
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Mingyi Wang | -- | 3038 | 2023-01-18 11:09:13 | | | |
| 2 | Jessie Wu | + 14 word(s) | 3052 | 2023-01-19 02:41:30 | | |
Video Upload Options
Medin, a small 50-amino acid peptide, is an internal cleaved product from the second discoidin domain of milk fat globule epidermal growth factor VIII (MFG-E8) protein. Medin has been reported as the most common amylogenic protein in the upper part of the arterial system, including aortic, temporal, and cerebral arterial walls in the elderly. Medin has a high affinity to elastic fibers and is closely associated with arterial degenerative inflammation, elastic fiber fragmentation, calcification, and amyloidosis. In vitro, treating with the medin peptide promotes the inflammatory phenotypic shift of both endothelial cells and vascular smooth muscle cells. In vitro, ex vivo, and in vivo studies demonstrate that medin enhances the abundance of reactive oxygen species and reactive nitrogen species produced by both endothelial cells and vascular smooth muscle cells and promotes vascular endothelial dysfunction and arterial stiffening. Immunostaining and immunoblotting analyses of human samples indicate that the levels of medin are increased in the pathogenesis of aortic aneurysm/dissection, temporal arteritis, and cerebrovascular dementia. Thus, medin peptide could be targeted as a biomarker diagnostic tool or as a potential molecular approach to curbing the arterial degenerative inflammatory remodeling that accompanies aging and disease.
1. Medin Induces Vascular Cell Inflammation
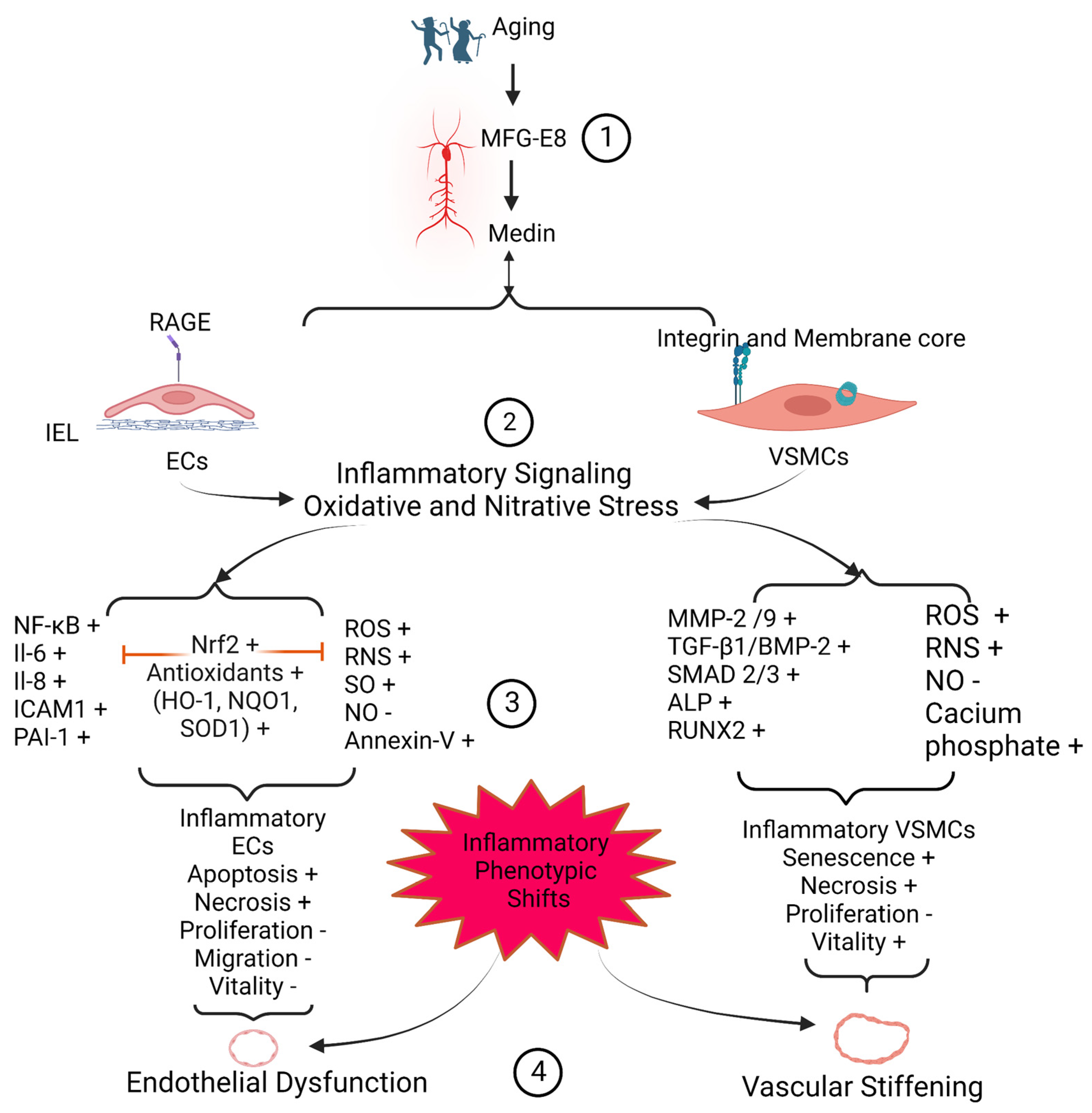
1.1. Endothelial Cells Inflammation and Viability
1.2. Vascular Smooth Muscle Cells Inflammation and Viability
2. Medin Deposition Impairs Vascular Function

2.1. Arterial Endothelial Disorder and Dysfunction
2.2. Arterial Microstructure Disorder and Stiffening
3. Medin Deposition Is Involved in the Pathogenesis of Vascular Diseases
