
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Masahiko Ogihara | -- | 1316 | 2023-01-19 06:44:37 | | | |
| 2 | Masahiko Ogihara | + 845 word(s) | 2161 | 2023-01-19 07:52:28 | | | | |
| 3 | Rita Xu | Meta information modification | 2161 | 2023-01-19 09:17:59 | | |
Video Upload Options
Researchers have studied whether growth factors, cytokines, hormones, neurotransmitters, and local hormones (autacoids) promote the proliferation of hepatic parenchymal cells (i.e., hepatocytes) using in vitro primary cultured hepatocytes. The indicators used for this purpose include changes in DNA synthesis activity, nuclear number, cell number, cell cycle, and gene expression. In addition, the intracellular signaling pathways from the plasma membrane receptors to the nucleus have been examined in detail for representative growth-promoting factors that have been found to promote DNA synthesis and cell proliferation of hepatocytes.
1. Introduction
2. Classification and Characteristics of Hepatocyte Growth Regulators
Mitogens can be defined as factors that promote the proliferation of hepatocytes by themselves, such as EGF, TGF-α, HGF, platelet-derived growth factor (PDGF), and IGF-I. Co-mitogens, by themselves, do not promote hepatocyte proliferation, but, when used in combination with mitogens, the co-mitogens enhance the activity of the mitogens, and these include adrenergic α- and β-agonists and glucagon. In addition, there are many indirect mitogens, such as TNF-α, IL-1β, prostaglandin (PG) E2, 5-HT, and GH, which indirectly promote hepatocyte proliferation by secreting autocrine factors. In contrast, some factors, such as transforming growth factor-β1 (TGF-β1) and glucocorticoids, strongly suppress mitogen-induced hepatocyte proliferation (i.e., they are inhibitory factors).
2.1 Mitogens
2.1.1 Direct Mitogens
EGF
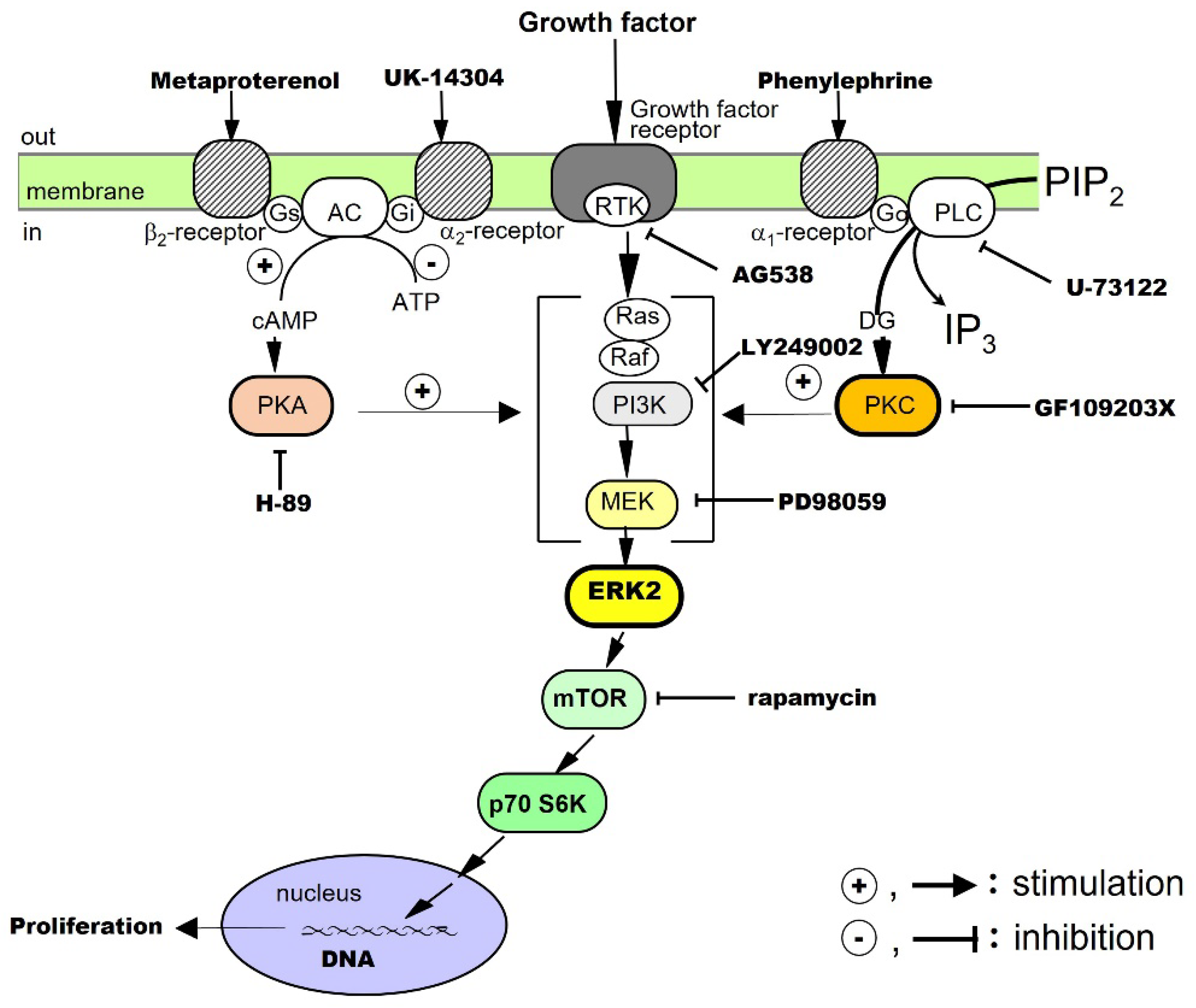
TGF-α
HGF
PDGF
Insulin
2.1.2. Co-Mitogens
Noradrenaline
2.1.3. Indirect Mitogens
IL-1β
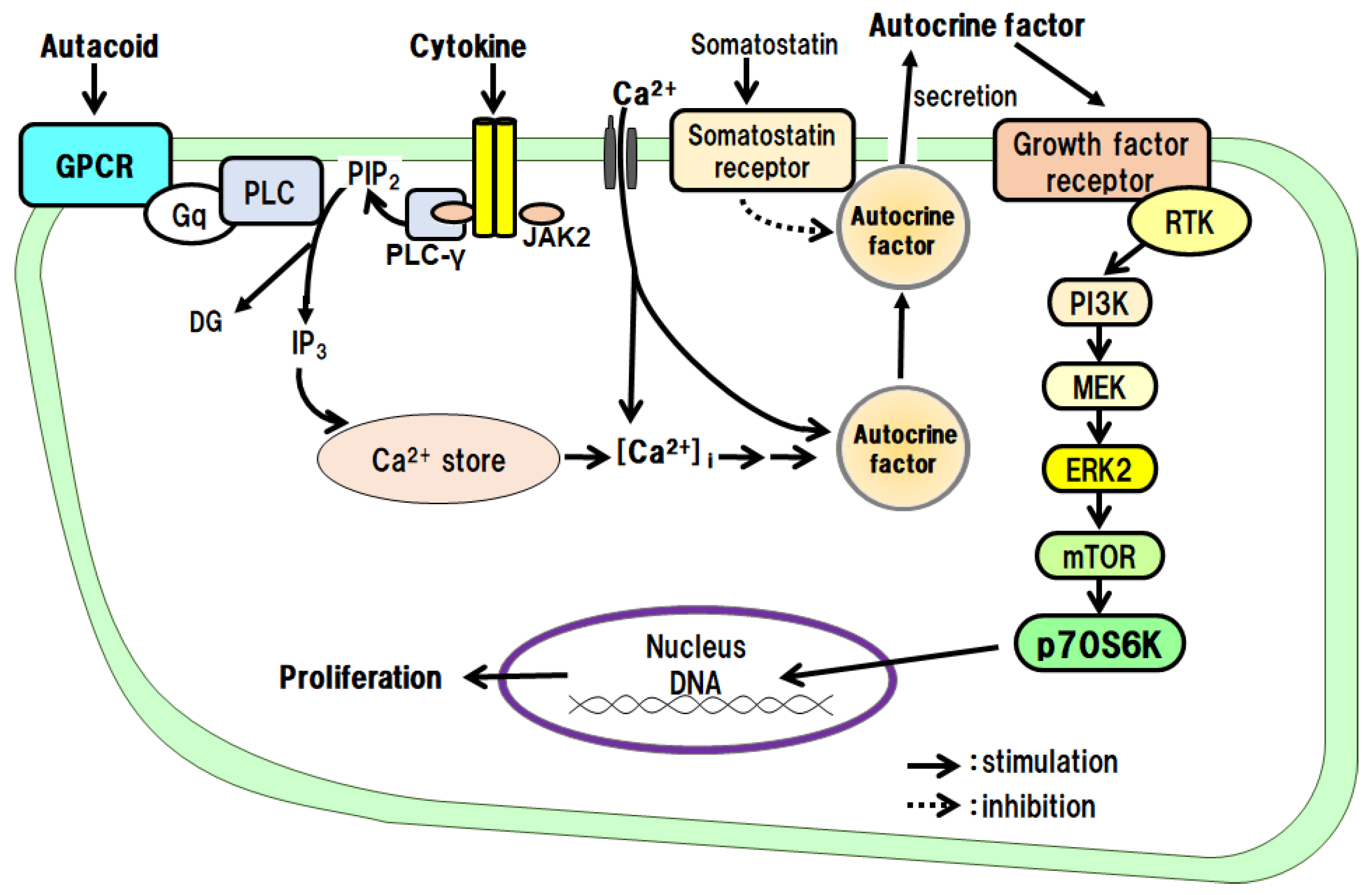
TNF-α
PGE2 and Prostacyclin (PGI2)
5-HT
GH
References
- Michchalopoulos, G.K.; DeFrances, M.C. Liver regeneration. Science 1997, 276, 60–66.
- Fausto, N.; Campbell, J.S.; Riehle, K.J. Liver Regeneration. Hepatology 2006, 43, S45–S53.
- Michalopoulos, G.K. Liver Regeneration. J. Cell. Physiol. 2007, 213, 286–300.
