Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | pan xinhui | -- | 4638 | 2023-02-08 10:08:11 | | | |
| 2 | Conner Chen | Meta information modification | 4638 | 2023-02-09 08:41:29 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Wang, X.; Hu, Q.; Tang, H.; Xinhui, P. Antitumour Activity of Natural Products Containing Isoxazole/Isoxazoline Moiety. Encyclopedia. Available online: https://encyclopedia.pub/entry/40972 (accessed on 07 February 2026).
Wang X, Hu Q, Tang H, Xinhui P. Antitumour Activity of Natural Products Containing Isoxazole/Isoxazoline Moiety. Encyclopedia. Available at: https://encyclopedia.pub/entry/40972. Accessed February 07, 2026.
Wang, Xiyue, Qingyun Hu, Hui Tang, Pan Xinhui. "Antitumour Activity of Natural Products Containing Isoxazole/Isoxazoline Moiety" Encyclopedia, https://encyclopedia.pub/entry/40972 (accessed February 07, 2026).
Wang, X., Hu, Q., Tang, H., & Xinhui, P. (2023, February 08). Antitumour Activity of Natural Products Containing Isoxazole/Isoxazoline Moiety. In Encyclopedia. https://encyclopedia.pub/entry/40972
Wang, Xiyue, et al. "Antitumour Activity of Natural Products Containing Isoxazole/Isoxazoline Moiety." Encyclopedia. Web. 08 February, 2023.
Copy Citation
Isoxazoles and isoxazolines are five-membered heterocyclic molecules containing nitrogen and oxygen. Isoxazole and isoxazoline are the most popular heterocyclic compounds for developing novel drug candidates. As biosynthetic technology has advanced, A growing number of natural products are being developed for cancer therapy as clinical candidates. As a result, scientists have created several isoxazole and isoxazoline derivatives with anti-cancer properties based on natural products.
isoxazole
isoxazoline
natural products
1. Introduction
Isoxazole is a heterocyclic compound with a five-membered ring that has oxygen and nitrogen atoms at the 1 and 2 positions, and their partially saturated analogs are known as isoxazoline. Many biologically active products contain derivatives of these heterocyclic compounds [1][2][3]. Derivatives containing isoxazole/isoxazoline fragments possess biological activities such as anticancer [4][5], anti-inflammatory [6][7], antibacterial [8][9], anti-Alzheimer’s disease [10][11], antioxidant [12][13], insecticidal [14], antifungal [15][16], and antidiabetic [17][18]. Isoxazolines and isoxazoles have unique electron-rich aromatic structures and have received much attention [19][20]. They make potential candidates for ring cleavage because of their weak nitrogen–oxygen bonds and aromatic character. Thus, isoxazoles and isoxazolines are particularly valuable intermediates in numerous synthetic methods of bioactive chemicals because this isoxazole ring system makes it easy to modify the substituents in their ring structures [5]. Their unusual architectures enable high-affinity binding to many targets or multiple distinct receptors, which aids in the development of innovative medications with original therapeutic applications. Therefore, chemists have been interested in developing and testing isoxazole- and isoxazoline-containing compounds with a variety of medicinal benefits [21].
In the drug discovery process, natural products play a significant role. Biologically active natural products can be obtained from plants, marine organisms, or microorganisms, and they are a vital source of drug discovery [22]. However, most directly extracted and isolated unmodified natural products cannot be directly used for clinical treatment of diseases due to the low pharmacological activity or excessive adverse effects of most natural products. Structural modifications can be used to improve the physicochemical properties and biological activity to reduce the adverse effects and improve the drug selectivity of natural products. They may also exhibit completely different biological activities from the parent [23]. Given the importance of the isoxazole/isoxazoline backbone in natural products and the remarkable bioactivity, an increasing number of studies have reported the modification of natural products by isoxazole/isoxazoline rings [24].
2. Antitumour Activity
Malignant tumors are extremely complex, and they can have a major impact on people’s life and health. The incidence of cancer has been on the rise, and despite the availability of many drugs and treatments for cancer, it remains one of the greatest threats to human health. Natural products or their derivatives make up over 65% of all anti-cancer medications. Natural products thus serve a crucial clinical role in the treatment of cancer. As biosynthetic technology has advanced, A growing number of natural products are being developed for cancer therapy as clinical candidates [25]. As a result, scientists have created several isoxazole and isoxazoline derivatives with anti-cancer properties based on natural products.
Maslinic acid (MA)(Figure 1) and oleanolic acid (OA) (Figure 2) can be isolated from the natural Olea europaea L. MA as well as OA have been found to have anti-cancer and anti-inflammatory properties. A number of isoxazole-containing pentacyclic triterpene derivatives were created and examined by Chouaïb and coworkers. The majority of the isoxazoles, especially those generated from MA, showed remarkable anti-cancer activity in tests on the cancer cell lines EMT-6 and SW480. Isoxazole derivatives of MA 1a, 1b, 1c, and 1d (Figure 1) showed better anti-cancer properties against SW480 cell line compared to the starting substrate MA (viability (%/control) = 9, 9, 10, and 10%, respectively, 30 µM). However, only compounds 1d and 1c showed higher activity than MA against EMT6 (breast) (viability (%/control) = 5 and 64%, respectively, 10 µM). The scientists also performed an anti-proliferative assessment on the cancer cell lines EMT-6 and SW480 and documented the synthesis of OA isoxazole derivatives. However, according to in vitro cytotoxicity testing, OA contains a more potent anti-proliferative active compound than its isoxazole derivatives [26]. In another study, A number of OA nitrogen heterocyclic derivatives with nitrogen heterocycles at C-2 and C-3 were created by Mallavadhani et al. According to the study, pyrimidine derivatives had much greater activity than isoxazoles derivative 2 (Figure 2) against seven cancerous cell lines. The pyrimidine derivatives stopped the cell cycle and caused apoptosis in MCF cells during the S phase, according to the flow cytometric study [27].
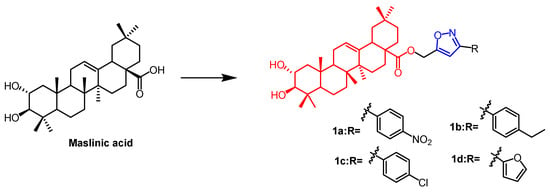
Figure 1. The chemical structure and derivative of maslinic. (The red marker in the figure indicates the parent structure of MA, and the blue marker indicates the structural modification of isoxazole.) [26].
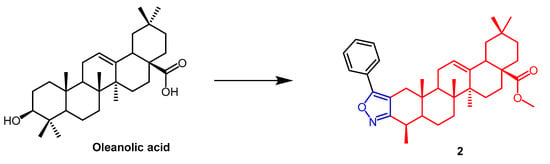
Figure 2. The chemical structure and derivatives of oleanolic. (The red marker in the figure indicates the parent structure of OA, and the blue marker indicates the structural modification of isoxazole.) The same explanation for the following figures [27].
Streptomyces sp. extract vegfrecine (Figure 3) as a VEGF receptor tyrosine kinase inhibitor. It exhibits potent in vitro inhibitory activity against VEGFR-1 and VEGFR-2 tyrosine kinases by blocking VEGFR-1 signaling which inhibits pathological angiogenesis associated with cancer and tumor metastasis. Adachi et al. synthesized a natural quinone compound containing an isoxazole ring through the structure of vegfrecine. Compound 3 (Figure 3), with a quinone ring thickened with an isoxazole ring, exhibited moderate inhibitory activity against VEGFR-1 tyrosine kinase (IC50 = 0.65 µM) and reduced inhibitory activity against VEGFR-2 tyrosine kinase compared to vegfrecine (IC50 = 7.1 µM). It is explained by the structure–activity relationship (SAR) that the isoxazole ring formed by the aminocarbonyl and amino groups which inhibits VEGFR-1 and VEGFR-2 tyrosine kinases by fixing the orientation of the aminocarbonyl and amino groups [28].
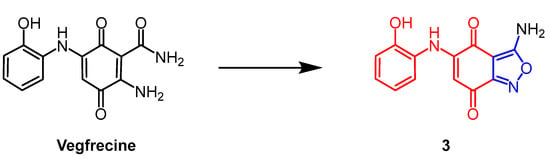
Figure 3. The chemical structure and derivatives of vegfrecine [28].
Tyrosol (Figure 4) is a natural phenolic compound obtained from various plants. In the study by Aissa et al., 3,5-disubstituted isoxazole derivatives (4a–e) (Figure 4) were synthesized from tyrosol, of which compounds 4c, 4b, and 4a showed the greatest antiproliferative properties with IC50 values of 67.6 µM, 42.8 µM, and 61.4 µM, respectively. The derivative 4c was superior to the positive drug than temozolomide (IC50 = 53.85 µM). The newly synthesized compounds exerted anticancer activity by inducing apoptosis in U87 cells. It was found that methyl, methoxy, or chloride substitutions on the R group of isoxazole derivatives enhanced their activity against U87 cells based on SAR studies [29].
