Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Kathy Tou | -- | 1248 | 2023-01-20 01:04:00 | | | |
| 2 | Conner Chen | -37 word(s) | 1211 | 2023-01-28 09:44:50 | | | | |
| 3 | Conner Chen | Meta information modification | 1211 | 2023-01-29 09:55:18 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Tou, K.; Cawley, A.; Bowen, C.; Bishop, D.P.; Fu, S. Lipids as Biomarkers for Equine Anti-Doping. Encyclopedia. Available online: https://encyclopedia.pub/entry/40429 (accessed on 07 February 2026).
Tou K, Cawley A, Bowen C, Bishop DP, Fu S. Lipids as Biomarkers for Equine Anti-Doping. Encyclopedia. Available at: https://encyclopedia.pub/entry/40429. Accessed February 07, 2026.
Tou, Kathy, Adam Cawley, Christopher Bowen, David P. Bishop, Shanlin Fu. "Lipids as Biomarkers for Equine Anti-Doping" Encyclopedia, https://encyclopedia.pub/entry/40429 (accessed February 07, 2026).
Tou, K., Cawley, A., Bowen, C., Bishop, D.P., & Fu, S. (2023, January 20). Lipids as Biomarkers for Equine Anti-Doping. In Encyclopedia. https://encyclopedia.pub/entry/40429
Tou, Kathy, et al. "Lipids as Biomarkers for Equine Anti-Doping." Encyclopedia. Web. 20 January, 2023.
Copy Citation
The approach to equine anti-doping is focused on the targeted detection of prohibited substances. However, as new substances are rapidly being developed, the need for complimentary methods for monitoring is crucial to ensure the integrity of the racing industry is upheld. Lipidomics is a growing field involved in the characterisation of lipids, their function and metabolism in a biological system. Different lipids have various biological effects throughout the equine system including platelet aggregation and inflammation.
lipidomics
review
analytical
equine
anti-doping
1. Biomarkers for Equine Anti-Doping
The current approach to equine anti-doping is focused on the targeted detection of prohibited substances [1]. However, as new substances are rapidly being developed, the need for complimentary methods of monitoring is important to ensure the integrity of the racing industry is upheld [1]. The use of biomarkers for the detection of doping abuse is a significant advancement for sports anti-doping. Teale et al. [2] define biomarkers as an “individual biological parameter or substance (metabolite, protein or transcript); the concentration of which is indicative of the use or abuse of a drug or therapy”. With the discovery of novel biomarkers for detecting doping abuse, the potential exists for a larger number of drugs to be indirectly detected and over longer periods of time. However, with indirect detection, there is the possibility of the method not being specific and the increased likelihood of inconsistent results [3]. An “omics” approach may provide an alternative to direct detection of doping as maintaining a contemporary scope of testing makes direct detection continually difficult due to availability of reference materials [4][5]. The use of metabolomics has been utilised in many different laboratories to measure metabolites at low levels relative to time-related biological responses of a drug administration [4][5][6]. This provides a framework for non-targeted detection, particularly for drugs that have a short half-life but long lasting effect on any individual system [4].
2. Lipids
Lipidomics is a growing field involved in the characterisation of lipids, their function and metabolism in a biological system [7][8]. Lipids are non-polar molecules with a diverse chemistry and functionality [9][10]. In conjunction with carbohydrates, lipids are the main energy source for equine striated muscles [11]. There are a number of different classes of lipids including monounsaturated fatty acids (MUFAs) and polyunsaturated fatty acids (PUFAs) [12][13]. MUFAs are lipids that have a single double bond present in the compound and usually only exist in seeds or marine organisms, however, are naturally rare [12]. PUFAs comparatively contain more than one double bond, are more commonly found [12] and have various biological effects including platelet aggregation and inflammation [14]. In animals, common PUFAs include arachidonic acid (AA), eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) [12]. The most relevant and important oxygenated products for the racing industry are lipids known as the eicosanoids [12]. Eicosanoids are a large subclass predominately defined by the 20 carbon chain containing over 100 lipid mediators including prostaglandins, thromboxanes, leukotrienes, hydroxy fatty acids and lipoxins [12][15] with the majority derived from AA, an omega-6 fatty acid [16]. Eicosanoids are believed to act as inflammatory mediators since they have the ability to mimic inflammatory symptoms and decrease in the presence of anti-inflammatory drugs [8][14]. Disruption of eicosanoids can cause a range of inflammatory pathological conditions including asthma, chronic obstructive pulmonary disease, fevers, pain, a range of cardiovascular diseases and cancers [15]. Eicosanoids are synthesised at the site of injury in order to control and regulate the inflammatory response [7].