- Michalopoulos, G.K. Hepatostat: Liver regeneration and normal liver tissue maintenance. Hepatology 2017, 65, 1384–1392.
- Rmilah, A.A.; Zhou, W.; Nelson, E.; Lin, L.; Amiot, B.; Nyberg, S.L. Understanding the marvels behind liver regeneration. WIREs Dev. Biol. 2019, 8, e340–e367.
- Yagi, S.; Hirata, M.; Miyachi, Y.; Uemoto, S. Liver regeneration after hepatectomy and partial liver transplantation. Int. J. Mol. Sci. 2020, 21, 8414–8434.
- 7. Ichihara, A.; Nakamura, T.; Noda, C.; Tanaka, K. Control of Enzyme Expression Deduced from Studies on Primary Cultures of Hepatocytes in Research in Isolated and Cultured Hepatocytes; Guillouzo, A., Guguen-Guillouzo, C., Eds.; John Libbey Eurotext Ltd./INSERM: Paris, France, 1986; pp. 187–208.
- Kimura, M.; Ogihara, M. Density-dependent proliferation of adult rat hepatocytes in primary culture induced by epidermal growth factor is potentiated by cAMP-elevating agents. Eur. J. Pharmacol. 1997, 324, 267–276.
- Mead, J.E.; Fausto, N. Transforming growth factor alpha may be a physiological regulator of liver regeneration by means of an autocrine mechanism. Proc. Natl. Acad. Sci. USA 1989, 86, 1558–1562.