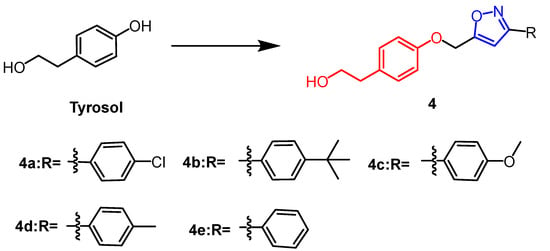
Figure 4. The chemical structure and derivatives of tyrosol [29].
A flavonoid derived from the seeds of Hydnocarpus wightiana Blume is called hydnocarpin (Hy) (Figure 5). It was reported that Hy exhibit antitumor effects [30]. Arya et al. modified Hy as the structural basis by introducing isoxazole rings to develop a series of new compounds. The new derivatives induce apoptosis and arrest the cell cycle at G2/M and S phases in human metastatic melanoma (A375) and human lung adenocarcinoma (A549) cells. One of the most potent compounds was compound 5 (Figure 5), which inhibited A375 at IC50 values of 3.6 and 0.76 µM at 24 h and 48 h, respectively, about 18–60 fold higher than Hy. In conclusion, the results indicate that attaching isoxazole to naturally occurring hydnocarpin enhanced the selectivity and cytotoxicity of the derivatives against A549 and A375 cells [31].
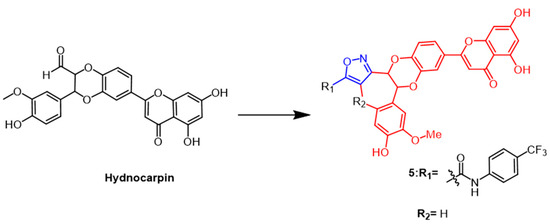
Figure 5. The chemical structure and derivatives of hydnocarpin [31].
Forskolin (Figure 6) is a natural product of the labdane diterpene. Its antiproliferative activity was shown to be mediated by the tumor suppressor protein p53. Therefore Burra et al. introduced new isoxazoles by 1,3-dipole cycloaddition reactions at C1-OH of forskolin and tested their activity against breast cancer cell lines. The parental forskolin was active against MCF-7 cells with an IC50 of 63.3 µM, but did not exhibit any anticancer activity against BT-474 cells (IC50 > 100 µM). Among the derivatives tested in this study, the compound 6 (Figure 6) with an acetyl group at the 7th position displayed the highest activity against MCF-7 and BT-474 cell lines, exhibiting an IC50 of 0.5 µM [32].
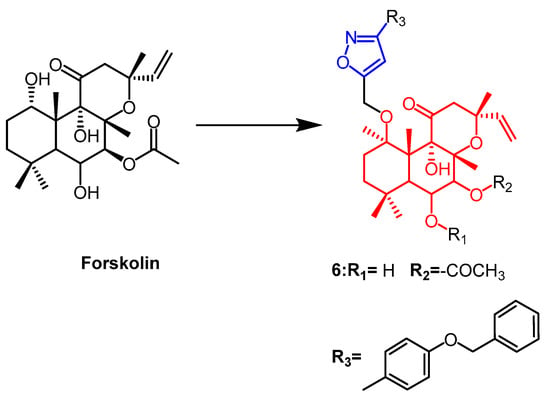
Figure 6. The chemical structure and derivatives of forskolin [32].
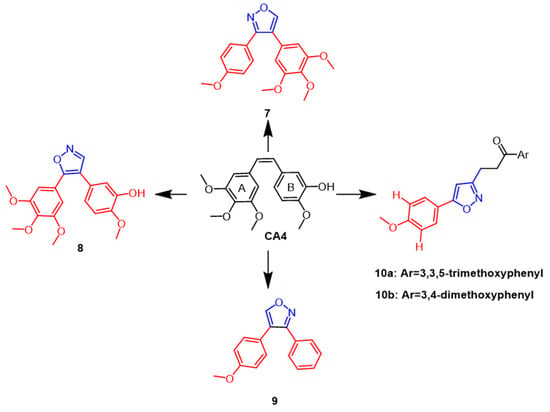
A combretastatin A-4 (CA4) (Figure 7) isolated from the bark of the African willow tree Combretum caffrum is being developed as a potent natural cytostatic agent that blocks and apoptoses cancer cells in the G2/M phase. According to SAR studies, the cis double bonds bound to 3,4,5-trimethoxyphenyl ring A as well as the 4-methoxyphenyl ring B are essential to CA4’s anti-mitotic microtubule destabilizing activity. Using in vivo sea urchin embryo experiments to examine the isoxazole ring modification to CA4, Chernysheva et al. came to the conclusion that CA4 showed anti-mitotic micro-tubule destabilization at a minimum effective concentration (MEC) of 0.002 µM. Chernysheva et al. modified CA4 by introducing isoxazole rings and evaluated them using in vivo sea urchin embryo assays and concluded that CA4 exhibited anti mitotic microtubule destabilization at a minimum effective concentration (MEC) of 0.002 µM, while isoxazole derivatives 7 and 9 (Figure 7) caused altered division in sea urchin embryos at 0.005 µM and 0.02 µM, respectively [33]. In the same year, a new series of CA4 derivatives, including diaryl pyrazoles, isoxazoles, and pyrroles, were reported in the literature. To evaluate the antimitotic efficacy of their drugs against microtubules, a panel of human cancer cells and in vivo sea urchin embryo experiments were performed. The strongest antimitotic agent among the isoxazole derivatives was discovered to be compound 8 (Figure 7) (EC = 0.001 µM), possessing better antiproliferative activity than CA4 (EC = 0.002 µM) while also exhibiting comparable cytotoxicity against human cancer cells. According to structure–activity relationship studies, in the 4,5-diarylisoxazole series, removing the 3-hydroxyl group from ring B decreases the antimitotic activity, and the 3-hydroxyl group is required for antiproliferative activity [34]. Silyanova and colleagues found that only the isoxazole heterocycle and the unsubstituted benzene ring next to the heteroatom conferred the appropriate conformation of the molecule to exert antiproliferative effects through the microtubule destabilization mode of action. The 4,5-diarylisoxazoles showed greater antimitotic activity than 3,4-diarylisoxazoles [35]. In 2018, isoxazole chalcone derivatives with structural similarities to CA4 were synthesized. According to the results, the new synthesized compounds 10a and 10b (Figure 7) exhibited potent cytotoxic activity against DU145 prostate cancer cell lines with IC50 values of 0.96 µM and 1.06 µM, respectively, compared to the positive control (IC50 =4.10 µM). According to structure–activity relationship studies, electron-giving on the benzene ring groups, such as methoxy substituents, enhanced the anticancer activity [36].
Indirubin is an active ingredient in Chinese medicine formulas with good anti-cancer properties. Meisoindigo (Figure 8) is derived from Indirubin and is effective against cancer. Therefore, Tang et al. synthesized a series of 3-subunit indoleacetamides using meisoindigo as a structural template. Different cancer cell lines were tested by researchers to see if they were cytotoxic. Such compounds arrest the cell cycle in the G1 phase and subsequently trigger cystatinase-dependent apoptosis. Compound 11 (Figure 8) showed the best activity in the series with IC50 values of 2.3, 2.7, 2.2, 3.6, and 3.6 µM against MCF-7, Hep3B, KB, SF-268, and MKN-48 cancer cell lines, respectively. In comparison with meisoindigo, compound 11 had better antiproliferative properties against these five cancer cell lines (IC50 = 25.5, 9.0, 19.2, 37.0, 36.7 µM). As a result of adding isoxazole to precursor compounds, their antiproliferative properties were enhanced [37].
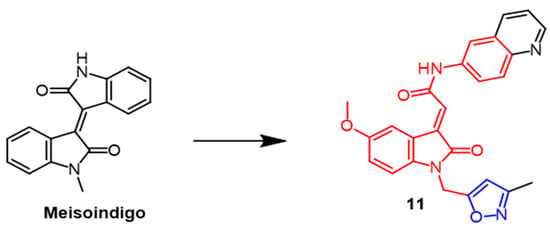
Figure 8. The chemical structure and derivatives of meisoindigo [37].
