49:58 | | | |
| 2 | Camila Xu | Meta information modification | 7011 | 2023-02-17 02:07:38 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Koina, I.M.; Sarigiannis, Y.; Hapeshi, E. Green Extraction Techniques for Active Ingredients in Tea. Encyclopedia. Available online: https://encyclopedia.pub/entry/41288 (accessed on 28 February 2026).
Koina IM, Sarigiannis Y, Hapeshi E. Green Extraction Techniques for Active Ingredients in Tea. Encyclopedia. Available at: https://encyclopedia.pub/entry/41288. Accessed February 28, 2026.
Koina, Ioulia Maria, Yiannis Sarigiannis, Evroula Hapeshi. "Green Extraction Techniques for Active Ingredients in Tea" Encyclopedia, https://encyclopedia.pub/entry/41288 (accessed February 28, 2026).
Koina, I.M., Sarigiannis, Y., & Hapeshi, E. (2023, February 16). Green Extraction Techniques for Active Ingredients in Tea. In Encyclopedia. https://encyclopedia.pub/entry/41288
Koina, Ioulia Maria, et al. "Green Extraction Techniques for Active Ingredients in Tea." Encyclopedia. Web. 16 February, 2023.
Copy Citation
The so-called “Green Extraction”—which is based on the design of different extraction processes for the reduction in energy consumption, as well as the usage of alternative solvents and renewable natural materials —was developed.
green solvents
green tea
extraction methods
catechins
HPLC
1. Introduction
The term “Green Chemistry” appeared in the early 1990s. In addition, in the last two decades, it has subsequently been adopted worldwide. The goal of green chemistry is a reduction in environmental pollution, and support for the principles of sustainable development, in order to protect the health of workers. Furthermore, the expected results of the implementation of green chemistry include: reduction in environmental pollution; reduction in the use of toxic and hazardous chemicals; the use of renewable energy sources; energy-saving techniques; cost reductions; an increase in security; the improvement of human health, and a better quality of human life.
According to Anastas and Warner, the most important aspect of green chemistry is the design of novel technological methods and synthetic pathways. In this context, the scientists focus on the prevention of pollution by relying on scientific and technological achievements instead of waste disposal [1].
It must be mentioned that the isolation of natural products by the chemical industries has entailed negative effects on the environment. For example, such a process has required the consumption of 50% of the total energy of the ongoing industrial process. For this reason, the relevant industries turned their attention to a greener approach in order to produce natural products. This was achieved by developing new and pioneering technologies in order to save energy, as well as to decrease the usage of solvents, or to instead promote the usage of alternative solvents [2]. The desire to use alternative solvents arose following the widespread application of organic petrochemical solvents. This is due to the fact that such solvents caused negative effects not only to the environment, human health, and safety, but also to the global economy [3]. For this reason, the so-called “Green Extraction”—which is based on the design of different extraction processes for the reduction in energy consumption, as well as the usage of alternative solvents and renewable natural materials—was developed [3]. Figure 1 illustrates a flow diagram of the extraction methods of bioactive substances, as well as their application in the food, pharmaceutical, and cosmetic industries [4].
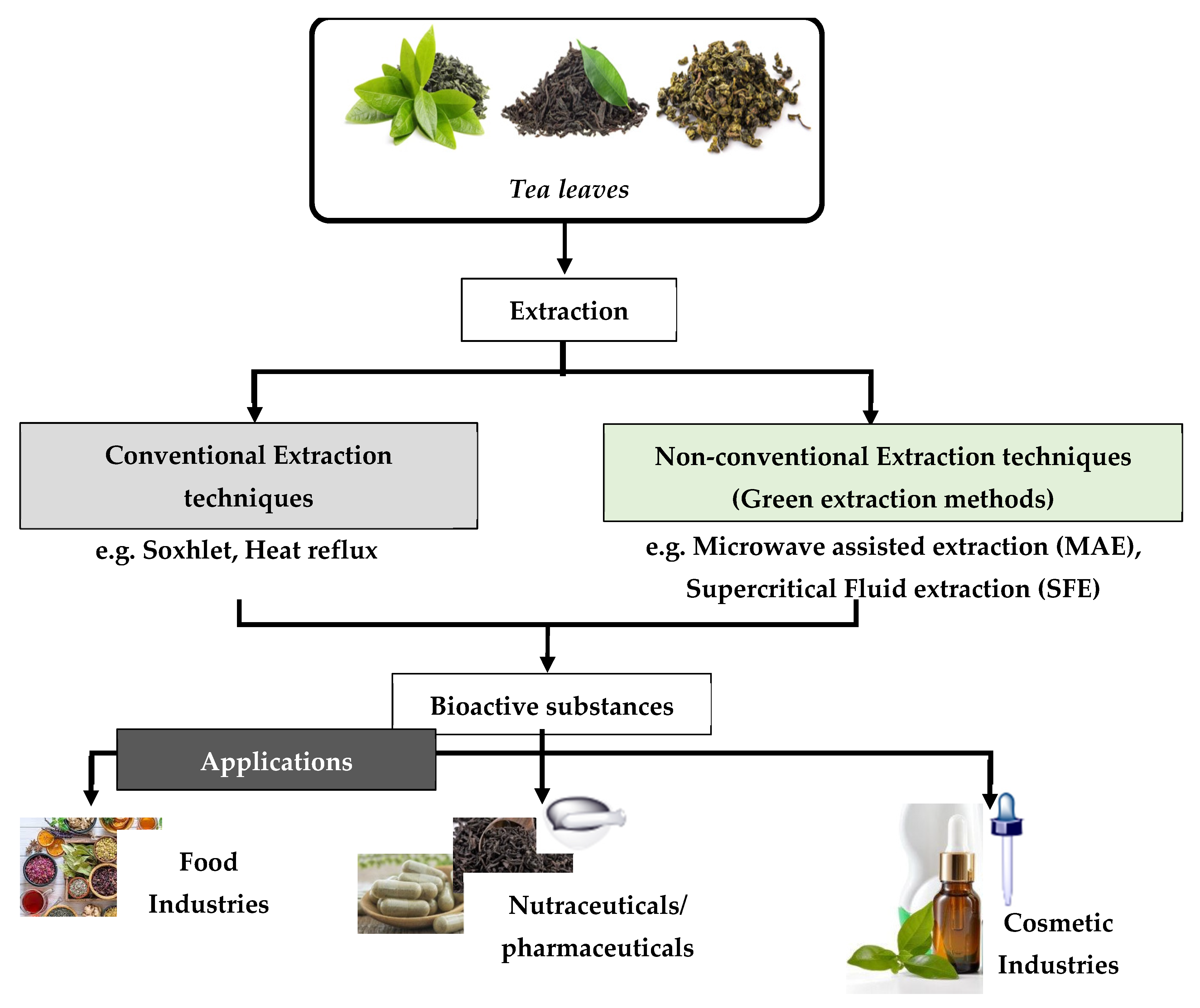
Figure 1. Schematic diagram regarding the extraction of bioactive compounds from tea leaves and their applications.
Polyphenols, polysaccharides, amino acids, alkaloids, organic acids, proteins, and volatile components are the main bioactive compounds that have been determined in various tea species [4]. In recent years—in respect of the elimination of the disadvantages of conventional extraction methods—novel non-conventional extraction methods have been developed, such as: ultrasound-assisted; microwave-assisted; high-pressure; pressurized hot water; supercritical fluid; and deep eutectic extraction. All of this information, regarding bioactive compounds and the extraction techniques used, is discussed below in greater detail.
2. Active Ingredients Contained in Tea
Tea is the most popular daily beverage throughout the world, with an estimated daily consumption of more than 3 billion cups [5]. Its health benefits have also been confirmed by preclinical and epidemiological studies.