- Kimura, M.; Ogihara, M. Stimulation by transforming growth factor-α of DNA synthesis and proliferation of adult rat hepatocytes in primary cultures: Modulation by α-and β-adrenoceptor agonists. J. Pharmacol. Exp. Ther. 1999, 291, 171–180.
- Lindroos, P.M.; Zarnegar, R.; Michalopoulos, G.K. Hepatocyte growth factor (hepatopoietin A) rapidly increases in plasma before DNA synthesis and liver regeneration stimulated by partial hepatectomy and carbon tetrachloride administration. Hepatology 1991, 13, 743–750.
- Naldini, L.; Vigna, E.; Narsimhan, R.P.; Gaudino, G.; Zarnegar, R.; Michalopoulos, G.K.; Comoglio, P.M. Hepatocyte growth factor (HGF) stimulates the tyrosine kinase activity of the receptor encoded by the proto-oncogene c-MET. Oncogene 1991, 6, 501–504.
- Stolz, D.B.; Mars, W.M.; Peterson, B.E.; Kim, T.H.; Michalopoulos, G.K. Growth factor signal transduction immediately after two-thirds partial hepatectomy in the rat. Cancer Res. 1999, 59, 3954–3960.
- Kimura, M.; Ogihara, M. Proliferation of adult rat hepatocytes by hepatocyte growth factor is potentiated by both phe-nylephrine and metaproterenol. J. Phamacol. Exp. Ther. 1997, 282, 1146–1154.
- Heldin, C.-H.; Westermark, B. Platelet-derived growth factor: Mechanism of action and possible in vivo function. Cell Regul. 1990, 1, 555–566.
- Kimura, M.; Ogihara, M. Proliferation of adult rat hepatocytes in primary cultures induced by platelet-derived growth factor is potentiated by phenylephrine. Jpn. J. Pharmacol. 1998, 76, 165–174.
- Leffert, H.L.; Koch, K.S.; Moran, T.; Rubalcaba, B. Hormonal control of rat liver regeneration. Gastroenterology 1979, 76, 1470–1482.
- Ito, Y.; Uchijima, Y.; Ariga, M.; Seki, T.; Takenaka, A.; Hakuno, F.; Takahashi, S.I.; Ariga, T.; Noguchi, T. Interaction between cAMP-dependent and insulin-dependent signal pathways in tyrosine phosphorylation in primary cultures of rat hepatocytes. Biochem. J. 1997, 324, 379–388.
- Kimura, M.; Ogihara, M. Proliferation of adult rat hepatocytes in primary cultures induced by insulin is potentiated by cAMP-elevating agents. Eur. J. Pharmacol. 1998, 327, 87–95.
- Cruise, J.L.; Knechtle, S.J.; Bollinger, R.R.; Kuhn, C.; Michalopoulos, G.K. Alpha 1-adrenergic effects and liver regeneration. Hepatology 1987, 7, 1189–1194.
- Olsen, P.S.; Poulsen, S.S.; Kirkegaard, P. Adrenergic effects on secretion of epidermal growth factor from Brunner’s glands. Gut. 1985, 26, 920–927.
- Broten, J.; Michalopoulos, G.; Peterson, B.; Cruise, J. Adrenergic stimulation of hepatocyte growth factor expression. Biochem. Biophys. Res. Commun. 1999, 262, 76–79.
- Boulton, R.; Woodman, A.; Calnan, D.; Selden, C.; Tam, F.; Hodgson, H. Nonparenchymal cells from regenerating rat liver generate interleukin-1alpha and -1beta: A mechanism of negative regulation of hepatocyte proliferation. Hepatology 1997, 26, 49–58.
- Kimura, M.; Moteki, H.; Ogihara, M. Involvement of endogenous transforming growth factor-α in signal transduction pathway for interleukin-1β-induced hepatocyte proliferation. Eur. J. Pharmacol. 2014, 745, 223–233.