Dai et al. identified many novel bromotyrosine-derived compounds from the Indonesian sponge. The isolated compound purpuramine N (Figure 9) was modified by oxidation of aromatic groups to produce derivative 12 (Figure 9) containing an isoxazoline fraction. Unfortunately, NIH3T3 cells (normal mouse fibroblasts) were inhibited by compound 12, so this compound was not further investigated. However, further studies could be conducted with compound 12 due to its moderate inhibition of aspartate protease BACE1 (memapsin-2) [38].
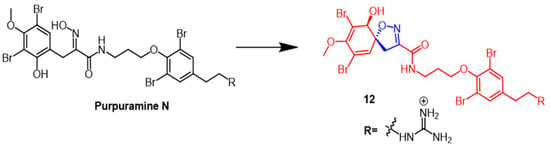
Figure 9. The chemical structure and derivatives of pupuramine N [38].
There are several biological activities associated with bis-indole alkaloids, which are sponge metabolites. Several novel bis-indolyl-isoxazoles and bis-indolyl-furans were synthesized and evaluated as antitumor agents in 10 human tumor cell lines, according to Diana et al. The most potent compound among the isoxazole derivatives was 13 (Figure 10), displaying mean IC50 values of 53.2 µM. Compound 13 was detected to have selective activity against A549 and LXFA 629L (lung) and UXF 1138L (uterine body). The results indicated that the bisindolyl isofuran derivatives exhibited more significant antitumor activity than isoxazole derivatives against human tumor cell lines [39].
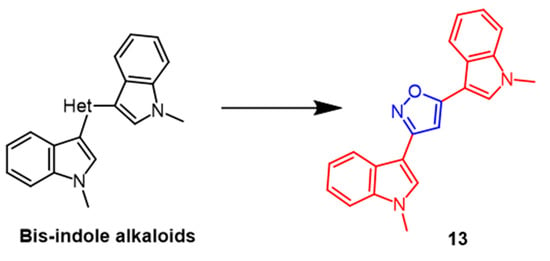
Figure 10. The chemical structure and derivatives of bis-indole alkaloids [39].
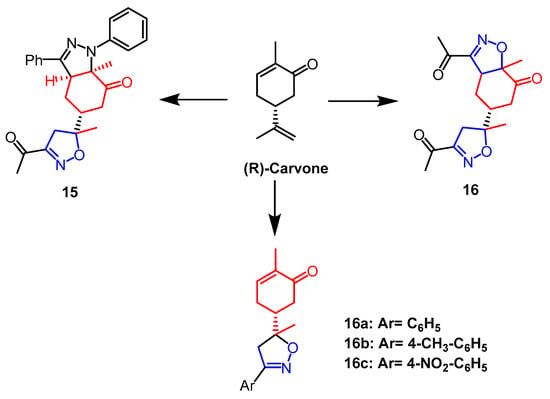
(R)-Carvone belongs to a group of monoterpenes that are present in many natural products and bioactive molecules. An array of derivatives of monoterpenes was synthesized by Fawzi et al. Through MTT assays, all synthesized molecules were evaluated for cytotoxicity against HT-1080, A-549, MCF-7, and MDA-MB-231 cells, which concluded that compound 15 (Figure 11), an isoxazole-pyrazole heterodimer, had no significant activity against all selected cancer cell lines without significant activity. In terms of growth inhibition, compound 14 (Figure 11) was the strongest with IC50 values of 22.47, 25.87, 19.19, and 20.79 µM, respectively. Analyses of the SAR process revealed that the two isoxazoline parts of compound 14 are responsible for the cytotoxicity of the compound on human cancer cells. Further studies by flow cytometry showed that compound 14 caused MCF-7 cancer cells and MDA-MB-231 cancer cells to arrest in the S and G2/M phases of the cell cycle, as well as induced early apoptosis of MCF-7 and MDA-MB-231 through caspase-3/7 activation [40]. In research by Oubella and colleagues, chiral isoxazolines and pyrazole derivatives with monoterpene backbones were efficiently synthesized from (R)-Carvone. Human HT1080, MCF-7, and A-549 cancer cells were used as test subjects for the newly synthesized monoterpene isoxazoline and pyrazole derivatives’ cytotoxic properties. Among them, isoxazoline derivatives 16a, 16b, and 16c (Figure 11) showed the best anticancer activity against HT1080 cells with IC50 values of 16.1 µM, 10.72 µM, and 9.02 µM, respectively. In contrast, pyrazole derivatives were less active in HT1080 cells, all with IC50 values over 100 µM. In HT-1080 cells, isoxazoline derivatives exhibited a greater anticancer activity than pyrazole derivatives [41].
(–)-α-Santonin is a sesquiterpene lactone compound derived from various Asian plants. Recently, it was shown by flow cytometry that naturally occurring santonin can cause G2/M phase arrest of SK-BR-3 cancer cells in the cell cycle while inhibiting the expression of cell cycle proteins A and B1 and also exerts anticancer effects by blocking the Raf/MEK/ERK pathway in breast cancer cells [42]. In a recent study, Khazir et al. synthesized new spirocyclic derivatives of the human santonin and tested their anticancer activity against cancer cell lines Among them, spiroisoxazoline derivative 17 (Figure 12) showed good activity against MCF-7 and A549 cell lines with IC50 values of 0.02 and 0.2 µM, respectively. As a result, compound 17 is promising as a new anticancer drug [43].
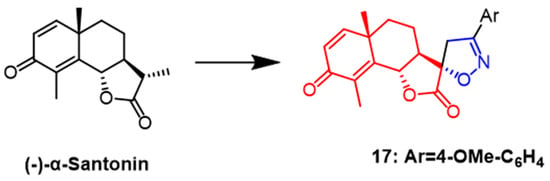
Figure 12. The chemical structure and derivatives of (–)-α-santonin [43].
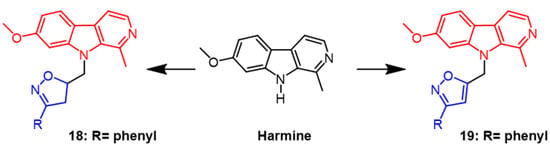
Harmine can be extracted from natural Peganum harmala seeds. According to research, it has significant cytotoxic activity against cancer cell lines and can induce the G2/M cell cycle arrest in breast cancer cells by regulating MAPK and AKT/FOXO3a signaling pathways [44]. Harmine was used as a scaffold to generate derivatives containing isoxazoline, and its cytotoxicity against MCF7 breast cancer and HCT116 colon cancer was assessed. As a result of the tests, Harbin had potent cytotoxicity on both cells with IC50 values of 0.7 µM and 1.3 µM, respectively. Among the synthesized derivatives, derivative 18 (Figure 13) with a benzene ring in the isoxazoline part showed the best activity with IC50 values of 9.7 µM and 0.2 µM, respectively. According to the report, the cytotoxic activity of the derivative isoxazoline part decreases when the aromatic system of the derivative bears’ methyl, methoxy, and Cl atoms in the para position, respectively [45]. Next, the group synthesized isoxazole derivatives from harmine and evaluated ovarian cancer (OCVAR-3), breast cancer (MCF-7), and colon cancer (HCT 116) cell lines using MTT assays. Among the synthesized derivatives, compound 19 (Figure 13) showed the best activity with IC50 values of 5.0, 16.0, and 5.0 μM, respectively. It can be seen that the isoxazoline derivatives showed more bioactivity in the colon cancer (HCT 116) cell line [46].
Plants contain betulin, which is a pentacyclic triterpene naturally occurring in many species. Lugiņina et al. synthesized dense heterocyclic derivatives based on this natural compound. The isoxazole ring was joined to the betulin scaffold by altering the triterpene ring A. Using the MTT assay, the cytotoxic activity of each derivative was evaluated against the human cancer cell lines RD TE32, A549, MS, HEp-2, and HCT 116. Among them, the N-acetyl triazole of betulin showed the strongest activity with IC50 2.3–7.5 µM, whereas the thickened isoxazole ring derivative 20 (Figure 14) was not as active as the triazole derivatives with IC50 values of 7.9–22.1 µM. The synthetic and cytotoxic activity of betulin derivatives containing the isoxazole fraction was reported by Lugiņina et al. Five tumor cell lines were used to test the effectiveness of all derivatives (A-549, MDA-MB-231, MCF-7, KB, and KB-VIN). Compound 21a (Figure 14) showed GI50 values of 11.05 ± 0.88 µM against the lung cancer cell line A549. Compounds 21b and 21c (Figure 14) were found against breast cancer MCF7 cells (11.47 ± 0.84 µM) and (14.51 ± 1.42 µM). Compound 21d (Figure 14) was found on cells of breast cancer MCF7 (12.49 ± 1.18 µM) and lung cancer A549 (13.15 ± 1.56 µM). The constitutive relationship indicated that compounds with hydrophilic substituents on the isoxazole ring had stronger cytotoxic activity [47].