Tea is one of the main sources of caffeine intake through an average diet. Several species of tea, such as black, green, and white tea are prepared from the leaves of the Camellia sinensis plant, which is grown in at least 30 countries around the world [6][7]. Indeed, it is well known that the birthplace of tea is China [8]. Regarding the total amount of produced tea: 78% is black tea, which is consumed mainly in Western countries and certain Asian countries; 20% is green tea, which is consumed in Asia, North America, and Middle Eastern countries; and 2% is oolong tea, which is mainly produced in South China [9]. The Portuguese, on behalf of the Dutch, brought the tea to Europe for the first time in 1610. Rooibos tea, also known as red tea, is another tea species that is grown in South Africa. This type of tea contains aspalithin, which is a unique substance in contrast with the other types of tea [10]. This popular decoction is the subject of study for several scientists, due to its multiple uses and its benefits in respect of human health. Numerous research studies have proved that these extracts demonstrate antifungal, antiviral, antioxidant, antimutagenic, and anti-inflammatory properties [11]. These benefits derive from certain various contained compounds, such as polyphenols, alkaloids, vitamins, tannins, and flavonoids [12].
The type of tea is determined from the treatment of the Camelia sinensis leaves, which are collected within the first three years of the plant’s life. In order to produce black tea, the leaves are oxidized from two to three days, which is unlike green tea where the leaves are not oxidized. For this reason, green tea includes a high content of polyphenols [13]. In the initial stages of black tea production, the leaves contain both catechins and oxidation enzymes, which are found in different places on the leaf. The characteristic flavor and color of black tea is based on the production of theaflavins and thearubgins [9]. The production of white tea differs from green tea, due to the different time at which the leaves of Camellia sinensis are harvested. Regarding white tea, the collection of the leaves is carried out at the early stage of the plant’s development. This is in contrast to green tea, which involves the collection of leaves to be carried out in a later stage of the plant’s development [14]. Concerning oolong tea, the leaves are oxidized but not to the same degree of oxidation that occurs in relation to black tea leaves (which are semi-fermented) [15].
The main groups of biomolecules that are contained in a tea are: catechins, phenolic acids, and alkaloids. All these components show different bioactivity, while most of the time a mixture of them presents synergistic activity. It is worth noting that catechins and phenolic acids are responsible for the pharmacological action of tea. In detail, tea biomolecules mainly consist of non-protein amino acids, such as: theanine; free sugars; methylxanthine or purine alkaloids (such as caffeine); theobromine; theophylline and theacrine; as well as phenolic acids, such as gallic acid. This is in addition to eight other catechins. Moreover, the following catechins are also present: (+)-catechin (C); (−)-epicatechin (EC); (−)-gallocatechin (GC); (−)-epigallocatechin (EGC); (−)-catechin gallate (CG); (−)-gallocatechin gallate (GCG); (−)-epicatechin gallate (ECG); (−)-epigallocatechin gallate (EGCG); (−)-epicatechin (EC); (−)-epigallocatechin (EGC); (−)-epicatechin-3-gallate (ECG); and (−)-epigallocatechin-3-gallate (EGCG) [16][17].
3. Extraction of Natural Products
The most important stage of the process, in respect of the conventional extraction of natural products, is found in the usage of an appropriate solvent where the plant material will be in direct contact [18]. Often, the plant material is pulverized or crushed in order to increase the surface area that will be in contact with the solvent under stirring. The duration of the contact with the raw material in conjunction with the solvents is an important factor for the enhancement of extraction efficiency. Furthermore, it must be mentioned that the processes of most conventional extraction methods, such as maceration, are performed under stirring for the facilitating of mass transfer, which is achieved as a result of the enhancement of the extraction efficiency of bioactive substances.
Regarding extraction, both dried biomass or plant material, as well as fresh biomass (wet biomass) can be used. In the latter case, the sample preparation takes longer. However, minimal alteration of the isolated bioactive compounds is observed, via using fresh biomass. In the other hand, the fresh plant material contains a huge proportion of water, more than 70%. This often causes a problem during handling of the material due to the dilution of the extraction solvent. In contrast, the dried plant material is pulverized more easily to the appropriate size before extraction, which also helps to control the solvent/plant material ratio. This offers a greater reproducibility during experiments than the use of fresh plant material [18]. Extraction efficiency can be improved through the optimization of the time regarding: the contact with the plant material; the extraction solvent; the type of the plant; the fresh or dried material; the process of crushing or pulverization.
It is known that the traditional extraction methods encompass the solid–liquid extraction (SLE) techniques via the use of solvent and leaching [19]. The conventional extraction methods are found in: Soxhlet extraction, hydro-distillation, heat-reflux method, and maceration [20]. However, in recent years, the conventional techniques are not used very often due to certain disadvantages, such as the use of toxic solvents, as well as a huge time and energy consumption [20][21][22]. During the last few decades, due the disadvantages of the conventional methods, the scientific community has turned its attention to the development of more cost-effective and greener extraction methods of bioactive compounds from various natural products, such as tea plants [6][19].
Table 1 presents a brief description of the conventional, non-conventional, or green extraction methods of bioactive compounds from natural products. As shown in Table 1, the major experimental parameters for the efficient extraction of natural products are found in the type of solvent used and the temperature of extraction.
Table 1. Conventional and non-conventional extraction methods of natural products.
| Method Classification | Extraction Method | Solvents | Pressure | Temperature | Extraction Time |
|---|---|---|---|---|---|
| Conventional extraction techniques | Soxhlet method | Organic solvents (ethanol, methanol, chloroform, acetone, etc.) | Atmospheric | Under heat (The range of temperature alters according to the used solvents) |
Long time |
| Maceration | Water, aqueous, and organic solvents | Atmospheric | Room temperature | Long time | |
| Reflux extraction | Aqueous and organic solvents | Atmospheric | Under heat (The range of temperature alters according to the used solvents) |
Moderate time | |
| Hydro-distillation | Water | Atmospheric | Under heat (100 °C) | Long time | |
| Decoction | Water | Atmospheric | Under heat (100 °C) | Moderate time | |
| Non-conventional extraction techniques or green extraction methods | Supercritical fluid extraction (SFE) | Supercritical fluid (usually S-CO2)/modifier | High (For example, CO2 is the most used solvent for SFE, with pressure at 74 bars) |
Near room temperature (For CO2, a critical temperature at 31 °C) |
Short time |
| Ultrasound-assisted extraction (UAE) | Water, aqueous, and organic solvents | Atmospheric | Room temperature or under heat (The range of temperature alters according to the used solvents) |
Short time | |
| Microwave-assisted extraction (MAE) | Water, aqueous, and organic solvents | Atmospheric | Room temperature | Short time (15–30 min) | |
| Pressurized liquid extraction (PLE) | Water, aqueous, and organic solvents | High (For example, water is the most common solvent in PLE, whereby the boiling point of water at 0.1 MPa is obtained at the critical point of water at 22.1 MPa) |
Under heat (Water: temperature ranging from 100 °C to 374 °C) |
Short time | |
| Enzyme-assisted extraction (EAE) | Water, aqueous, and organic solvents | Atmospheric | Room temperature, or heated after enzyme treatment (For example, when using a pectinolytic method, the temperature is 50 °C) |
Moderate time | |
| Pulsed electric field extraction (PEFE) | Water, aqueous, and organic solvents | Atmospheric | Room temperature or under heat (The range of temperature alters according to the used solvents) |
Short time |
Regarding the selection of the extraction solvents, various parameters—such as solubility of the active ingredients in them, their cost, and their security—should be considered. As is widely known, the polar bioactive compounds, such as flavonols, dissolve in polar solvents, while the non-polar ones, such as squalene, dissolve in non-polar solvents [21].