3.1. Arterial Aneurysms and Dissections
3.2. Temporal Arteritis
3.3. Vascular-Related Cognitive Impairment and Dementia
References
- Miura, Y.; Tsumoto, H.; Iwamoto, M.; Yamaguchi, Y.; Ko, P.; Soejima, Y.; Yoshida, S.; Toda, T.; Arai, T.; Hamamatsu, A.; et al. Age-associated proteomic alterations in human aortic media. Geriatr. Gerontol. Int. 2019, 19, 1054–1062.
- Migrino, R.Q.; Davies, H.A.; Truran, S.; Karamanova, N.; Franco, D.A.; Beach, T.G.; Serrano, G.E.; Truong, D.; Nikkhah, M.; Madine, J. Amyloidogenic medin induces endothelial dysfunction and vascular inflammation through the receptor for advanced glycation endproducts. Cardiovasc. Res. 2017, 113, 1389–1402.
- Davies, H.A.; Phelan, M.M.; Wilkinson, M.C.; Migrino, R.Q.; Truran, S.; Franco, D.A.; Liu, L.N.; Longmore, C.J.; Madine, J. Oxidative Stress Alters the Morphology and Toxicity of Aortic Medial Amyloid. Biophys. J. 2015, 109, 2363–2370.
- Karamanova, N.; Truran, S.; Serrano, G.E.; Beach, T.G.; Madine, J.; Weissig, V.; Davies, H.A.; Veldhuizen, J.; Nikkhah, M.; Hansen, M.; et al. Endothelial Immune Activation by Medin: Potential Role in Cerebrovascular Disease and Reversal by Monosialoganglioside-Containing Nanoliposomes. J. Am. Heart Assoc. 2020, 9, e014810.
- Madine, J.; Middleton, D.A. Comparison of aggregation enhancement and inhibition as strategies for reducing the cytotoxicity of the aortic amyloid polypeptide medin. Eur. Biophys. J. 2010, 39, 1281–1288.
- Karamanova, N.; Truran, S.; Weissig, V.; Madine, J.; Davies, H.; Guzman-Villanueva, D.; Migrino, R. Amyloidogenic Medin Impairs Endothelial Cell Autophagy and Viability that is Reversed by Monosialoganglioside Nanoliposomes. FASEB J. 2018, 32, 846.816.
- Wang, M.; Jiang, L.; Monticone, R.E.; Lakatta, E.G. Proinflammation: The key to arterial aging. Trends Endocrinol. Metab. 2014, 25, 72–79.
- Wang, M.; Monticon, R.; McGraw, K. Proinflammation, profibrosis, and arterial aging. Aging Med. 2020, 3, 159–168.