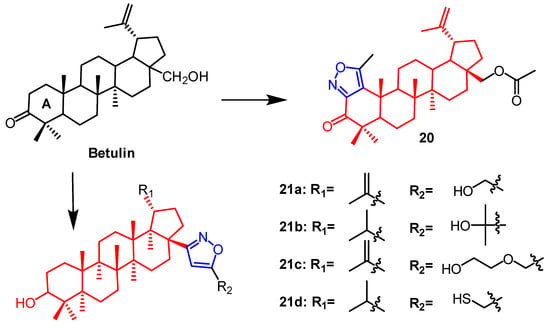
Figure 14. The chemical structure and derivatives of betulin [47].
AD-1 (Figure 15) is a novel ginsenoside discovered to induce G0/G1 cell cycle arrest, apoptosis, and ROS production. Ma et al. introduced various heterocycles containing nitro groups at the C-2 and C-3 positions to synthesize AD-1 derivatives. The AD-1 IC50 value of 14.38 μM for isoxazole derivative 22 (Figure 15) showed solid cellular activity, which enriched our approach to studying the synthesis of AD-1 derivatives [48]. Smirnova et al. synthesized a Dipterocarpus alatus derivative 23 containing the isoxazole fraction and evaluated its cholinesterase inhibitory activity and cytotoxicity. Compound 23 (Figure 16) was found to be cytotoxic to MCF7 (breast cancer) (EC50 = 16.2 µM) and mildly inhibited the enzyme AChE with 15.9% inhibition. This study provides some reference for the structural modification of D. alatus [49].
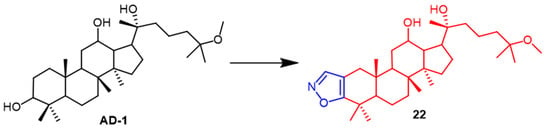
Figure 15. The chemical structure and derivatives of AD-1 [48].
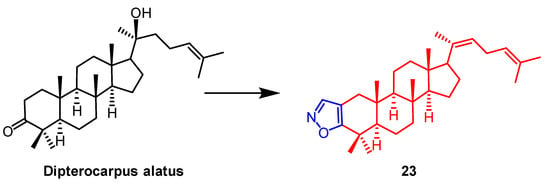
Figure 16. The chemical structure and derivatives of Dispterocarous alatus [49].
The roots of a few wild yams contain large amounts of diosgenin. Diosgenin has received a lot of attention as a possible anticancer drug in recent years. Yildiz et al. synthetically modified the diosgenin backbone to obtain some new derivatives. The findings demonstrated that among these compounds, The pyridine-containing compound had the highest efficacy against human breast cancer (MCF-7), with an IC50 value of 5.72 µM. Compound 24 (Figure 17), containing the isoxazole fraction, also showed potent anticancer activity against human breast cancer (MCF-7) and lung adenocarcinoma (A549) with IC50 values of 9.15 ± 1.30 µM and 14.92 ± 1.70 µM, which was superior to the parent compound diosgenin (IC50 = 26.91 ± 1.84 µM and 36.21 ± 2.42 µM). It can be inferred that the A-ring substituted isoxazole and pyrazole fractions may enhance the anticancer activity of diosgenin derivatives [50].
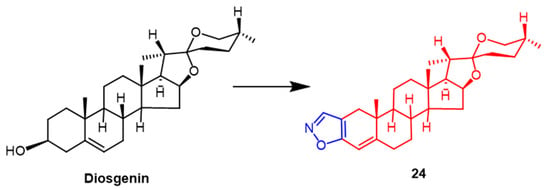
Figure 17. The chemical structure and derivatives of diosgenin [50].
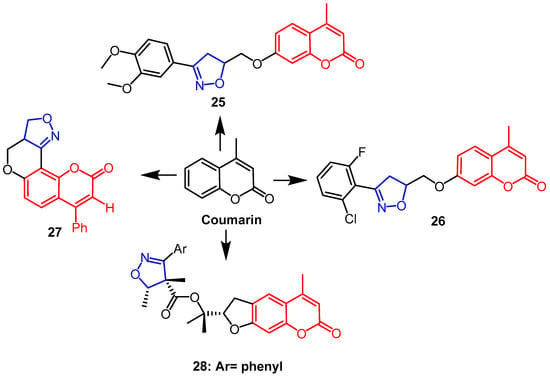
In an in vitro MTS experiment, Lingaraju et al. synthesized coumarin-isoxazoline adducts and assessed their capacity to cause cytotoxicity in human melanoma cancer cell line (UACC 903) and fibroblast normal cell line (FF2441). The findings revealed that compounds 25 and 26 (Figure 18) exhibited better cytotoxicity against UACC 903, with IC50 values of 1.5 µM and 4.5 µM, respectively. Compound 26 was the most active, which may be due to the presence of chlorine and fluorine substitutions in the ortho-positions of the phenyl ring of isoxazoline. Because compound 25 has 3,4-dimethoxy on the benzene ring of the isoxazoline ring, it is more selective for melanoma cancer cells than normal cells, which may explain why compound 25 was the leading contender in this series [51]. Another team synthesized isoxazoline/isoxazole fused coumarin analogs and evaluated their cytotoxicity against human colorectal cancer (Colo-205), human hepatocellular liver cancer (HepG2) and human cervical cancer (HeLa). The findings revealed that compound 27 (Figure 18) had more sensitive activity against the HepG2 cell line than Colo-205 and HeLa cell lines, and it had the highest anti-proliferative activity (IC50 ≤ 50 µM) against all cell lines [52]. (–)-Deltoin is a coumarin-containing natural product extracted from flowers of Ferula lutea (Poir.). As potential anticancer agents based on (–)-deltoin, Znati et al. synthesized coumarin derivatives containing isoxazoline backbones. When it came to the human colon cell line HCT-116, compound 28 (Figure 18) was the most effective (IC50 = 3.3 µM), four times as effective as the parent chemical (IC50 = 14.3 µM). This finding suggests that the introduction of the isoxazoline fraction yielded better anticancer potential [53].
A significant family of compounds known as C-glycosides is present in many natural product architectures and exhibits a wide range of biological activities. Compound 29 (Figure 19), with an IC50 value of 0.67 M, showed the greatest cytotoxicity against MCF-7 breast cancer cells among a series of C-glycoside-linked pyrazoline and isoxazole derivatives synthesized by Kumari et al. It can be seen that pyrazoline partially favors the C-glycoside derivatives in COX-2 enzyme inhibition. The addition of the isoxazole ring to the pyrazoline structure further improves the compounds’ biological activity [54].
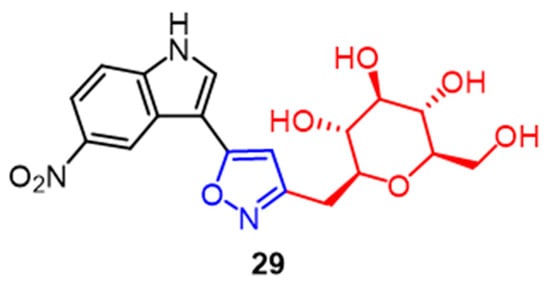
Figure 19. The chemical structure and derivatives of c-glycosides [54].
The spiro-pyrrolidine-oxindole ring system has specific structural properties and potent biological activities. A number of isoxazole derivatives of spiroazolidine-oxindole were created by Liu et al. Using an MTT assay, and they assessed the derivatives’ cytotoxic effects on human leukemia cell K562, human prostate cancer cell PC-3, and human lung cancer cell A549. The outcomes demonstrated that compound 30 (Figure 20) exhibited considerable cytotoxicity against these three cell lines, K562, A549, and PC-3, with IC50 values of 10.7 µM, 21.5 µM, and 13.1 µM, respectively. Isoxazole was added to the sporozoite–oxindole complex, and it inhibited cancer cell proliferation as well as or better than cisplatin (up to 2.1-fold) [55].
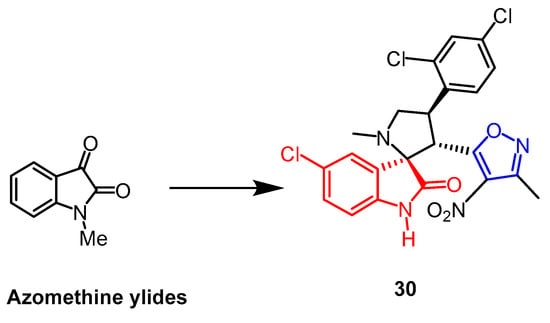
Figure 20. The chemical structure and derivatives of azomethine ylides [55].