AA is released from membrane phospholipids through the activation of phospholipase A2 enzyme (PLA2) [14]. It can be further converted into other eicosanoids in the cascade (Figure 1). In a non-targeted sense, it should be possible to utilise the AA cascade to determine which lipids are being affected following drug administration. This cascade includes prostaglandin D2 (PGD2) [17], prostaglandin E2 (PGE2) [18], prostaglandin F2α (PGF2α) [7], thromboxane B2 (TXB2) [19], 11-dehydro thromboxane B2 (11-Dehydro TXB2) [20], 6-keto prostaglandin F1α (6-Keto PGF1α) [21], 15(S)-hydroxyeicosatetraenoic acid (15-HETE) [7], 5(S)-hydroxyeicosatetraenoic acid (5-HETE) [22], leukotriene B4 (LTB4) [23][24][25], leukotriene D4 (LTD4) [23][25] and leukotriene E4 (LTE4) [23][25]. The analogues of AA are also of interest including arachidonoyl ethanolamide (AEA) [26][27] and oleoyl ethanolamide (OEA) [26].
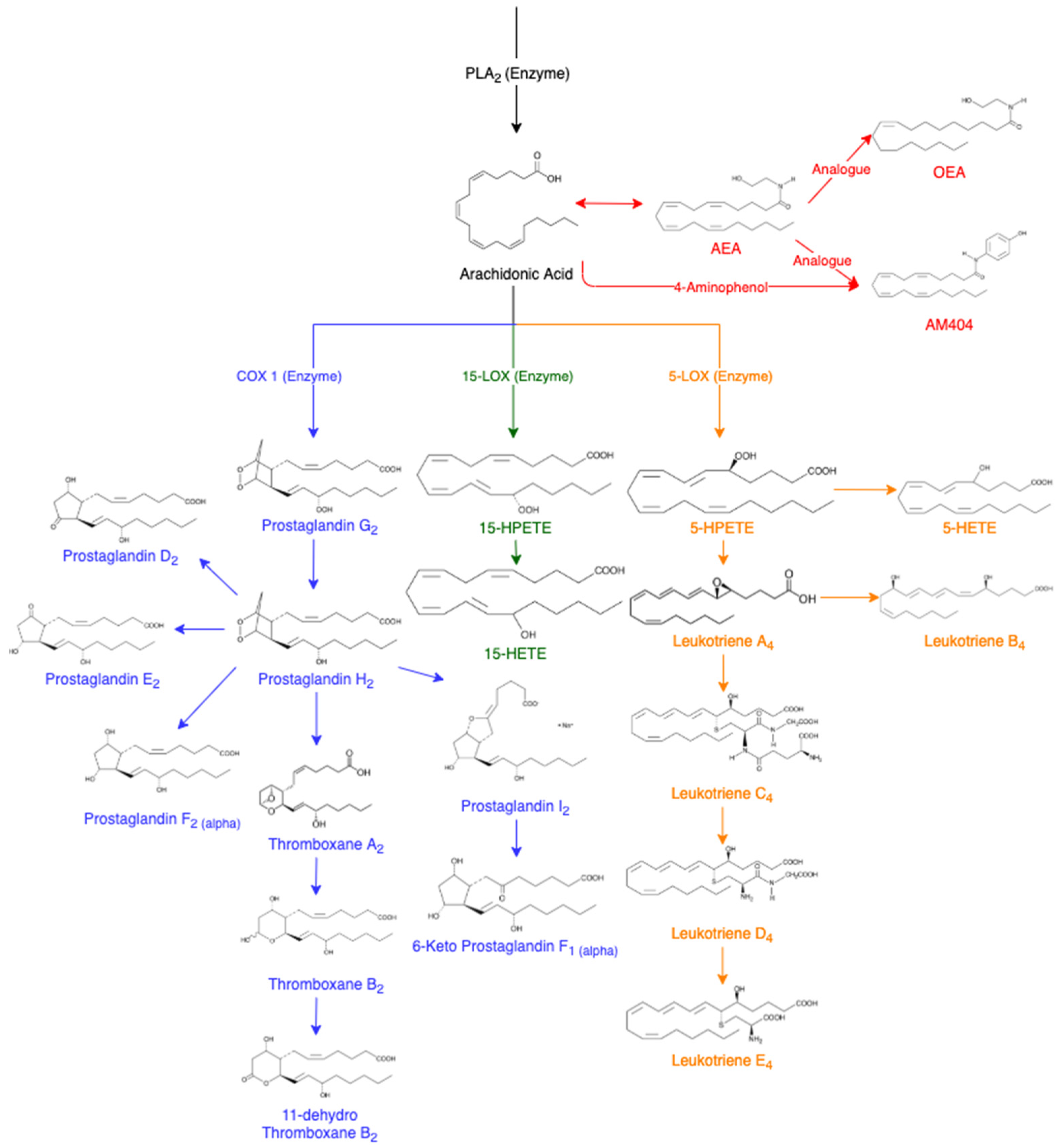
Figure 1. Arachidonic Acid Cascade, adapted from various sources [7][8][14][17][18][19][20][21][22][23][24][25][26][27][28][29]. Abbreviations of eicosanoids: arachidonoyl ethanolamide (AEA), oleoyl ethanolamide (OEA), prostaglandin (PG), thromboxane (Tx), hydroperoxyeicosatetraenoic acids (HPETES), hydroxyeicosatetraenoic acid (HETE) and leukotriene (LT).