3.1. Conventional Extraction Methods
3.1.1. Conventional Maceration Extraction Using Water as Solvent
For the extraction of bioactive compounds using conventional maceration extraction, both hot and cold water have been used. The experimental factors that affect the concentration of the extracted compounds are found in the temperature of the extraction and the time of processing. A high temperature of extraction appears to increase the content of the bioactive compounds due to the probable promotion in the wetting of the tea samples and the increased of the solubility of the bioactive compounds. It is worthwhile to mention that the higher yield of bioactive compounds is associated with a longer extraction time. However, a longer extraction time in hot water causes thermal degradation of these biomolecules [23].
Although the extraction using cold water appears to present a higher yield for the extraction of bioactive molecules than hot water extraction, the process time when using cold water was much longer than the extraction time that is incurred when using hot water. Consequently, the longer extraction time that occurs when using cold water increases the risk of microbial contamination. This phenomenon may obstruct the utilization of these extracts within the food and the pharmaceutical industries [23].
As already mentioned above, the size of the tea particles is another important experimental factor that affects the extraction yield [21]. According to Bindes et al., a comparison of unground tea leaves and the ground tea leaves shows that the ground leaves increase the yields of bioactive compounds, especially polyphenols, by 14% [24]. Furthermore, the small particle diameter results the increase in the contact of tea samples with solvents. The decreased particle diameter is associated with the increased specific area, thus contributing to the increase in the contact area between tea samples and solvents [23]. It is noteworthy to mention that the particle size must be optimized. This is due to the fact that the tea leaves with particle sizes lower than 0.15 mm showed a decrease in the content of polyphenols, which was due to the formation of agglomerates. However, the optimization of the particle sizes of tea leaves should be studied in the future [24].
Conclusively, the usage of water as a solvent for the extraction of bioactive molecules is primarily determined by the issue of safety. However, as mentioned above, conducting extraction via using both hot and cold water shows several drawbacks. An extraction through using hot water causes a decomposition of the bioactive molecules; while, at the same time, the extraction time when using cold water is much longer than for hot-water-based extraction. Regarding the minimization of the drawbacks of the extraction method when using a water as a solvent, various organic solvents have been used.
3.1.2. Conventional Maceration Extraction Using Organic Solvents
The type of the used organic solvent for the purposes of extraction is dependent on the solubility of the bioactive compounds. For example, the comparison of chloroform, ethyl acetate, water, and methanol showed that methanol was the most appropriate solvent for the extraction of the polyphenols. It must be mentioned that the efficiency of the extraction in respect of the bioactive compounds increased up to an 8 h extraction time. In respect of a further extraction time, it was found that this slightly increased the efficiency, without substantial changes in the process. However, as is widely known, the longtime process of extraction should be prevented, in the interest of a lower cost and energy. Although extraction via using organic solvents has presented a lower cost and ease in respect of operations, there are, regardless, various disadvantages. These disadvantages may include: the existence of impurities in the extracts; the difficulty found in purifying the impurities; and the toxicity of the appropriate organic solvents. Therefore, one of the major drawbacks of using organic compounds is found in environmental pollution. For this reason, the development of greener extraction methods is a pressing requirement in order to address this issue [23].
In a general sense, maceration is characterized by the simplicity of the operation. However, the major disadvantages of such an approach are found in the low extraction yield and longer time that is required in completing the process [19].
3.1.3. Decoction
Decoction is considered another conventional method. This is where the extracts contain only substances that are soluble in water, due to the fact of water being used as a solvent. Although decoction presents a higher extraction efficiency for water-soluble substances, it cannot be used for the extraction of thermolabile substances [19]. As Zhang et al. reported, the high temperature of the extraction may enhance the dissolution of the water-soluble bioactive substances in comparison with approaches that utilize room temperature during the maceration process [19]. However, one of the major drawbacks of this method is that the high temperature used in the extraction can cause a transformation of certain bioactive compounds.
3.1.4. Hydro Distillation
The hydro distillation method is the oldest method that is still used for the extraction of essential oils and phenolics. Hydrolysis, hydro diffusion, and decomposition via heating are the main processes that are performed during the hydro distillation method. The disadvantages of such a method are found in the: long extraction time, high extraction temperature (which is, as a result, unsuitable for heat-sensitive compounds), and high energy consumption.
3.1.5. Reflux Extraction
The process of reflux extraction is conducted at a constant temperature, while the solvent is evaporated and condensed, repeatedly. The selection of the extraction temperature is dependent on the solvent that is used [21]. Indeed, when compared with percolation and maceration, the reflux extraction method demands a lesser volume of solvent and a smaller extraction time; however, the extraction efficiency is increased as a result [19].
3.1.6. Soxhlet Extraction
It is known that the Soxhlet extraction method is an automatic, continuous extraction method, which is based on the operating principle of the reflux method and siphoning. This conventional method incorporates the advantages of reflux extraction and percolation [19]. The selection of the extraction temperature depends on the used solvent, while process time can range from a few hours to up to 48 h. The main disadvantages of the Soxhlet method are found in low efficiency, the large volumes of the solvents that are required, the long extraction time, and the high extraction temperature. Due to the high extraction temperature, the Soxhlet method is not only unsuitable for thermolabile ingredients, but also increases the possibility of a thermal transformation of the bioactive compounds [21].
According to Zhang et al., the temperature of the extraction is one of the most important factors for the purposes of efficient extraction. The solubility and the diffusion of the active ingredients of the plants are enhanced via using a high temperature during the extraction. However, the high temperature may trigger a loss of the solvent, a creation of impurities in the extracts, and a possible decay of thermosensitive substances [19]. One of the drawbacks of the conventional extraction method is in the longer period of time that is required to conduct the extraction process, which thus results in a large energy cost. Based on all of the above, the development of greener extraction methods is thus encouraged.
3.2. Non-Conventional or Green Extraction Methods
During the few last decades, in order to overcome many of the drawbacks of the conventional extraction methods, green extraction techniques have been developed. Green extraction techniques present various advantages over the conventional approaches, such as a reduction in the consumption of the extraction solvent, the usage of non-hazardous substances, a reduction in the extraction time, and the consumption of less energy [19][21][22]. In recent years, the use of green extraction methods has become the new trend for the isolation of active ingredients from natural products, especially in respect of tea plants due to the fact that they promote a greater environmental sustainability [20]. The developed green extraction methods are: pressurized liquid extraction (PLE); microwave-assisted extraction (MAE); supercritical fluid extraction (SFE); ultrasound-assisted extraction (UAE); enzyme-assisted extraction (EAE); as well as the usage of eutectic mixtures and supercritical fluids that have been applied alone, or in a combination with the above mentioned techniques [7][19][21][22]. A brief description of the various green extraction methods used for natural products and, especially, for tea is provided in Table 1 and Table 2. Today, many scientific references highlight the advantages of the green extraction techniques over the conventional methods in terms of the extraction of bioactive compounds [7][19][20][21][22][23][24][25][26][27][28][29]. Table 2 details the advantages and the disadvantages of these advanced extraction techniques. In addition to their advantages, these methods also present their own disadvantages, such as: the non-uniformity in the distribution of ultrasound energy in respect of the UAE method; the lack of homogeneous results in respect of heating for the MAE method; the possible presence of inhibitors that effect the enzyme activity for the EAE method [22][25]. Moreover, the application of green extraction methods on an industrial scale is not easily achieved due to their disadvantages as reported in Table 2. Therefore, the challenge for the scientific community is to reduce their disadvantages and to implement all these green techniques on an industrial scale.