It was reported that a series of isoxazole derivatives were synthesized using naturally occurring andrographolide as a backbone by Mokenapelli et al. The cytotoxicity of the derivatives was also evaluated against HCT15, HeLa, and DU145 cell lines. Compounds 31a, 31b, 31c, 31d, and 32 (Figure 21) exhibited significant cytotoxicity against the three cancer cell lines with IC50 values below 40 µg/mL. Therefore, isoxazoline derivatives of andrographolide at the C-14 position are promising as anticancer agents [56].
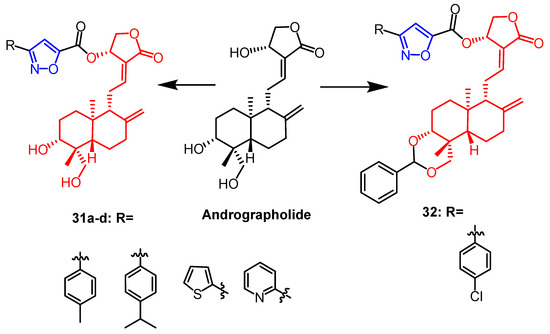
Figure 21. The chemical structure and derivatives of andrographolide [56].
Eugenol is a naturally occurring phenolic monoterpene obtained from clove oil having a variety of biological properties. Using eugenol as a scaffold, Oubella et al. created 1,2,3-triazole mixed isoxazoline derivatives. They next tested and assessed the derivatives’ in vitro anticancer activity against the fibrosarcoma (HT-1080), breast cancer (MCF-7 and MDA-MB-231), and lung cancer (A-549) cell lines. The mixed compounds 33a–d (Figure 22) exhibited more significant cytotoxicity than the triazole derivatives (IC50 = 15–29 µg/mL) against the three cancer cell lines. According to preliminary structural investigations, the simultaneous presence of 1,2,3-triazole and isoxazole produced stronger anticancer effects. Follow-up studies showed that the most potent compound 33a, induced apoptosis through the activation of caspase-3/7, leading to cell cycle arrest in A-549 cancer cells in the G2/M phase [57].
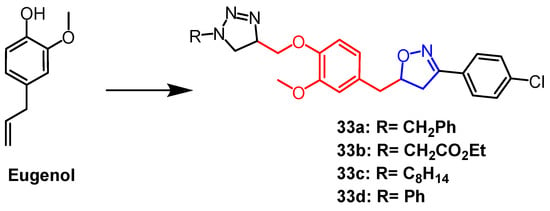
Figure 22. The chemical structure and derivatives of eugenol [57].
Sclareo is a natural product with anticancer activity. As one of the isoxazoline derivatives of sclareol, derivative 34a (Figure 23) showed the strongest anticancer activity with cytotoxic activity (IC50 = 13.20–21.16 µM) against human hepatocellular carcinoma (HepG2), human cholangiocarcinoma (HuCCA-1) and human lung adenocarcinoma (A549) cell lines. When compared to the natural parent chemical sclareol, the findings demonstrated that derivative 34a increased cytotoxicity against cancer cell lines (IC50 = 49.89–70.40 µM). This could be due to the structure of the derivative showing a significant hydrophobicity (due to the aliphatic skeleton) and hydrogen bonding ability (–OH), leading to increased cellular uptake of the compound, resulting in cytotoxicity [58].
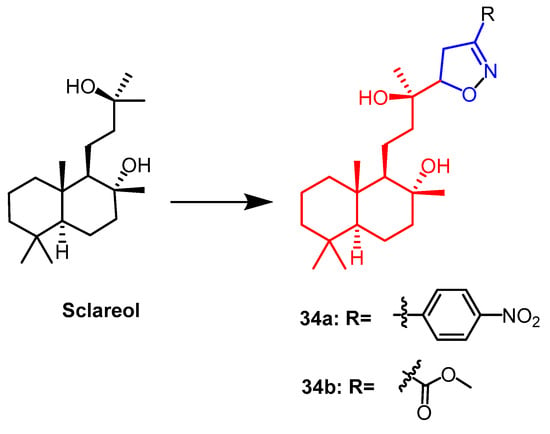
Figure 23. The chemical structure and derivatives of sclareol [58].
Pratap et al. synthesized a series of artemisinin derivatives containing spiroisoxazoline. They evaluated the antiproliferative activity of the newly synthesized derivatives against the human lung cancer cell line (A-549), human colon cancer cell line (HCT-15), and human liver cancer cell line (Hep-G2) by MTT assay. Among all compounds, compound 35a (Figure 24) was found to show significant cytotoxicity against all selected cell lines with IC50 values of 32.43, 4.04, and 46.30 µM, respectively. it can be seen that compound 35a was more sensitive against colon cancer cell line (HCT-15). Compound 35a containing the spiroisoxazoline fraction (IC50 = 4.04 µM) was nine times more active against HCT-15 cell line than the positive drug 5-fluorouracil (IC50 = 35.53 µM). Follow-up DNA cell cycle analysis showed that 35a inhibited cell proliferation in the G2/M phase. It was also found that compound 35b (Figure 24) had significant activity against P. falciparum (IC50 = 0.1 µM) [59].
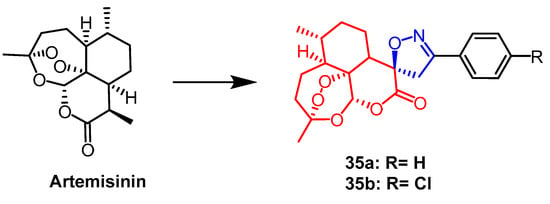
Figure 24. The chemical structure and derivatives of artemisinin [59].
Bromopyrrole alkaloids are an essential family of marine alkaloids with a wide range of biological activities. Rane et al. synthesized a series of isoxazole-containing bromopyrrolidine alkaloid derivatives and evaluated their in vitro antiproliferative activity against five human cancer cell lines by MTT assay. Among them, compound 36a (Figure 25) exhibited the most potent anti-cancer activity. It was able to selectively inhibit oral cancer cell line KB403 with an IC50 of 2.45 µM, whereas compound 36b (Figure 25) (IC50 = 16.58 µM) was found to be selectively cytotoxic against colon cancer cells CaCO2. Therefore, introducing brominated pyrroles into isoxazoles could enhance the anticancer activity of such compounds [60].
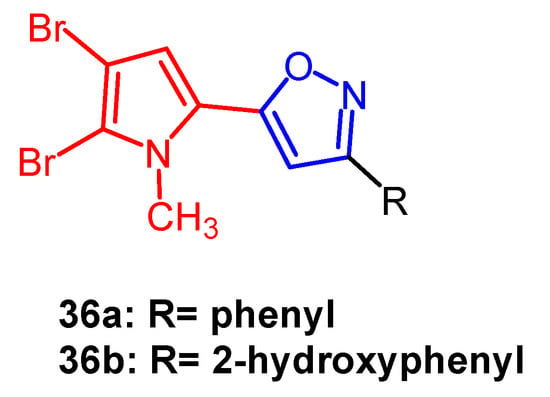
Figure 25. The chemical structure and derivatives of bromopyrrole [60].
Methyl β-orsellinate is a highly functionalized natural phenolic molecule with a variety of biological properties that is present in plants. A series of isoxazole derivatives of methyl β-orsellinate were synthesized by Reddy et al., and four human cancer cell lines as well as the normal cell line HEK-293T (embryonic kidney) were evaluated for their antiproliferative activity in vitro. The majority of these artificial compounds demonstrated antiproliferative efficacy. The most effective combination was found to be compound 37 (Figure 26), whose IC50 against the MCF-7 breast cancer cell line was found to be 5 times higher (IC50 = 7.9 ± 0.07 µM) than the parent compound (IC50 = 46.63 ± 0.11 µM). The constitutive relationship analysis indicated that chlorine substitution at the benzene ring para position gave the compound a better anticancer potential. Compound 37 was shown by flow cytometric analysis to induce apoptosis and arrest the cell cycle in the G2/M phase [61].
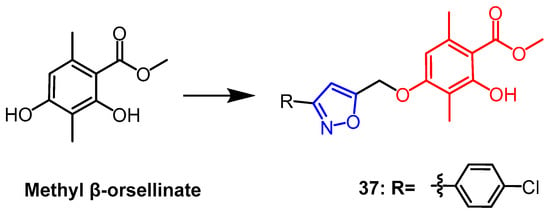
Figure 26. The chemical structure and derivatives of methyl β-orsellinate [61].