AEA is an endogenous cannabinoid ligand that has binding activity resulting in pharmacological effects of tetrahydrocannabinol (THC) such as euphoria and calmness [26][27]. In a variety of cells, cannabinoid agonists have caused an increase in the amount of AA [26]. This has been hypothesised to result from the combination of PLA2 and acyltransferase inhibition [26]. AA can also be converted into N-(4-hydroxyphenyl) arachidonylamide (AM404) which displays analgesic properties and the ability to lower body temperature [28][29]. AM404 is produced when acetaminophen is metabolised in the body to produce p-aminophenol, which is then conjugated with AA [29]. AM404 has been reported to inhibit the cyclooxygenase (COX) pathways leading to the decreased formation of PGE2, demonstrating effectiveness as a COX-2 enzyme inhibitor to reduce the production of prostaglandins by consumption of AA [29]. The COX and lipoxygenase (LOX) pathways are of particular interest for equine anti-doping due to their augmentation following anti-inflammatory treatments.
Prostaglandins are monocarboxylic acids with two side chains at carbon 7 and 8 attached to a central, five-membered ring [14]. Prostaglandins have oxygen-containing substituents in various positions in the molecule with the naming of the prostaglandins ranging from PGA to PGI depending on the basis of the substituents in the ring, and further sub-grouped into three series depending on the degree of unsaturation [14]. Prostaglandins are one of the key compounds in the generation of the inflammatory response due to an increase in concentration in inflamed tissue [30]. Prostaglandins are formed from AA being converted by the COX enzyme to prostaglandin G2 and H2 [17]. Prostaglandin endoperoxide-D-isomerase can convert PGH2 into a mixture of PGD2, PGE2 and PGF2α [17]. PGH2 can also produce prostacyclin (PGI2) that may further metabolise to a more stable compound, 6-keto F1α [19]. PGEs and PGIs have been identified to mimic the signs of inflammation caused by vasodilation and swelling due to an increase in vascular permeability [14].
Prostaglandins can also convert into thromboxane A2 (TXA2), an unstable intermediate in the production of TXB2, which further metabolises to form 11-dehydro TXB2 [20]. Thromboxanes are the major products of prostaglandin endoperoxides in platelets, lungs and the spleen [14]. The production of new platelets could potentially have a high capacity to further increase the synthesis of TXA2, leading to increased amounts of TXB2 and 11-dehydro TXB2 [19]. The use of non-steroidal anti-inflammatory drugs (NSAIDs) results in PGI2 and thromboxane synthesis being inhibited [19].
Using the LOX pathways, AA converts into esterified hydroperoxyeicosatetraenoic acids (HPETEs) [31]. HPETEs are further reduced to their corresponding hydroxyeicosatetraenoic acids (HETEs) [7]. For example, using the 15-LOX enzyme, AA will metabolise into 15-HPETE, and then further metabolises to 15-HETE. Similarly, the 5-LOX enzyme converts AA to 5-HPETE and further to 5-HETE. The leukotrienes are an oxygenated metabolite of polyunsaturated fatty acids, but the initial formation is catalysed by lipoxygenases [23]. Leukotrienes are produced from AA using the 5-lipoxygenase (5-LOX) enzyme to produce Leukotriene A4 (LTA4), an unstable epoxide. LTA4 is hydrolysed to LTB4 or conjugated with glutathione to yield Leukotriene C4 (LTC4) and its metabolites LTD4 and LTE4. Leukotrienes are known for their strong vascular effect with the most effective being LTB4, in comparison to LTC4 and LTD4 [14][15]. In the presence of more leukocytes, the leukotrienes also have a role in the inflammatory process by increasing blood pressure [23].
