The current approach to equine anti-doping is focused on the targeted detection of prohibited substances [
1]. However, as new substances are rapidly being developed, the need for complimentary methods of monitoring is important to ensure the integrity of the racing industry is upheld [
1]. The use of biomarkers for the detection of doping abuse is a significant advancement for sports anti-doping. Teale et al. [
2] define biomarkers as an “individual biological parameter or substance (metabolite, protein or transcript); the concentration of which is indicative of the use or abuse of a drug or therapy”. With the discovery of novel biomarkers for detecting doping abuse, the potential exists for a larger number of drugs to be indirectly detected and over longer periods of time. However, with indirect detection, there is the possibility of the method not being specific and the increased likelihood of inconsistent results [
3].
An “omics” approach may provide an alternative to direct detection of doping as maintaining a contemporary scope of testing makes direct detection continually difficult due to availability of reference materials [5, 6]. The use of metabolomics has been utilised in many different laboratories to measure metabolites at low levels relative to time-related biological responses of a drug administration[3, 5, 6]. This provides a framework for non-targeted detection, particularly for drugs that have a short half-life but long lasting effect on any individual system[5].
2. Lipids
Lipidomics is a growing field involved in the characterisation of lipids, their function and metabolism in a biological system [
7,
8]. Lipids are non-polar molecules with a diverse chemistry and functionality [
9,
10]. In conjunction with carbohydrates, lipids are the main energy source for equine striated muscles [
11]. There are a number of different classes of lipids including monounsaturated fatty acids (MUFAs) and polyunsaturated fatty acids (PUFAs) [
12,
13]. MUFAs are lipids that have a single double bond present in the compound and usually only exist in seeds or marine organisms, however, are naturally rare [
12]. PUFAs comparatively contain more than one double bond, are more commonly found [
12] and have various biological effects including platelet aggregation and inflammation [
14]. In animals, common PUFAs include arachidonic acid (AA), eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) [
12]. The most relevant and important oxygenated products for the racing industry are lipids known as the eicosanoids [
12]. Eicosanoids are a large subclass predominately defined by the 20 carbon chain containing over 100 lipid mediators including prostaglandins, thromboxanes, leukotrienes, hydroxy fatty acids and lipoxins [
12,
15] with the majority derived from AA, an omega-6 fatty acid [
16]. Eicosanoids are believed to act as inflammatory mediators since they have the ability to mimic inflammatory symptoms and decrease in the presence of anti-inflammatory drugs [
8,
14]. Disruption of eicosanoids can cause a range of inflammatory pathological conditions including asthma, chronic obstructive pulmonary disease, fevers, pain, a range of cardiovascular diseases and cancers [
15]. Eicosanoids are synthesised at the site of injury in order to control and regulate the inflammatory response [
7].
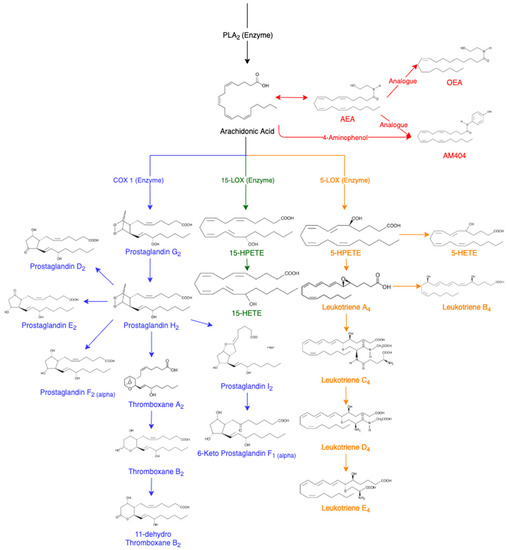
AA is released from membrane phospholipids through the activation of phospholipase A
2 enzyme (PLA
2) [
14]. It can be further converted into other eicosanoids in the cascade (
Figure 1). In a non-targeted sense, it should be possible to utilise the AA cascade to determine which lipids are being affected following drug administration. This cascade includes prostaglandin D
2 (PGD
2) [
17], prostaglandin E
2 (PGE
2) [
18], prostaglandin F
2α (PGF
2α) [
7], thromboxane B
2 (TXB
2) [
19], 11-dehydro thromboxane B
2 (11-Dehydro TXB
2) [
20], 6-keto prostaglandin F
1α (6-Keto PGF
1α) [
21], 15(S)-hydroxyeicosatetraenoic acid (15-HETE) [
7], 5(S)-hydroxyeicosatetraenoic acid (5-HETE) [
22], leukotriene B
4 (LTB
4) [
23,
24,
25], leukotriene D
4 (LTD
4) [
23,
25] and leukotriene E
4 (LTE
4) [
23,
25]. The analogues of AA are also of interest including arachidonoyl ethanolamide (AEA) [
26,
27] and oleoyl ethanolamide (OEA) [
26].