The mono-acetate of goniodiol-7-monoacetate was obtained from ethyl acetate extract of Goniothalamus wynaadensis Bedd. Goniodiol diacetate was transformed into a new isoxazoline derivative by Talimarada et al. The MTT test was used to measure the derivatives’ cytotoxic activity against the human cancer cell lines MDA-MB-231, SKOV3, PC-3, and HCT-15, as well as the normal human cell line HEK 293. All isoxazoline derivatives were inhibiting cancer cells (EC50 < 10 µM) without damaging normal cell lines, with compound 38 (Figure 27) showing the strongest activity, compared to the positive control drug vincristine (EC50 = 9.02 µM, 7.00 µM), and compound 38 (EC50 = 6.83 µM, 6.88 µM) on SKOV3 and MDA-MB-231 cell lines exhibited better cytotoxicity. The data suggest that the presence of saturated lactones is essential for activity and that changes in the electron-absorbing or electron-donating groups on the aryl rings of all derivatives have little effect on enhancing cytotoxicity. Further molecular biology studies showed compound 38 stalled the cell cycle in the S phase [62].
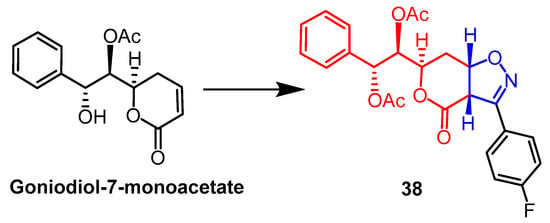
Figure 27. The chemical structure and derivatives of goniodiol-7-monoacetate [62].
A number of spiroisoxazoline derivatives based on the natural substance 1-hydroxy alantolactone have recently been created by Tang et al. Among them, compound 39 (Figure 28) exhibited the most potent antitumor activity with IC50 values of 2.7-5.1 µM against HeLa, PC-3, HEp-2, and HepG2 cells, respectively, which were superior to the parent compound 1β-hydroxy alantolactone (IC50 = 3.2–6.4 µM). Preliminary conformational analysis indicated that oxidation of the lead compound C1-OH would show greater cytotoxicity; the double bond at the C5–C6 position might be more effective for activity. At the same time, the C1-OH esterified derivative would be less potent. Further studies revealed that compound 39 would concentration-dependently inhibit TNF-α-induced NF-κB signaling in PC-3 cancer cells and lead to G2/M phase arrest of PC-3 cancer cells in the cell cycle [63].
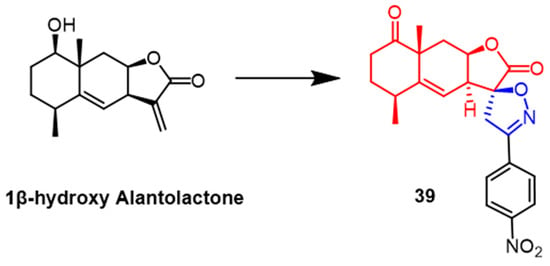
Figure 28. The chemical structure and derivatives of 1β-hydroxy alantolactone [63].
Curcumin is an extremely potent natural product with numerous biological effects. Researchers improved the stability of the compounds by using heterocyclic substitution of the diketone group of curcumin. One of the isoxazole curcumin derivatives, 40 (Figure 29), exhibited potent antitumor activity, with compound 40 (IC50 = 3.97 µM) showing more significant cytotoxicity against the breast cancer cell line (MCF7) compared to the parent compound curcumin (IC50 = 21.89 µM). In addition, compound 40 consistently showed better docking fractions than the other compounds and curcumin. As indicated by preliminary conformational analyses, curcumin’s biological activity was enhanced by the introduction of the isoxazole ring, and isoxazole curcumin is promising as an anti-breast cancer drug [64].
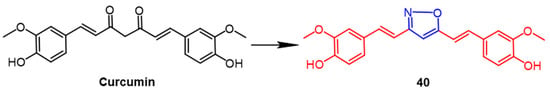
Figure 29. The chemical structure and derivatives of curcumin [64].
References
- Kumar, G.; Shankar, R. 2-Isoxazolines: A Synthetic and Medicinal Overview. Chemmedchem 2021, 16, 430–447.
- Pandhurnekar, C.P.; Pandhurnekar, H.C.; Mungole, A.J.; Butoliya, S.S.; Yadao, B.G. A review of recent synthetic strategies and biological activities of isoxazole. J. Heterocycl. Chem. 2022, 60, 1–29.
- Tilvi, S.; Singh, K.S. Synthesis of Oxazole, Oxazoline and Isoxazoline Derived Marine Natural Products: A Review. Curr. Org. Chem. 2016, 20, 898–929.
- Arya, G.C.; Kaur, K.; Jaitak, V. Isoxazole derivatives as anticancer agent: A review on synthetic strategies, mechanism of action and SAR studies. Eur. J. Med. Chem. 2021, 221, 113511.
- Kaur, K.; Kumar, V.; Sharma, A.K.; Gupta, G.K. Isoxazoline containing natural products as anticancer agents: A review. Eur. J. Med. Chem. 2014, 77, 121–133.
- Abu-Hashem, A.A.; El-Shazly, M. Synthesis of New Isoxazole-, Pyridazine-, Pyrimidopyrazines and their Anti-Inflammatory and Analgesic Activity. Med. Chem. 2018, 14, 356–371.
- Mota, F.V.B.; Neta, M.S.D.; Franco, E.D.; Bastos, I.; da Araujo, L.C.C.; da Silva, S.C.; de Oliveira, T.B.; Souza, E.K.; de Almeida, V.M.; Ximenes, R.M.; et al. Evaluation of anti-inflammatory activity and molecular docking study of new aza-bicyclic isoxazoline acylhydrazone derivatives. Medchemcomm 2019, 10, 1916–1925.
- Aarjane, M.; Slassi, S.; Ghaleb, A.; Tazi, B.; Amine, A. Synthesis, biological evaluation, molecular docking and in silico ADMET screening studies of novel isoxazoline derivatives from acridone. Arab. J. Chem. 2021, 14, 103057.
- Shaik, A.; Bhandare, R.R.; Palleapati, K.; Nissankararao, S.; Kancharlapalli, V.; Shaik, S. Antimicrobial, Antioxidant, and Anticancer Activities of Some Novel Isoxazole Ring Containing Chalcone and Dihydropyrazole Derivatives. Molecules 2020, 25, 1047.
- Rastegari, A.; Safavi, M.; Vafadarnejad, F.; Najafi, Z.; Hariri, R.; Bukhari, S.N.A.; Iraji, A.; Edraki, N.; Firuzi, O.; Saeedi, M.; et al. Synthesis and evaluation of novel arylisoxazoles linked to tacrine moiety: In vitro in vivo biological activities against Alzheimer’s disease. Mol. Divers. 2022, 26, 409–428.
- Patil, P.; Thakur, A.; Sharma, A.; Flora, S.J.S. Natural products and their derivatives as multifunctional ligands against Alzheimer’s disease. Drug Dev. Res. 2020, 81, 165–183.
- Gul, M.; Eryilmaz, S. Synthesis, Antioxidant Activity and Theoretical Investigation of Isoxazolines Derivatives of Monoterpenoids. Lett. Org. Chem. 2019, 16, 501–510.
- Pothuri, V.V.; Machiraju, P.V.S.; Rao, V.S.S. Synthesis and Biological Activity of Some Novel Derivatives of 4-dioxin-7-yl)isoxazole-3-yl]benzoic Acid. Russ. J. Gen. Chem. 2020, 90, 889–894.
- Huang, S.S.; Zhu, B.B.; Wang, K.H.; Yu, M.; Wang, Z.W.; Li, Y.Q.; Liu, Y.X.; Zhang, P.L.; Li, S.J.; Li, Y.L.; et al. Design, synthesis, and insecticidal and fungicidal activities of quaternary ammonium salt derivatives of a triazolyphenyl isoxazoline insecticide. Pest Manag. Sci. 2022, 78, 2011–2021.
- Trefzger, O.S.; Barbosa, N.V.; Scapolatempo, R.L.; das Neves, A.R.; Ortale, M.; Carvalho, D.B.; Honorato, A.M.; Fragoso, M.R.; Shuiguemoto, C.Y.K.; Perdomo, R.T.; et al. Design, synthesis, antileishmanial, and antifungal biological evaluation of novel 3,5-disubstituted isoxazole compounds based on 5-nitrofuran scaffolds. Arch. Der Pharm. 2020, 353, 1900241.
- Zhang, T.; Dong, M.Y.; Zhao, J.J.; Zhang, X.F.; Mei, X.D. Synthesis and antifungal activity of novel pyrazolines and isoxazolines derived from cuminaldehyde. J. Pestic. Sci. 2019, 44, 181–185.