References
- Fragkaki, A.G.; Kioukia-Fougia, N.; Kiousi, P.; Kioussi, M.; Tsivou, M. Challenges in detecting substances for equine anti-doping. Drug Test. Anal. 2017, 9, 1291–1303.
- Teale, P.; Barton, C.; Driver, P.M.; Kay, R.G. Biomarkers: Unrealized potential in sports doping analysis. Bioanalysis 2009, 1, 1103–1118.
- Narduzzi, L.; Dervilly, G.; Audran, M.; Le Bizec, B.; Buisson, C. A role for metabolomics in the antidoping toolbox? Drug Test. Anal. 2020, 12, 677–690.
- Stojiljkovic, N.; Paris, A.; Garcia, P.; Popot, M.-A.; Bonnaire, Y.; Tabet, J.-C.; Junot, C. Evaluation of horse urine sample preparation methods for metabolomics using LC coupled to HRMS. Bioanalysis 2014, 6, 785–803.
- Reichel, C. OMICS-strategies and methods in the fight against doping. Forensic Sci. Int. 2011, 213, 20.
- Narduzzi, L.; Dervilly, G.; Audran, M.; Le Bizec, B.; Buisson, C. A role for metabolomics in the antidoping toolbox? Drug Test. Anal. 2020, 12, 677–690.
- Mangal, D.; Uboh, C.E.; Soma, L.R. Analysis of bioactive eicosanoids in equine plasma by stable isotope dilution reversed-phase liquid chromatography/multiple reaction monitoring mass spectrometry. Rapid Commun. Mass Spectrom. 2011, 25, 585–598.
- Dass, C. Characterization of Lipids. In Fundamentals of Contemporary Mass Spectrometry; John Wiley & Sons: New York, NY, USA, 2007; pp. 423–451.
- López-Bascón, M.A.; Calderón-Santiago, M.; Díaz-Lozano, A.; Camargo, A.; López-Miranda, J.; Priego-Capote, F. Development of a qualitative/quantitative strategy for comprehensive determination of polar lipids by LC–MS/MS in human plasma. Anal. Bioanal. Chem. 2020, 412, 489–498.
- Koelmel, J.P.; Li, X.; Stow, S.M.; Sartain, M.J.; Murali, A.; Kemperman, R.; Tsugawa, H.; Takahashi, M.; Vasiliou, V.; Bowden, J.A.; et al. Lipid Annotator: Towards Accurate Annotation in Non-Targeted Liquid Chromatography High-Resolution Tandem Mass Spectrometry (LC-HRMS/MS) Lipidomics Using A Rapid and User-Friendly Software. Metabolites 2020, 10, 101.
- Nolazco Sassot, L.; Villarino, N.F.; Dasgupta, N.; Morrison, J.J.; Bayly, W.M.; Gang, D.; Sanz, M.G. The lipidome of Thoroughbred racehorses before and after supramaximal exercise. Equine Vet. J. 2019, 51, 696–700.
- Harwood, J.L.; Frayn, K.N.; Murphy, D.J.; Michell, R.H.; Gurr, M.I. Lipids: Biochemistry, Biotechnology and Health; John Wiley & Sons, Incorporated: Hoboken, UK, 2016.
- Fahy, E.; Cotter, D.; Sud, M.; Subramaniam, S. Lipid classification, structures and tools. Biochim. Et Biophys. Acta. Mol. Cell Biol. Lipids 2011, 1811, 637–647.
- Granström, E. The arachidonic acid cascade. Inflammation 1984, 8, S15–S25.
- Thakare, R.; Chhonker, Y.S.; Gautam, N.; Nelson, A.; Casaburi, R.; Criner, G.; Dransfield, M.T.; Make, B.; Schmid, K.K.; Rennard, S.I.; et al. Simultaneous LC–MS/MS analysis of eicosanoids and related metabolites in human serum, sputum and BALF. Biomed. Chromatogr. 2018, 32, e4102.
- Toewe, A.; Balas, L.; Durand, T.; Geisslinger, G.; Ferreirós, N. Simultaneous determination of PUFA-derived pro-resolving metabolites and pathway markers using chiral chromatography and tandem mass spectrometry. Anal. Chim. Acta 2018, 1031, 185–194.