Table 2. The main operative conditions, advantages, and disadvantages of the main green techniques for the purposes of extracting bioactive compounds from natural products.
| Techniques | Main Operative Conditions | Advantages | Disadvantages |
|---|---|---|---|
| Ultrasound-assisted extraction (UAE) | The frequency range is 20 to 40 kHz; in addition, the intensities range from 10–1000 W/cm2. |
|
|
| Microwave-assisted extraction (MAE) | Magnetic and electric fields oscillate between 0.3 to 300 GHz. |
|
|
| Supercritical fluid extraction (SFE) | CO2, with ethanol as a co-solvent, at 40–60 °C and a range of 350–500 bar. CO2, which is the most utilized solvent for the SFE method, is used with a pressure at 7.39 MPa and a critical temperature at 31.3 °C. |
|
|
| Enzyme-assisted extraction (EAE) | Enzymes that are required for the method: cellulase, pectinesterase, hemicellulase, fructosyltransferase, pectinase, a-amylase, and protease in the solvent extraction. Examples of the method use celluzyme at 2500 ppm, as well as pectinolytic enzymes at 50 °C, 5000 ppm. |
|
|
| Pressurized liquid extraction (PLE) | Elevated temperatures under reduced pressures are used (thus, elevating the temperatures of employed solvents above their atmospheric boiling points). The range of temperature is dependent on the solvent used. |
|
|
| Pulsed electric field extraction (PEF) and high-voltage electrical discharges (HVED) | It is a non-thermal process. High-voltage pulses in the range of 20–80 kV/cm |
|
|
| High-hydrostatic pressure extraction (HHPE) | Introduction of a sample in a chamber with a high pressure, which is 100 to 1000 MPa, or higher (depending on the exposure time), at temperatures from 50–200 °C for short time periods (i.e., 5–10 min). |
|
|
| Deep eutectic solvents (DESs) | Using solvents with melting points that are lower than 100 °C, at a low pressure with high viscosity. |
|
|
3.3. Green Solvents
According to Choi and Verpoorte, an extraction in conjunction with a solvent is a crucial step in the sample preparation of natural products in the industrial production of antioxidants, aromas, antimicrobial compounds, etc. [30][31]. Although alcohols such as methanol and ethanol are the most common solvents used for the extraction of bioactive compounds from natural products, several other solvents are also used, such as chloroform, carbon tetrachloride, acetone, acetonitrile [4][21]. It is known that the extraction solvents that are produced from non-renewable resources are unsafe to the environment and thus are harmful to human health. In order to designate a solvent as a green solvent, there must be an account for not only the assessment of the environment, health, and safety, but also the energy demand. The main desired properties regarding the alternative solvents for green extraction are those of low toxicity, high solvency, and biodegradability. Moreover, the solvents used should originate from renewable resources and be recyclable—which, as a result, entails smaller environmental impacts [31]. The discovery of a suitable solvent is a challenge due to difficulty of appropriate selection. The factors to consider are dependent on the species of the plant, the target biomolecules, and the extraction method to be used.
Criteria of Green Solvent Selection
Some of the solvents used in the conventional methods are toxic, and as a result they pose a risk to both to the environment and to human health. For this reason, during the last few decades, more environmentally friendly solvents have been discovered and applied. According to Luczynska et al., the major principles of green chemistry are in the design of safer chemicals and more easily degradable products, as well as the use of renewable raw materials. The selection of extraction solvents must consider: low toxicity, the ability of quick mass transfer; and a low boiling point [2]. As Jessop reported, the main objective of the development of green solvents is in the reduction of toxic substances and the use of safer products [32]. In respect of this, certain green solvents are presented in Table 3.
Table 3. Solvents for green extraction.
| Solvent | Application | Solvent Polarity |
|---|---|---|
| Water/subcritical H2O | Steam distillation (essential oils); Microwave-assisted distillation (essential oils); Extraction by sub-critical water (aromas) |
Polar, weakly polar, and non-polar |
| CO2/supercritical CO2 | Supercritical fluid extraction (extraction of tea) | Weakly polar and non-polar |
| Ionic liquids (ILs)/Eutectic solvents (DES, NADES) | Ultrasound-assisted extraction | Polar and non-polar |
| Natural solvents from biomass/Agrosolvents | Ethanol (pigments and antioxidants); Glycerol (polyphenols) terpenes, such as d-limonene (fats and oils) |
Polar, weakly polar, and non-polar |
| Solvent-free | Microwave hydro-diffusion and gravity (antioxidants and essential oils); Pulse electric field (antioxidants and pigments) |
Polar and weakly polar |
To date, ethanol, acetone, and chloroform are the most used solvents in the extraction of natural products, especially in respect of tea leaves [4][33]. As was mentioned above, various organic solvents may be toxic, necessitating the development of new “green” media with environmentally friendly requirements for the purposes of a sustainable environment.
The selection of the solvent is a crucial step for green extraction. It is worthwhile to mention that the good practices guidelines must be accounted for. At first, the evaluation of the possibility regarding the use of a “solvent-free” technique must be performed. Further, a 100% natural, agro-solvent must be used, especially when knowing the potentially related risks. The solvents that may be toxic and affect human health must be avoided. In addition, solvents with low volatile organic compounds and high-rate recyclability must be used. Moreover, solvents that reduce energy consumption and the cost of process must be used. One of the major issues is found in the suitability of the solvent within the respective industry that the extraction is being conducted for, as well as in respect of the maximal recovery of the solvent at the end of the procedure [31].
Water is considered a green solvent due to the fact that it is non-toxic, non-flammable, low cost, and environmentally friendly. As it is known as a polar solvent, it can extract polar compounds. However, it is not a good solvent for non-polar and semi-polar compounds [31][34]. An alternative solvent is subcritical water, which is water under different conditions of temperature, i.e., between the boiling point and critical point of water (which is found at 100 °C at 1 bar and 374 °C at 221 bar), as well in higher pressures where it is stable in a liquid state. The method that utilizes this solvent is the subcritical water extraction (SWE) technique. Indeed, subcritical water demonstrates a lower polarity, as a result of the hydrophobic organic compounds that are soluble in it [35]. Moreover, subcritical water can be used not only for the extraction of bioactive compounds, but also for the chromatographic analysis of the target analytes. Currently, the method of subcritical water extraction is popular due to the usage of such an excellent alternative solvent. Indeed, subcritical water—when compared to the conventional organic solvents—allows, through its application, for the extraction of various bioactive compounds, such as phenolic compounds and fatty acids [31][36].
Certain researchers have turned their attention to developing new solvents, such as DESs, which are considered to be promising substitutes of the more conventional solvents [4]. The usage of eutectic mixtures and supercritical fluids is considered to be a green solution and could eliminate the problem of using toxic solvents. Moreover, DESs are used for the extraction of bioactive compounds from tea species due their advantages over conventional solvents. For example, their low toxicity, thermal stability, low vapor pressure, biodegradability, and its low cost [4][31][37][38].
DESs are obtained from the combination of hydrogen bond donors (HBDs) and hydrogen bond acceptors (HBAs). The mixture of these components leads to the preparation of DESs with a lower melting point due to the hydrogen bonds and Van der Waals interactions that are formed [25][39]. In addition, DESs are mainly a mixture of quaternary ammonium salt (HBA) with metal salts (HBD). The mixing of specific HBA and HBD results in two types of DESs, i.e., hydrophilic and hydrophobic DESs. Hydrophilic DESs are created by the combination of quaternary salt with metal chloride or with a hydrated metal chloride. Hydrophobic DESs are a mixture of quaternary salt or metal chloride in conjunction with a hydrogen bond donor [25][38]. The most used DESs are a combination of choline chloride (ChCl) (as the HBA), with acetic acid and oxalic acid, combined with glycerol, xylitol, urea (as the HBD).