- Li, Z.; Liu, C.X.; Shi, W.; Cai, X.G.; Dai, Y.X.; Liao, C.; Huang, W.L.; Qian, H. Identification of highly potent and orally available free fatty acid receptor 1 agonists bearing isoxazole scaffold. Bioorganic Med. Chem. 2018, 26, 703–711.
- Fettach, S.; Thari, F.Z.; Hafidi, Z.; Karrouchi, K.; Bouathmany, K.; Cherrah, Y.; El Achouri, M.; Benbacer, L.; El Mzibri, M.; Sefrioui, H.; et al. Biological, toxicological and molecular docking evaluations of isoxazoline-thiazolidine-2,4-dione analogues as new class of anti-hyperglycemic agents. J. Biomol. Struct. Dyn. 2021, 12, 1072–1084.
- Sysak, A.; Obminska-Mrukowicz, B. Isoxazole ring as a useful scaffold in a search for new therapeutic agents. Eur. J. Med. Chem. 2017, 137, 292–309.
- Tugrak, M.; Gul, H.I.; Bandow, K.; Sakagami, H.; Gulcin, I.; Ozkay, Y.; Supuran, C.T. Synthesis and biological evaluation of some new mono Mannich bases with piperazines as possible anticancer agents and carbonic anhydrase inhibitors. Bioorganic Chem. 2019, 90, 103095.
- Bhardwaj, S.; Bendi, A.; Singh, L. A Study on Synthesis of Chalcone Derived-5-Membered Isoxazoline and Isoxazole Scaffolds. Curr. Org. Synth. 2022, 19, 643–663.
- Chopra, B.; Dhingra, A.K. Natural products: A lead for drug discovery and development. Phytother. Res. 2021, 35, 4660–4702.
- Yao, H.; Liu, J.K.; Xu, S.T.; Zhu, Z.Y.; Xu, J.Y. The structural modification of natural products for novel drug discovery. Expert Opin. Drug Discov. 2017, 12, 121–140.
- Farooq, S.; Ngaini, Z. Synthesis of Benzalacetophenone-based Isoxazoline and Isoxazole Derivatives. Curr. Org. Chem. 2022, 26, 679–692.
- Wang, H.B.; He, Y.; Jian, M.L.; Fu, X.G.; Cheng, Y.H.; He, Y.J.; Fang, J.; Li, L.; Zhang, D. Breaking the Bottleneck in Anticancer Drug Development: Efficient Utilization of Synthetic Biology. Molecules 2022, 27, 7480.
- Chouiab, K.; Romdhane, A.; Delemasure, S.; Dutartre, P.; Elie, N.; Touboul, D.; Ben Jannet, H.; Hamza, M.A. Regiospecific synthesis, anti-inflammatory and anticancer evaluation of novel 3,5-disubstituted isoxazoles from the natural maslinic and oleanolic acids. Ind. Crops Prod. 2016, 85, 287–299.
- Mallavadhani, U.V.; Vanga, N.R.; Jeengar, M.K.; Naidu, V.G.M. Synthesis of novel ring-A fused hybrids of oleanolic acid with capabilities to arrest cell cycle and induce apoptosis in breast cancer cells. Eur. J. Med. Chem. 2014, 74, 398–404.
- Adachi, H.; Nosaka, C.; Atsumi, S.; Nakae, K.; Umezawa, Y.; Sawa, R.; Kubota, Y.; Nakane, C.; Shibuya, M.; Nishimura, Y. Structure-activity relationships of natural quinone vegfrecine analogs with potent activity against VEGFR-1 and-2 tyrosine kinases. J. Antibiot. 2021, 74, 734–742.
- Aissa, I.; Abdelkafi-Koubaa, Z.; Chouaib, K.; Jalouli, M.; Assel, A.; Romdhane, A.; Harrath, A.H.; Marrakchi, N.; Ben Jannet, H. Glioblastoma-specific anticancer activity of newly synthetized 3,5-disubstituted isoxazole and 1,4-disubstituted triazole-linked tyrosol conjugates. Bioorg. Chem. 2021, 114, 105071.
- Mathai, B.M.; Joseph, M.M.; Maniganda, S.; Nair, J.B.; Arya, J.S.; Karunakaran, V.; Radhakrishnan, K.V.; Maiti, K.K. Guanidinium rich dendron-appended hydnocarpin exhibits superior anti-neoplastic effects through caspase mediated apoptosis. RSC Adv. 2016, 6, 52772–52780.
- Arya, J.S.; Joseph, M.M.; Sherin, D.R.; Nair, J.B.; Manojkumar, T.K.; Maiti, K.K. Exploring Mitochondria-Mediated Intrinsic Apoptosis by New Phytochemical Entities: An Explicit Observation of Cytochrome c Dynamics on Lung and Melanoma Cancer Cells. J. Med. Chem. 2019, 62, 8311–8329.
- Burra, S.; Voora, V.; Rao, C.P.; Vijay Kumar, P.; Kancha, R.K.; David Krupadanam, G.L. Synthesis of novel forskolin isoxazole derivatives with potent anti-cancer activity against breast cancer cell lines. Bioorg. Med. Chem. Lett. 2017, 27, 4314–4318.
- Chernysheva, N.B.; Maksimenko, A.S.; Andreyanov, F.A.; Kislyi, V.P.; Strelenko, Y.A.; Khrustalev, V.N.; Semenova, M.N.; Semenov, V.V. Regioselective synthesis of 3,4-diaryl-5-unsubstituted isoxazoles, analogues of natural cytostatic combretastatin A4. Eur. J. Med. Chem. 2018, 146, 511–518.
- Semenova, M.N.; Demchuk, D.V.; Tsyganov, D.V.; Chernysheya, N.B.; Samet, A.V.; Silyanova, E.A.; Kislyi, V.P.; Maksimenko, A.S.; Varakutin, A.E.; Konyushkin, L.D.; et al. Sea Urchin Embryo Model As a Reliable in Vivo Phenotypic Screen to Characterize Selective Antimitotic Molecules. Comparative evaluation of Combretapyrazoles, -isoxazoles,-1,2,3-triazoles, and -pyrroles as Tubulin-Binding Agents. ACS Comb. Sci. 2018, 20, 700–721.
- Silyanova, E.A.; Ushkarov, V.I.; Samet, A.V.; Maksimenko, A.S.; Koblov, I.A.; Kislyi, V.P.; Semenova, M.N.; Semenov, V.V. A comparative evaluation of monomethoxy substituted o-diarylazoles as antiproliferative microtubule destabilizing agents. Mendeleev Commun. 2022, 32, 120–122.
- Thiriveedhi, A.; Nadh, R.V.; Srinivasu, N.; Kaushal, K. Novel Hybrid Molecules of Isoxazole Chalcone Derivatives: Synthesis and Study of In Vitro Cytotoxic Activities. Lett. Drug Des. Discov. 2018, 15, 576–582.
- Chiou, C.-T.; Lee, W.-C.; Liao, J.-H.; Cheng, J.-J.; Lin, L.-C.; Chen, C.-Y.; Song, J.-S.; Wu, M.-H.; Shia, K.-S.; Li, W.-T. Synthesis and evaluation of 3-ylideneoxindole acetamides as potent anticancer agents. Eur. J. Med. Chem. 2015, 98, 1–12.
- Dai, J.; Parrish, S.M.; Yoshida, W.Y.; Yip, M.L.R.; Turkson, J.; Kelly, M.; Williams, P. Bromotyrosine-derived metabolites from an Indonesian marine sponge in the family Aplysinellidae (Order Verongiida). Bioorg. Med. Chem. Lett. 2016, 26, 499–504.
- Diana, P.; Carbone, A.; Barraja, P.; Kelter, G.; Fiebig, H.-H.; Cirrincione, G. Synthesis and antitumor activity of 2,5-bis(3′-indolyl)-furans and 3,5-bis(3′-indolyl)-isoxazoles, nortopsentin analogues. Bioorg. Med. Chem. 2010, 18, 4524–4529.
- Fawzi, M.; Oubella, A.; Bimoussa, A.; Bamou, F.Z.; Khdar, Z.A.; Auhmani, A.; Riahi, A.; Robert, A.; Morjani, H.; Itto, M.Y.A. Design, synthesis, evaluation of new 3-acetylisoxazolines and their hybrid analogous as anticancer agents: In vitro and in silico analysis. Comput. Biol. Chem. 2022, 98, 107666.
- Oubella, A.; Ait Itto, M.Y.; Auhmani, A.; Riahi, A.; Robert, A.; Daran, J.-C.; Morjani, H.; Parish, C.A.; Esseffar, M.H. Diastereoselective synthesis and cytotoxic evaluation of new isoxazoles and pyrazoles with monoterpenic skeleton. J. Mol. Struct. 2019, 1198, 126924.