- Giles, H.; Leff, P. The biology and pharmacology of PGD2. Prostaglandins 1988, 35, 277–300.
- Jackson, C.A.; Colahan, P.T.; Rice, B. Use of a Commercially Available Prostaglandin E2 Enzyme-Linked Immunosorbent Assay for Non-Steroidal Anti-Inflammatory Drug Screening. In Proceedings of the 16th International Conference of Racing Analysts and Veterinarians, Tokyo, Japan, 21–27 October 2006; Volume 16, pp. 477–482.
- Lees, P.; Ewins, C.P.; Taylor, J.B.O.; Sedgwick, A.D. Serum thromboxane in the horse and its inhibition by aspirin, phenylbutazone and flunixin. Br. Vet. J. 1987, 143, 462–476.
- Lopez, L.R.; Guyer, K.E.; Torre, I.G.D.L.; Pitts, K.R.; Matsuura, E.; Ames, P.R. Platelet thromboxane (11-dehydro-Thromboxane B2) and aspirin response in patients with diabetes and coronary artery disease. World J. Diabetes 2014, 5, 115–127.
- Johnson, R.A.; Morton, D.R.; Kinner, J.H.; Gorman, R.R.; McGuire, J.C.; Sun, F.F.; Whittaker, N.; Bunting, S.; Salmon, J.; Moncada, S.; et al. The chemical structure of prostaglandin X (prostacyclin). Prostaglandins 1976, 12, 915–928.
- Connolly, P.J.; Wetter, S.K.; Beers, K.N.; Hamel, S.C.; Chen, R.H.K.; Wachter, M.P.; Ansell, J.; Singer, M.M.; Steber, M.; Ritchie, D.M.; et al. N-Hydroxyurea and hydroxamic acid inhibitors of cyclooxygenase and 5-lipoxygenase. Bioorganic Med. Chem. Letters 1999, 9, 979–984.
- Samuelsson, B.; Dahlen, S.-E.; Lindgren, J.A.; Rouzer, C.A.; Serhan, C.N. Leukotrienes and lipoxins: Structures, biosynthesis, and biological effects. Science 1987, 237, 1171–1176.
- McMillan, R.M.; Foster, S.J. Leukotriene B4 and inflammatory disease. Agents Actions 1988, 24, 114–119.
- Goodman, L.; Coles, T.B.; Budsberg, S. Leukotriene inhibition in small animal medicine. J. Vet. Pharmacol. Ther. 2008, 31, 387–398.
- Felder, C.C.; Briley, E.M.; Axelrod, J.; Simpson, J.T.; Mackie, K.; Devane, W.A. Anandamide, an endogenous cannabimimetic eicosanoid, binds to the cloned human cannabinoid receptor and stimulates receptor-mediated signal transduction. Proc. Natl. Acad. Sci. USA 1993, 90, 7656–7660.
- Beltramo, M.; Stella, N.; Calignano, A.; Lin, S.Y.; Makriyannis, A.; Piomelli, D. Functional role of high-affinity anandamide transport, as revealed by selective inhibition. Science 1997, 277, 1094–1097.
- Sharma, C.V.; Long, J.H.; Shah, S.; Rahman, J.; Perrett, D.; Ayoub, S.S.; Mehta, V. First evidence of the conversion of paracetamol to AM404 in human cerebrospinal fluid. J. Pain Res. 2017, 10, 2703–2709.
- Högestätt, E.D.; Jönsson, B.A.G.; Ermund, A.; Andersson, D.A.; Björk, H.; Alexander, J.P.; Cravatt, B.F.; Basbaum, A.I.; Zygmunt, P.M. Conversion of Acetaminophen to the Bioactive N-Acylphenolamine AM404 via Fatty Acid Amide Hydrolase-dependent Arachidonic Acid Conjugation in the Nervous System. J. Biol. Chem. 2005, 280, 31405–31412.
- Ricciotti, E.; FitzGerald, G.A. Prostaglandins and Inflammation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 986–1000.
- Wang, B.; Wu, L.; Chen, J.; Dong, L.; Chen, C.; Wen, Z.; Hu, J.; Fleming, I.; Wang, D.W. Metabolism pathways of arachidonic acids: Mechanisms and potential therapeutic targets. Signal Transduct. Target. Ther. 2021, 6, 94.
More
Information
Subjects:
Chemistry, Analytical
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
560
Revisions:
3 times
(View History)
Update Date:
29 Jan 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