The implementation of non-conventional extraction methods—such as the ultrasound-assisted technique when using DESs as a solvent—have shown a higher extraction efficiency of bioactive compounds from natural products when compared to the UAE method when used with traditional solvents (e.g., ethanol). The advantages of a DES-based ultrasound-assisted extraction method are found in a decrease in the volume of solvents, a decrease in the consumption of energy, and an enhancement in the extraction yield of bioactive substances [25][40]. Moreover, according to Luo et al., the implementation of UAE-DES to the extraction of green tea has shown that the total phenolic content, the antioxidant activity, and the concentration of major catechins were enhanced when compared with those values achieved by more conventional methods [4].
In recent years, there has been an attempt to replace synthetic DESs with the corresponding natural sources of DESs, which are called natural deep eutectic solvents (NADESs). The formation of NADESs are a result of a combination of two or more natural substances, such as organic acids (e.g., lactic and citric acids), amino acids, sugars (e.g., sucrose and glucose), choline chloride. [41]. Similar to DESs, NADESs present a lower melting point than the traditional solvents. However, NADESs are also non-toxic, biodegradable, and sustainable when compared with synthetic DESs. Due to their advantages, the extracted bioactive compounds that are obtained as a result of using NADESs are considered safe to be used in the cosmetic and pharmaceutical industries [42][43][44].
In the effort of minimizing the use of solvents, solvent-free techniques have been developed. Indeed, solvent-free extraction techniques are popular as they can be utilized in the co-extraction of both lipophilic and hydrophilic substances, in addition to lipids, volatile and non-volatile substances. The advantages of solvent-free techniques are the decrease in risk associated with the organic solvents, the low cost, reduction in the risk of overpressure, and the ability to be scaled up [31][45]. Currently, various modern techniques based on solvent-free extraction, such as a new microwave solvent-free extraction technique called microwave hydro-diffusion and gravity (MHG), and pulsed electric fields (PEF) have been developed. These techniques improve the efficiency of the extraction as they reduce the extraction time and energy required, as well as eliminate the process step of treating wastewater. Moreover, according to Chemat et al., microwave hydro-diffusion and gravity (MHG) was developed for the extraction of various bioactive compounds, such as antioxidants and essential oils. In respect of this, the plant material is placed inside in a microwave reactor without any solvent. The microwaves increase the internal heating of the water of the plant material, thereby leading to the rupture of receptacles and glands due to the expansion of the cells [3].
4. Analytical Techniques for the Separation of Active Ingredients of Tea
Table 4 presents examples of the analytical methodologies that apply for the isolation of the bioactive molecules from the various species of tea. The specific study showed that catechins, alkaloids (such as caffeine), gallic acid, and flavonoids were the major extracted bioactive molecules that were isolated from several tea species. It is worth noting that the main isolated compound of Rooibos tea, which thrives in South Africa, was di-hydrochalcone aspalathin. In contrast, caffeine was not found in this tea [10]. Moreover, as has been demonstrated in this research, green tea has been widely studied at a rate of 52% of the available research studies, followed by black tea at 26%, and then white tea and oolong tea at 11% each. This, therefore, shows the importance of green tea due to certain valuable properties, such as its antiviral, antioxidant, antimutagenic, and anti-inflammatory traits.
Table 4. Extraction and analysis methods for the determination of the bioactive molecules in various types of tea.
| Type of Tea | Extraction Method | Analysis Method | References |
|---|---|---|---|
| White tea (Camellia Sinensis, Theaceae) | MAE; UAE; DLLME; Extraction with a hydro-alcoholic solution and evaporation. |
HPLC-UV; HPLC-PDA; RP-HPLC; GLC; GC/MS. |
[6][12][46][47][48][49][50] |
| Black tea (Camellia Sinensis, Theaceae) | MAE; UAE; DLLME; HRE. Heating extraction with hydro-alcoholic solution and evaporation |
HPLC/UV; HPLC/PDA; RP-HPLC/ECD; HPLC/DAD; HPLC/MS; UV-Vis; GLC; GC/MS; UHPLC/PDA or DAD; GC/FID. |
[12][14][46][47][48][49][51][52][53][54][55][56][57][58][59] |
| Green tea (Camellia Sinensis, Theaceae) | MAE; UAE; DLLME; HRE; Heating extraction; Stirring extraction; Extraction with a hydro-alcoholic solution and evaporation; Citric acid water extraction; WE-HT; SWE; PEF; IPL; Microwave mediated acetylation; SE; UPE; US and agitation extraction techniques; Conventional hot water/UAE; UAE-DES; V-LPD; SFE; SCCO2 extraction.High hydrostatic pressure extraction. |
HPLC/UV; HPLC/PDA; HPLC/MS; UV-Vis; GLC; GC/MS; RP-HPLC/ECD. UHPLC/PDA or DAD; HPLC-MS/MS; SEM; GC-FID; TLC; HRMS; FT-IR; 1H and 13C NMR; Colorimetric analysis. |
[4][8][11][12][14][29][46][47][48][49][50][51][52][53][54][55][57][59][60][61][62][63][64][65][66][67][68][69][70][71][72][73] |
| Red tea (Rooibos *) (Aspalathus Linearis, Fabaceae) | MAE | HPLC/PDA | [10] |
| Green mate tea (Ilex Paraguariensis) | MAE | RP-HPLC/PDA | [47] |
| Roasted mate tea ((Ilex Paraguariensis) | MAE | RP-HPLC/PDA | [47] |
| Yellow tea (Camellia sinensis L.) | MAE; UAE. |
RP-HPLC/PDA | [47][74] |
| Oolong tea (Camellia Sinensis, Theaceae) | MAE; UAE; HRE; Thermal extraction; Extraction with a hydro-alcoholic solution and evaporation. |
HPLC/PDA; GC/MS; UHPLC/DAD; GC/FID. |
[46][48][52][57][59][70] |
| Fujian oolong tea (Camellia Sinensis) (produced in the Wuyi Mountain of northern Fujian) |
MAE UAE |
HPLC/PDA | [46][48] |
| Jiangxi oolong tea (Camellia Sinsensis) | MAE UAE |
HPLC/PDA | [46][52] |
| Pu-erh tea (Camellia Sinensis var. assamica) | MAE | HPLC/PDA; UV-Vis. |
[46] |
| Jasmine tea (a mixture of leaves of Camellia Sinsensis with jasmine flowers (Jasminum officinale). |
MAE | HPLC/PDA; UV-Vis. |
[46] |
| Lipton tea (decaffeinated green tea and green tea lemon and ginseng) | MAE | UV-Vis; GC/MS. |
[75][76] |
Abbreviations—* HPLC/PDA: high performance liquid chromatography tandem photodiode array; UPLC: ultra-performance liquid chromatography; UHPLC: ultra-high-performance liquid chromatography; RP-HPLC: reverse phase high-performance liquid chromatography; TLC: thin-layer chromatography; GC: gas chromatography; MS: mass spectrometry; Gas/FID: gas chromatography/flame ion detector; Gas/MS: gas chromatography/mass spectrometry; UV-Vis: ultraviolet-visible spectrometry; HPLC-MS/MS: high-performance liquid chromatography tandem mass spectrometry; MAE: microwave-assisted extraction; UAE: ultrasound-assisted extraction; SFE: supercritical fluid extraction; DLLME: dispersive liquid–liquid microextraction; SE: Soxhlet extraction; and HRE: heat-reflux extraction.
As can be seen from Table 4, several different extraction techniques—such as ultrasonic-assisted extraction, ultrasonic-assisted extraction with DESs, the microwave-assisted-extraction method, and the reflux method—have been used for the isolation of bioactive molecules on different tea species. As mentioned above, a great effort has been made by scientists to develop green techniques for the extraction of bioactive molecules from several natural products. For this reason, in the last few decades, the application of green extraction methods, such as UAE-DESs, MAE, and SFE have been reported [4][8][25].