- Yamada, Y.; Kirillova, I.; Peschon, J.J.; Fausto, N. Inhibition of liver growth by tumor necrosis factor: Deficient liver regeneration in mice lacking type I tumor necrosis factor receptor. Pro. Natl. Acad. Sci. USA 1997, 94, 1441–1446.
- Okamoto, H.; Kimura, M.; Watanabe, N.; Ogihara, M. Tumor necrosis factor (TNF) receptor-2-mediated DNA synthesis and proliferation in primary cultures of adult rat hepatocytes: The involvement of endogenous transforming growth factor-α. Eur. J. Pharmacol. 2009, 604, 12–19.
- Refsnes, M.; Thoresen, G.H.; Dajani, O.F.; Christoffersen, T. Stimulation of hepatocyte DNA synthesis by prostaglandin E2 and prostaglandin F2α additivity with the effect of norepinephrine, and synergism with epidermal growth factor. J. Cell. Physiol. 1994, 159, 35–40.
- Kimura, M.; Osumi, S.; Ogihara, M. Stimulation of DNA synthesis and proliferation by prostaglandins in primary cultures of adult rat hepatocytes. Eur. J. Pharmacol. 2000, 404, 259–271.
- Lesurtel, M.; Graf, R.; Aleil, B.; Walther, B.; Tian, Y.; Jochum, W.; Gaget, C.; Bader, M.; Clavien, P. Platelet-derived serotonin mediates liver regeneration. Science 2006, 312, 104–107.
- Naito, K.; Tanaka, C.; Mitsuhashi, M.; Moteki, H.; Kimura, M.; Natsume, H.; Ogihara, M. Signal transduction mechanism for serotonin 5-HT2B receptor-mediated DNA synthesis and proliferation in primary cultures of adult rat hepatocytes. Biol. Pharm. Bull. 2016, 39, 121–129.
- Naito, K.; Moteki, H.; Kimura, M.; Natsume, H.; Ogihara, M. Serotonin 5-HT2B receptor-stimulated DNA synthesis and pro-liferation are mediated by autocrine secretion of transforming growth factor-α in primary cultures of adult rat hepatocytes. Biol. Pharm. Bull. 2016, 39, 570–577.
- Naito, K.; Kurihara, K.; Moteki, H.; Kimura, M.; Natsume, H.; Ogihara, M. Effect of selective serotonin (5-HT)2B receptor agonist BW723C86 on epidermal growth factor/transforming growth factor-α receptor tyrosine kinase and ribosomal p70 S6 kinase activities in primary cultures of adult rat hepatocytes. Biol. Pharm. Bull. 2019, 42, 631–637.
- Herrington, J.; Carter-Su, C. Signaling pathways activated by the growth hormone receptor. Trends. Endocrinol. Metab. 2001, 12, 252–257.
- Pennisi, P.A.; Kopchick, J.J.; Thorgeirsson, S.; LeRoith, D.; Yakar, S. Role of growth hormone (GH) in liver regeneration. En-docrinology 2004, 145, 4748–4755.
- Kurihara, K.; Moteki, H.; Ogihara, M.; Kimura, M. Growth hormone signaling pathway leading to the induction of DNA synthesis and proliferation in primary cultured hepatocytes of adult rats. J. Pharm. Pharm. Sci. 2021, 24, 1–15.
- Kurihara, K.; Moteki, H.; Kimura, M.; Ogihara, M. Autocrine secretion of insulin-like growth factor-I mediates growth hor-mone-stimulated DNA synthesis and proliferation in primary cultures of adult rat hepatocytes. Eur. J. Pharmacol. 2021, 891, 173753. https://doi.org/10.1016.
- Kimura, M.; Ogihara, M. Effects of insulin-like growth factor I and II on DNA synthesis and proliferation in primary cultures of adult rat hepatocytes. Eur. J. Pharmacol. 1998, 354, 271–281.