- Wu, Z.Q.; Wang, C.C.; Huang, M.Z.; Tao, Z.H.; Yan, W.J.; Du, Y.Q. Naturally Occurring Sesquiterpene Lactone-Santonin, Exerts Anticancer Effects in Multi-Drug Resistant Breast Cancer Cells by Inducing Mitochondrial Mediated Apoptosis, Caspase Activation, Cell Cycle Arrest, and by Targeting Ras/Raf/MEK/ERK Signaling Pathway. Med. Sci. Monit. 2019, 25, 3676–3682.
- Khazir, J.; Singh, P.P.; Reddy, D.M.; Hyder, I.; Shafi, S.; Sawant, S.D.; Chashoo, G.; Mahajan, A.; Alam, M.S.; Saxena, A.K.; et al. Synthesis and anticancer activity of novel spiro-isoxazoline and spiro-isoxazolidine derivatives of α-santonin. Eur. J. Med. Chem. 2013, 63, 279–289.
- Ock, C.W.; Kim, G.D. Harmine Hydrochloride Mediates the Induction of G2/M Cell Cycle Arrest in Breast Cancer Cells by Regulating the MAPKs and AKT/FOXO3a Signaling Pathways. Molecules 2021, 26, 6714.
- Filali, I.; Bouajila, J.; Znati, M.; Bousejra-El Garah, F.; Ben Jannet, H. Synthesis of new isoxazoline derivatives from harmine and evaluation of their anti-Alzheimer, anti-cancer and anti-inflammatory activities. J. Enzyme Inhib. Med. Chem. 2015, 30, 371–376.
- Filali, I.; Romdhane, A.; Znati, M.; Jannet, H.B.; Bouajila, J. Synthesis of New Harmine Isoxazoles and Evaluation of their Potential Anti-Alzheimer, Anti-inflammatory, and Anticancer Activities. Med. Chem. 2016, 12, 184–190.
- Luginina, J.; Linden, M.; Bazulis, M.; Kumpins, V.; Mishnev, A.; Popov, S.A.; Golubeva, T.S.; Waldvogel, S.R.; Shults, E.E.; Turks, M. Electrosynthesis of Stable Betulin-Derived Nitrile Oxides and their Application in Synthesis of Cytostatic Lupane-Type Triterpenoid-Isoxazole Conjugates. Eur. J. Org. Chem. 2021, 2021, 2557–2577.
- Ma, L.; Miao, D.Y.; Lee, J.J.; Li, T.; Chen, Y.; Su, G.Y.; Zhao, Y.Q. Synthesis and biological evaluation of heterocyclic ring-fused dammarane-type ginsenoside derivatives as potential anti-tumor agents. Bioorganic Chem. 2021, 116, 105365.
- Smirnova, I.E.; Kazakova, O.B.; Loesche, A.; Hoenke, S.; Csuk, R. Evaluation of cholinesterase inhibitory activity and cytotoxicity of synthetic derivatives of di- and triterpene metabolites from Pinus silvestris and Dipterocarpus alatus resins. Med. Chem. Res. 2020, 29, 1478–1485.
- Erdagi, S.I.; Yildiz, U. Synthesis, Structural Analysis and Antiproliferative Activity of Nitrogen-Containing Hetero Spirostan Derivatives: Oximes, Heterocyclic Ring-Fused and Furostanes. Chemistryselect 2022, 7, e202200439.
- Lingaraju, G.S.; Balaji, K.S.; Jayarama, S.; Anil, S.M.; Kiran, K.R.; Sadashiva, M.P. Synthesis of new coumarin tethered isoxazolines as potential anticancer agents. Bioorg. Med. Chem. Lett. 2018, 28, 3606–3612.
- Krishna, C.; Bhargavi, M.V.; Rao, Y.J.; Krupadanam, G.L.D. Synthesis of pyrano isoxazoline/isoxazole annulated coumarins via intramolecular nitrile oxide cycloaddition and their cytotoxicity. Russ. J. Gen. Chem. 2017, 87, 1857–1863.
- Znati, M.; Debbabi, M.; Romdhane, A.; Ben Jannet, H.; Bouajila, J. Synthesis of new anticancer and anti-inflammatory isoxazolines and aziridines from the natural (-)-deltoin. J. Pharm. Pharmacol. 2018, 70, 1700–1712.
- Kumari, P.; Mishra, V.S.; Narayana, C.; Khanna, A.; Chakrabarty, A.; Sagar, R. Publisher Correction: Design and efficient synthesis of pyrazoline and isoxazole bridged indole C-glycoside hybrids as potential anticancer agents. Sci. Rep. 2020, 10, 10095.
- Liu, X.-W.; Yao, Z.; Yang, J.; Chen, Z.-Y.; Liu, X.-L.; Zhao, Z.; Lu, Y.; Zhou, Y.; Cao, Y. 1,3-Dipolar cycloaddition enabled isoxazole-fused spiropyrrolidine oxindoles syntheses from 3-methyl-4-nitro-5-alkenyl-isoxazoles and azomethine ylides. Tetrahedron 2016, 72, 1364–1374.
- Mokenapelli, S.; Yerrabelli, J.R.; Das, N.; Roy, P.; Chitneni, P.R. Synthesis and cytotoxicity of novel 14α-O-(andrographolide-3-subsitutedisoxazole-5-carboxylate) derivatives. Nat. Prod. Res. 2021, 35, 3738–3744.
- Oubella, A.; Taia, A.; Byadi, S.; Ait Lahcen, M.; Bimoussa, A.; Essaber, M.; Podlipnik, C.; Morjani, H.; Ait Itto, M.Y.; Aatif, A. Chemical profiling, cytotoxic activities through apoptosis induction in human fibrosarcoma and carcinoma cells, and molecular docking of some 1,2,3-triazole-isoxazoline hybrids using the eugenol as a precursors. J. Biomol. Struct. Dyn. 2022, 40, 1–13.
- Phanumartwiwath, A.; Kesornpun, C.; Sureram, S.; Hongmanee, P.; Pungpo, P.; Kamsri, P.; Punkvang, A.; Eurtivong, C.; Kittakoop, P.; Ruchirawat, S. Antitubercular and antibacterial activities of isoxazolines derived from natural products: Isoxazolines as inhibitors of Mycobacterium tuberculosis InhA. J. Chem. Res. 2021, 45, 1003–1015.
- Pratap, S.; Naaz, F.; Reddy, S.; Jha, K.K.; Sharma, K.; Sahal, D.; Akhter, M.; Nayakanti, D.; Kumar, H.M.S.; Kumari, V.; et al. Anti-proliferative and anti-malarial activities of spiroisoxazoline analogues of artemisinin. Arch. Pharm. 2019, 352, 1800192.
- Rane, R.A.; Sahu, N.U.; Gutte, S.D.; Mahajan, A.A.; Shah, C.P.; Bangalore, P. Synthesis and evaluation of novel marine bromopyrrole alkaloid-based hybrids as anticancer agents. Eur. J. Med. Chem. 2013, 63, 793–799.
- Reddy, S.T.; Mendonza, J.J.; Makani, V.K.K.; Bhadra, M.P.; Uppuluri, V.M. Synthesis of some novel methyl β-orsellinate based 3, 5-disubstituted isoxazoles and their anti-proliferative activity: Identification of potent leads active against MCF-7 breast cancer cell. Bioorg. Chem. 2020, 105, 104374.
- Talimarada, D.; Sharma, A.; Wakhradkar, M.G.; Dhuri, S.N.; Gunturu, K.C.; Sundaram, V.N.N.; Holla, H. Synthesis, DFT analysis and in-vitro anti-cancer study of novel fused bicyclic pyranone isoxazoline derivatives of Goniodiol-diacetate-a natural product derivative. Fitoterapia 2022, 163, 105316.
- Tang, J.-J.; He, Q.-R.; Dong, S.; Guo, X.; Wang, Y.-G.; Lei, B.-L.; Tian, J.-M.; Gao, J.-M. Diversity Modification and Structure-Activity Relationships of Two Natural Products 1β-hydroxy Alantolactone and Ivangustin as Potent Cytotoxic Agents. Sci. Rep. 2018, 8, 1722.
- Rodrigues, F.C.; Kumar, N.V.A.; Hari, G.; Pai, K.S.R.; Thakur, G. The inhibitory potency of isoxazole-curcumin analogue for the management of breast cancer: A comparative in vitro and molecular modeling investigation. Chem. Pap. 2021, 75, 5995–6008.
More
Information
Subjects:
Pharmacology & Pharmacy
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.7K
Revisions:
2 times
(View History)
Update Date:
09 Feb 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No