Figure 1. Arachidonic Acid Cascade, adapted from various sources [
7,
8,
14,
17,
18,
19,
20,
21,
22,
23,
24,
25,
26,
27,
28,
29]. Abbreviations of eicosanoids: arachidonoyl ethanolamide (AEA), oleoyl ethanolamide (OEA), prostaglandin (PG), thromboxane (Tx), hydroperoxyeicosatetraenoic acids (HPETES), hydroxyeicosatetraenoic acid (HETE) and leukotriene (LT).
AEA is an endogenous cannabinoid ligand that has binding activity resulting in pharmacological effects of tetrahydrocannabinol (THC) such as euphoria and calmness [
26,
27]. In a variety of cells, cannabinoid agonists have caused an increase in the amount of AA [
26]. This has been hypothesised to result from the combination of PLA
2 and acyltransferase inhibition [
26]. AA can also be converted into N-(4-hydroxyphenyl) arachidonylamide (AM404) which displays analgesic properties and the ability to lower body temperature [
28,
29]. AM404 is produced when acetaminophen is metabolised in the body to produce
p-aminophenol, which is then conjugated with AA [
29]. AM404 has been reported to inhibit the cyclooxygenase (COX) pathways leading to the decreased formation of PGE
2, demonstrating effectiveness as a COX-2 enzyme inhibitor to reduce the production of prostaglandins by consumption of AA [
29]. The COX and lipoxygenase (LOX) pathways are of particular interest for equine anti-doping due to their augmentation following anti-inflammatory treatments.
Prostaglandins are monocarboxylic acids with two side chains at carbon 7 and 8 attached to a central, five-membered ring [
14]. Prostaglandins have oxygen-containing substituents in various positions in the molecule with the naming of the prostaglandins ranging from PGA to PGI depending on the basis of the substituents in the ring, and further sub-grouped into three series depending on the degree of unsaturation [
14]. Prostaglandins are one of the key compounds in the generation of the inflammatory response due to an increase in concentration in inflamed tissue [
30]. Prostaglandins are formed from AA being converted by the COX enzyme to prostaglandin G
2 and H
2 [
17]. Prostaglandin endoperoxide-D-isomerase can convert PGH
2 into a mixture of PGD
2, PGE
2 and PGF
2α [
17]. PGH
2 can also produce prostacyclin (PGI
2) that may further metabolise to a more stable compound, 6-keto F
1α [
19]. PGEs and PGIs have been identified to mimic the signs of inflammation caused by vasodilation and swelling due to an increase in vascular permeability [
14].
Prostaglandins can also convert into thromboxane A
2 (TXA
2), an unstable intermediate in the production of TXB
2, which further metabolises to form 11-dehydro TXB
2 [
20]. Thromboxanes are the major products of prostaglandin endoperoxides in platelets, lungs and the spleen [
14]. The production of new platelets could potentially have a high capacity to further increase the synthesis of TXA
2, leading to increased amounts of TXB
2 and 11-dehydro TXB
2 [
19]. The use of non-steroidal anti-inflammatory drugs (NSAIDs) results in PGI
2 and thromboxane synthesis being inhibited [
19].
Using the LOX pathways, AA converts into esterified hydroperoxyeicosatetraenoic acids (HPETEs) [
31]. HPETEs are further reduced to their corresponding hydroxyeicosatetraenoic acids (HETEs) [
7]. For example, using the 15-LOX enzyme, AA will metabolise into 15-HPETE, and then further metabolises to 15-HETE. Similarly, the 5-LOX enzyme converts AA to 5-HPETE and further to 5-HETE. The leukotrienes are an oxygenated metabolite of polyunsaturated fatty acids, but the initial formation is catalysed by lipoxygenases [
23]. Leukotrienes are produced from AA using the 5-lipoxygenase (5-LOX) enzyme to produce Leukotriene A
4 (LTA
4), an unstable epoxide. LTA
4 is hydrolysed to LTB
4 or conjugated with glutathione to yield Leukotriene C
4 (LTC
4) and its metabolites LTD
4 and LTE
4. Leukotrienes are known for their strong vascular effect with the most effective being LTB
4, in comparison to LTC
4 and LTD
4 [
14,
15]. In the presence of more leukocytes, the leukotrienes also have a role in the inflammatory process by increasing blood pressure [
23].