Table 4 also presents the analytical methods for the determination of the bioactive ingredients in the various types of tea. As is widely known, the substances in the extracts after using the different extraction methods are complex due to the fact that they contain a variety of bioactive molecules that require separation, purification, and identification [19]. From the literature review, it was clearly observed that chromatography and, more specifically, column chromatography are the most used analytical methods to identify the active ingredients of the tea. More specifically, regarding the determination of bioactive molecules, the most used analytical method is high-performance liquid chromatography (HPLC) combined with a photodiode array detector (PDA) and mass spectrophotometry (MS), at a usage rate of around 80%. This is followed by the UPLC and GC methods at 12% and 8%, respectively. Based on this, the selective chromatographic techniques and, mainly, liquid chromatography are widespread. Further, they are used for the determination of a cocktail of active ingredients that are extracted.
Sereshti et al. reported a simple ultrasound-assisted micro-solid phase extraction method based on graphene oxide (GO) nanoabsorbents (UAD-m-SPE); further, this was applied for the extraction of bioactive compounds, such as theophylline, theobromine, and caffeine from black, green, and white tea. The determination of these active ingredients was performed using HPLC-UV. Moreover, based on the specific research work, the following concentrations of active compounds were observed: For green tea: 30,0031.1 ng/mL caffeine, 462.4 ng/mL theophylline, and 1378.0 ng/mL theobromine; for black tea: 48,214.2 ng/mL caffeine, 471.4 ng/mL theophylline, and 6378.9 ng/mL theobromine; while for white tea: 18,161.5 ng/mL caffeine, 555.2 ng/mL theophylline, and 1641.5 ng/mL theobromine. These results clearly show the importance of the determination of the bioactive compounds in different tea species, as the type of bioactive compounds, their concentration, and thus their bioactivity are dependent on the tea species [12].
Novak et al. isolated, from green and black tea, the active compounds of gallic acid, EGC, C, EC, EGCG, and ECG while using the UAE method with water as the solvent. Japanese green tea showed: 1.435 mg/g gallic acid, 18.23 mg/g EGC, 0.785 mg/g C, 1.778 mg/g EC, 11.43 mg/g EGCG, and 3.058 mg/g ECG. Chinese green tea showed 0.711 mg/g gallic acid, 31.62 mg/g EGC, 5.764 mg/g C, 21.06 mg/g EC, 61.36 mg/g EGCG, and 33, 20 mg/g ECG [54]. In this research, it was clearly seen that the concentration of the active ingredients depended on both the type of tea and the place of origin, which is also related to the climatic conditions.
Luo et al. reported the development of a green method, UAE-DES, which was used to extract the antioxidant polyphenols from green tea. Regarding this, DES ChCl-glycerol was selected as the green solvent. The application of the UAE-DES method demonstrated higher antioxidant activity and a concentration of catechins ((−)-EGC, (−)-EC, (−)-EGCG, and (−)-ECG)) when compared to the other extraction methods—i.e., UAE with ethanol, ethanol extraction, and hot water extraction. The results of this research support the use of this green technique for the extraction of antioxidant polyphenols from green tea, thereby showing its potential application in the food and pharmaceutical industries [4]. Moreover, according to Zhang et al., the use of DESs demonstrate a high extraction yield, i.e., 97% for epigallocatechin gallate and up to 82.7% for catechin [73].
According to Ghasemzadeh-Mohammadi et al., a comparison between the UAE and MAE methods in respect of a study of polyphenols, as well as of antioxidant activity, was performed. The utilization of the MAE method presented a higher efficiency than the UAE technique. Furthermore, the utilization of MAE showed a 125 ± 5 mg gallic acid/g dry weight and a 56 mg/g of phenol, while the UAE method showed a 96 ± 6 mg gallic acid/g dry weight of total polyphenols and 66 mg/g of phenol [77].
Thus, by taking the above into account, as well as the results of different research studies, the combination of MAE/UAE was shown to possess a higher extraction efficiency together instead of when each extraction technique was conducted alone. Moreover, for the combination of the MAE/ UAE techniques with a green solvent, the chitosan/ascorbic acid solvents appeared to be the best approach for the extraction of catechins from green tea [8].
References
- Anastas, P.; Eghbali, N. Green chemistry: Principles and practice. Chem. Soc. Rev. 2010, 39, 301–312.
- Luczynska, G.; Pena-Pereira, F.; Tobiszewski, M.; Namieśnik, J. Expectation-maximization model for substitution of missing values characterizing greenness of organic solvents. Molecules 2018, 28, 1292–1300.
- Chemat, F.; Vian, M.A.; Cravotto, G. Green Extraction of Natural Products: Concept and Principles. Int. J. Mol. Sci. 2012, 13, 8615–8627.
- Luo, Q.; Zhang, J.R.; Li, H.B.; Wu, D.T.; Geng, F.; Corke, H.; Wei, X.L.; Ren-You Gan, R.Y. Green Extraction of Antioxidant Polyphenols from Green Tea (Camellia sinensis). Antioxidants 2020, 9, 785–799.
- Chen, L.; Zhou, Z.X. Variations of main quality components of tea genetic resources (Camellia sinensis (L.) O. Kuntze) preserved in the China national germplasm tea repository. Plant Food Hum. Nutr. 2005, 60, 31–35.
- Li, F.; Wei, Y.L.; Liang, L.; Huang, L.L.; Yu, G.Y.; Li, Q.H. A novel low-molecular-mass pumpkin polysaccharide: Structural characterization, antioxidant activity, and hypoglycemic potential. Carbohydr. Polym. 2021, 251, 117090.
- Banerjee, S.; Chatterjee, J. Efficient extraction strategies of tea (Camellia sinensis) biomolecules. J. Food Sci. Technol. 2015, 52, 3158–3168.
- Fujioka, K.; Salaheldin, T.A.; Godugu, K.; Meyers, H.V.; Mousa, S.A. Edible Green Solvent for Optimized Catechins Extraction from Green Tea Leaves: Anti-Hypercholesterolemia. J Pharm. Pharmacol. Res. 2022, 6, 80–92.
- Kanwar, J.; Taskeen, M.; Mohammad, I.; Huo, C.; Chan, T.H.; Dou, Q.P. Recent advances on tea polyphenols. Front Biosci. 2012, 4, 111–131.
- van Heerden, F.R.; van Wyk, B.E.; Viljoen, A.M.; Steenkamp, P.A. Phenolic variation in wild populations of Aspalathus linearis (rooibos tea). Biochem. Syst. Ecol. 2003, 31, 885–895.
- Zhang, H.; Tang, B.; Row, K. Extraction of Catechin Compounds from Green Tea with a New Green Solvent. Chem. Res. Chin. Univ. 2014, 30, 37–41.
- Sereshti, H.; Khosraviani, M.; Samadi, S.; Amini-Fazl, M.S. Simultaneous determination of theophylline, theobromine and caffeine in different tea beverages by graphene-oxide based ultrasonic-assisted dispersive micro solid-phase extraction combined with HPLC-UV. RSC Adv. 2014, 4, 47114–47120.
- Botten, D.; Fugallo, G.; Fraternali, F.; Molteni, C. Structural Properties of Green Tea Catechins. J. Phys. Chem. B 2015, 119, 12860–12867.
- Chin, J.M.; Merves, M.L.; Goldberger, B.A.; Sampson-Cone, A.; Cone, E.J. Caffeine content of brewed teas. J. Anal. Toxicol. 2008, 32, 702–704.
- Ng, K.W.; Cao, Z.J.; Chen, H.B.; Zhao, Z.Z.; Zhu, L.; Yi, T. Oolong tea: A critical review of processing methods, chemical composition, health effects, and risk. Crit. Rev. Food Sci. Nutr. 2017, 58, 2957–2980.
- Unachukwu, U.J.; Ahmed, S.; Kavalier, A.; Lyles, J.T.; Kennelly, E.J. White and green teas (Camellia sinensis var. sinensis): Variation in phenolic, methylxanthine, and antioxidant profiles. J. Food Sci. 2010, 75, 541–548.
- Peng, L.; Song, X.; Shi, X.; Li, J.; Ye, C. An improved HPLC method for simultaneous determination of Phenolic compounds purine alkaloids and theanine in Camellia species. J. Food Compos. Anal. 2008, 21, 559–563.
- Mason, T.J.; Chemat, F.; Vinatoru, M. The Extraction of Natural Products using Ultrasound or Microwaves. Curr. Org. Chem 2011, 15, 237–247.
- Zhang, Q.W.; Lin, L.G.; Ye, W.C. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018, 13, 20–46.
- Ameer, K.; Shahbaz, H.M.; Kwon, J.H. Green Extraction Methods for Polyphenols from Plant Matrices and Their Byproducts: A Review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 295–315.
- García, S.L.R.; Raghavan, V. Green extraction techniques from fruit and vegetable waste to obtain bioactive compounds—A review. Crit. Rev. Food Sci. Nutr. 2022, 62, 6446–6466.
- Mena- García, A.; Ruiz-Matute, A.I.; Soria, A.C.; Sanz, M.L. Green techniques for extraction of bioactive carbohydrates. TrAC Trend Anal. Chem. 2019, 119, 115612–115622.
- Li, H.; Guo, H.; Luo, Q.; Wu, D.T.; Zou, L.; Liu, Y.; Li, H.B.; Gan, R.Y. Current extraction, purification, and identification techniques of tea polyphenols: An updated review. Crit. Rev. Food Sci. Nutr. 2021, 1–19.
- Bindes, M.M.M.; Cardoso, V.L.; Reis, M.H.M.; Boffito, D.C. Maximisation of the polyphenols extraction yield from green tea leaves and sequential clarification. J. Food Eng. 2019, 241, 97–104.
- Calderón-Oliver, M.; Ponce-Alquicira, E. Environmentally Friendly Techniques and Their Comparison in the Extraction of Natural Antioxidants from Green Tea, Rosemary, Clove, and Oregano. Molecules 2021, 26, 1869–1889.
- Panja, P. Green extraction methods of food polyphenols from vegetable materials. Curr. Opin. Food Sci. 2018, 23, 173–182.
- Vinatoru, M.; Mason, T.J.; Calinescu, I. Ultrasonically assisted extraction (UAE) and microwave assisted extraction (MAE) of functional compounds from plant materials. Trend Anal. Chem. 2017, 97, 159–178.
- Tiwari, B.K. Ultrasound: A clean, green extraction technology. Trend Anal. Chem. 2015, 71, 100–109.
- Both, S.; Chemat, F.; Strube, J. Extraction of polyphenols from black tea—Conventional and ultrasound assisted extraction. Ultrason. Sonochem. 2014, 21, 1030–1034.
- Choi, Y.H.; Verpoorte, R. Metabolomics: What you see is what you extract. Phytochem. Anal. 2014, 25, 289–290.
- Chemat, F.; Vian, M.A.; Ravi, H.K.; Khadhraoui, B.; Hilali, S.; Perino, S.; Tixier, A.S.F. Review of Alternative Solvents for Green Extraction of Food and Natural Products: Panorama, Principles, Applications and Prospects. Molecules 2019, 24, 3007–3034.
- Jessop, P.G. Searching for green solvents. Green Chem. 2011, 13, 1391–1398.
- Wu, L.; Li, L.; Chen, S.; Wang, L.; Lin, X. Deep eutectic solvent-based ultrasonic-assisted extraction of phenolic compounds from Moringa oleifera L. leaves: Optimization, comparison and antioxidant activity. Sep. Purif. Technol. 2020, 247, 117014–117025.
- Mihaylova, D.; Lante, A. Water an Eco-Friendly Crossroad in Green Extraction: An Overview. Open Biotechnol. J. 2019, 13, 155–162.
- Carr, A.G.; Mammucari, R.; Foster, N.R. A review of subcritical water as a solvent and its utilization for the processing of hydrophobic organic compounds. Chem. Eng. J. 2011, 172, 1–17.
- Zakaria, S.M.; Kamal, S.M.M.; Harun, M.R.; Omar, R.; Siajam, S.I. Subcritical water technology for extraction of phenolic compounds from Chlorella sp. microalgae and assessment on its antioxidant activity. Molecules 2017, 22, 1105–1119.
- Li, J.; Han, Z.; Zou, Y.; Yu, B. Efficient extraction of major catechins in Camellia sinensis leaves using green choline chloride-based deep eutectic solvents. RSC Adv. 2015, 5, 93937–93944.
- Cai, C.; Li, F.; Liu, L.; Tan, Z. Deep eutectic solvents used as the green media for the efficient extraction of caffeine from Chinese dark tea. Sep. Purif. Technol. 2019, 227, 115723–115731.
- Dai, Y.; Van Spronsen, J.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.H. Natural deep eutectic solvents as new potential media for green technology. Anal. Chim. Acta 2013, 766, 61–68.
- Zhang, L.; Wang, M. Optimization of deep eutectic solvent-based ultrasound-assisted extraction of polysaccharides from Dioscorea opposita Thunb. Int. J. Biol. Macromol. 2017, 95, 675–681.
- Espino, M.; Fernández, M.A.; Gomez, F.J.V.; Silva, M.F. Natural designer solvents for greening analytical chemistry. TRaC Trend Anal. Chem. 2016, 76, 126–136.
- Paiva, A.; Craveiro, R.; Aroso, I.; Martins, M.; Reis, R.L.; Duarte, A.R.C. Natural Deep Eutectic Solvents—Solvents for the 21st Century. ACS Sustain. Chem. Eng. 2014, 2, 1063–1071.
- Neil Winterton, N. The green solvent: A critical perspective. Clean Technol. Environ. Policy 2021, 23, 2499–2522.
- Lavaud, A.; Laguerre, M.; Bitric, S.; Fabiano Tixier, A.-S.; Roller, M.; Chemat, F. International. Patent WO 2016/162703 Al, 10 April 2015. Available online: https://worldwide.espacenet.com/publicationDetails/biblio?DB=EPODOC&II=4&ND=3&adjacent=true&locale=fr_EP&FT=D&date=20180406&CC=CN&NR=107889468A&KC=A# (accessed on 17 August 2019).
- Kerton, F.M.; Mariotte, R. Alternative Solvents for Green Chemistry, 2nd ed.; Royal Society of Chemistry: Croydon, UK, 2013; pp. 1–325.
- Zuo, Y.; Chen, H.; Deng, Y. Simultaneous determination of catechins, caffeine and gallic acids in green, oolong, black and pu-erh teas using HPLC with a photodiode array detector. Talanta 2002, 57, 307–316.
- Komes, D.; Horžić, D.; Belščak, A.; Kovačević Ganič, K.; Baljak, A. Determination of caffeine content in tea and maté tea by using different methods. Czech J. Food Sci. 2009, 27, S213–S216.
- Sereshti, H.; Samadi, S.; Jalali-Heravi, M. Determination of volatile components of green, black, oolong and white tea by optimized ultrasound-assisted extraction-dispersive liquid–liquid microextraction coupled with gas chromatography. J. Chromatogr. A 2013, 1280, 1–7.
- Rostagno, M.A.; Manchón, N.; D’Arrigo, M.; Guillamón, E.; Villares, A.; García-Lafuente, A.; Ramos, A.; Martínez, J.A. Fast and simultaneous determination of phenolic compounds and caffeine in teas, mate, instant coffee, soft drink and energetic drink by high-performance liquid chromatography using a fused-core column. Anal. Chim. Acta 2011, 685, 204–211.
- Wei, K.; Wang, L.Y.; Zhou, J.; He, W.; Zeng, J.M.; Jiang, Y.W.; Cheng, H. Comparison of catechins and purine alkaloids in albino and normal green tea cultivars (Camellia sinensis L.) by HPLC. Food Chem. 2012, 130, 720–724.
- Lee, K.J.; Lee, S.H. Extraction behavior of caffeine and EGCG from green and black tea. Biotechn. Bioprocess. Eng. 2008, 13, 646–649.
- Bae, I.K.; Ham, H.M.; Jeong, M.H.; Kim, D.H.; Kim, H.J. Simultaneous determination of 15 phenolic compounds and caffeine in teas and mate using RP-HPLC/UV detection: Method development and optimization of extraction process. Food Chem. 2015, 172, 469–475.
- Rahim, A.A.; Nofrizal, S.; Saad, B. Rapid tea catechins and caffeine determination by HPLC using microwave-assisted extraction and silica monolithic column. Food Chem. 2014, 147, 262–268.
- Novak, I.; Šeruga, M.; Komorsky-Lovrić, Š. Characterisation of catechins in green and black teas using square-wave voltammetry and RP-HPLC-ECD. Food Chem. 2010, 122, 1283–1289.
- Scoparo, C.T.; de Souza, L.M.; Dartora, N.; Sassaki, G.L.; Gorin, P.A.J.; Iacomini, M. Analysis of Camellia sinensis green and black teas via ultra high performance liquid chromatography assisted by liquid–liquid partition and two-dimensional liquid chromatography (size exclusion × reversed phase). J. Chromatogr., A 2012, 1222, 29–37.
- Yuda, N.; Tanaka, M.; Suzuki, M.; Asano, Y.; Ochi, H.; Iwatsuki, K. Polyphenols Extracted from Black Tea (Camellia sinensis) Residue by Hot-Compressed Water and Their Inhibitory Effect on Pancreatic Lipasein vitro. J. Food Sci. 2012, 77, H254–H261.
- Jiang, H.; Engelhardt, U.H.; Thräne, C.; Maiwald, B.; Stark, J. Determination of flavonol glycosides in green tea, oolong tea and black tea by UHPLC compared to HPLC. Food Chem. 2015, 183, 30–35.
- Fernando, C.D.; Soysa, P. Extraction Kinetics of phytochemicals and antioxidant activity during black tea (Camellia sinensis L.) brewing. Nutr. J. 2015, 31, 14–74.
- Xu, L.L.; Chen, Y.; Chen, Z.Q.; Gao, X.D.; Wang, C.L.; Panichayupakaranant, P.; Chen, H.X. Ultrafiltration isolation, physicochemical characterization, and antidiabetic activities analysis of polysaccharides from green tea, oolong tea, and black tea. J. Food Sci. 2020, 85, 4025–4032.
- Wang, H.; Helliwell, K.; You, X. Isocratic elution system for the determination of catechins, caffeine and gallic acid in green tea using HPLC. Food Chem. 2000, 68, 115–121.
- Vuong, Q.V.; Tan, S.P.; Stathopoulos, C.E.; Roach, P.D. Improved extraction of green tea components from teabags using the microwave oven. J. Food Compos. Anal. 2012, 27, 95–101.
- He, X.; Li, J.; Zhao, W.; Liu, R.; Zhang, L.; Kong, X. Chemical fingerprint analysis for quality control and identification of Ziyang green tea by HPLC. Food Chem. 2015, 171, 405–411.
- Demir, E.; Serdar, G.; Sökmen, M. Comparison of Some Extraction Methods for Isolation of Catechins and Caffeine from Turkish Green Tea. Int. J. Second. Metab. 2015, 2, 16–25.
- Hwang, H.J.; Kim, Y.G.; Chung, M.S. Improving the Extraction of Catechins of Green Tea (Camellia sinensis) by Subcritical Water Extraction (SWE) Combined with Pulsed Electric Field (PEF) or Intense Pulsed Light (IPL) Pretreatment. Foods 2021, 10, 3092–3105.
- Perva-Uzunalic, A.; Skerget, M.; Knez, Z.; Weinreich, B.; Otto, F.; Gruner, S. Extraction of active ingredients from green tea (Camellia sinensis): Extraction efficiency of major catechins and caffeine. Food Chem. 2006, 96, 597–605.
- Selvi, I.K.; Nagarajan, S. Separation of catechins from green tea (Camellia sinensis L.) by microwave assisted acetylation, evaluation of antioxidant potential of individual components and spectroscopic analysis. Food Sci. Technol. 2018, 91, 391–397.
- Pan, X.; Niu, G.; Liu, H. Microwave-assisted extraction of tea polyphenols and tea caffeine from green tea leaves. Chem. Eng. Process 2003, 42, 129–133.
- Ayyildiz, S.S.; Karadeniz, B.; Sagcan, N.; Bahar, B.; Abdullah, A.; Alasalvar, C. Optimizing the extraction parameters of epigallocatechin gallate using conventional hot water and ultrasound assisted methods from green tea. Food Bioprod. Process. 2018, 111, 37–44.
- Jun, X.; Deji, S.; Shou, Z.; Bingbing, L.; Ye, L.; Rui, Z. Characterization of polyphenols from green tea leaves using a high hydrostatic pressure extraction. Int. J. Pharm. 2009, 382, 139–143.
- Das, P.R.; Eun, J.B. A comparative study of ultra-sonication and agitation extraction techniques on bioactive metabolites of green tea extract. Food Chem. 2018, 253, 22–29.
- Xiang, B.; Zhou, X.; Qin, D.; Xi, J. Vesicle-enhanced liquid-phase pulsed discharge extraction of polyphenols from green tea leaves. Innov. Food Sci. Emerg. 2021, 74, 102839.
- Sökmen, M.; Demir, E.; Alomar, S.Y. Optimization of sequential supercritical fluid extraction (SFE) of caffeine and catechins from green tea. J. Supercrit. Fluids 2018, 133, 171–176.
- Bermejo, D.V.; Ibánez, E.; Reglero, G.; Fornari, T. Effect of cosolvents (ethyl lactate, ethyl acetate and ethanol) on the supercritical CO2 extraction of caffeine from green tea. J. Supercrit. Fluids 2016, 107, 507–512.
- Wang, H.S.; Chen, J.R.; Ren, P.F.; Zhang, Y.W.; Onayango, S.O. Ultrasound irradiation alters the spatial structure and improves the antioxidant activity of the yellow tea polysaccharide. Ultrason. Sonochem. 2021, 70, 105355.
- Edwards, Q.A.; Hinkson, S.A.S.; Garner-O’Neale, L.D.; Kulikov, S.M. Quantification of caffeine in selected beverages via gas chromatography-mass spectroscopy. Int. J. Chem. Sci. 2015, 13, 133–142.
- Sharif, R.; Ahmad, S.W.; Anjum, H.; Ramzan, N.; Malik, S.R. Effect of infusion time and temperature on decaffeination of tea using liquid-liquid extraction technique. J. Food Process. Eng. 2014, 37, 46–52.
- Ghasemzadeh-Mohammadi, V.; Zamani, B.; Afsharpour, M.; Mohammadi, A. Extraction of caffeine and catechins using microwave-assisted and ultrasonic extraction from green tea leaves: An optimization study by the IV-optimal design. Food Sci. Biotechnol. 2017, 26, 1281–1290.
More
Information
Subjects:
Chemistry, Analytical
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
4.9K
Entry Collection:
Extraction Techniques in Sample Preparation
Revisions:
2 times
(View History)
Update Date:
17 Feb 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