- Li, B.Y.; Li, X.L.; Cai, Q.; Gao, H.Q.; Cheng, M.; Zhang, J.H.; Wang, J.F.; Yu, F.; Zhou, R.H. Induction of lactadherin mediates the apoptosis of endothelial cells in response to advanced glycation end products and protective effects of grape seed procyanidin B2 and resveratrol. Apoptosis 2011, 16, 732–745.
- Li, B.Y.; Li, X.L.; Gao, H.Q.; Zhang, J.H.; Cai, Q.; Cheng, M.; Lu, M. Grape seed procyanidin B2 inhibits advanced glycation end product-induced endothelial cell apoptosis through regulating GSK3beta phosphorylation. Cell Biol. Int. 2011, 35, 663–669.
- Nerelius, C.; Gustafsson, M.; Nordling, K.; Larsson, A.; Johansson, J. Anti-amyloid activity of the C-terminal domain of proSP-C against amyloid beta-peptide and medin. Biochemistry 2009, 48, 3778–3786.
- Whitehead, M.; Yusoff, S.; Ahmad, S.; Schmidt, L.; Mayr, M.; Madine, J.; Middleton, D.; Shanahan, C.M. Vascular smooth muscle cell senescence accelerates medin aggregation via small extracellular vesicle secretion and extracellular matrix reorganization. Aging Cell 2022, e13746.
- Degenhardt, K.; Wagner, J.; Skodras, A.; Candlish, M.; Koppelmann, A.J.; Wild, K.; Maxwell, R.; Rotermund, C.; von Zweydorf, F.; Gloeckner, C.J.; et al. Medin aggregation causes cerebrovascular dysfunction in aging wild-type mice. Proc. Natl. Acad. Sci. USA 2020, 117, 23925–23931.
- Haggqvist, B.; Naslund, J.; Sletten, K.; Westermark, G.T.; Mucchiano, G.; Tjernberg, L.O.; Nordstedt, C.; Engstrom, U.; Westermark, P. Medin: An integral fragment of aortic smooth muscle cell-produced lactadherin forms the most common human amyloid. Proc. Natl. Acad. Sci. USA 1999, 96, 8669–8674.
- Larsson, A.; Malmstrom, S.; Westermark, P. Signs of cross-seeding: Aortic medin amyloid as a trigger for protein AA deposition. Amyloid 2011, 18, 229–234.
- Peng, S.; Larsson, A.; Wassberg, E.; Gerwins, P.; Thelin, S.; Fu, X.; Westermark, P. Role of aggregated medin in the pathogenesis of thoracic aortic aneurysm and dissection. Lab. Invest. 2007, 87, 1195–1205.
- Davies, H.A.; Madine, J.; Middleton, D.A. Comparisons with amyloid-beta reveal an aspartate residue that stabilizes fibrils of the aortic amyloid peptide medin. J. Biol. Chem. 2015, 290, 7791–7803.
- Ni, Y.-Q.; Li, S.; Lin, X.; Wang, Y.-J.; He, J.-Y.; Song, W.-L.; Xiang, Q.-Y.; Zhao, Y.; Li, C.; Wang, Y.; et al. Exosomal MFGE8 from high glucose induced endothelial cells is involved in calcification/senescence of vascular smooth muscle cells. bioRxiv 2021.
- Younger, S.; Jang, H.; Davies, H.A.; Niemiec, M.J.; Garcia, J.G.N.; Nussinov, R.; Migrino, R.Q.; Madine, J.; Arce, F.T. Medin Oligomer Membrane Pore Formation: A Potential Mechanism of Vascular Dysfunction. Biophys. J. 2020, 118, 2769–2782.
- Larsson, A.; Peng, S.; Persson, H.; Rosenbloom, J.; Abrams, W.R.; Wassberg, E.; Thelin, S.; Sletten, K.; Gerwins, P.; Westermark, P. Lactadherin binds to elastin--a starting point for medin amyloid formation? Amyloid 2006, 13, 78–85.
- Peng, S.; Westermark, G.T.; Naslund, J.; Haggqvist, B.; Glennert, J.; Westermark, P. Medin and medin-amyloid in ageing inflamed and non-inflamed temporal arteries. J. Pathol. 2002, 196, 91–96.
- Davies, H.A.; Caamano-Gutierrez, E.; Chim, Y.H.; Field, M.; Nawaytou, O.; Ressel, L.; Akhtar, R.; Madine, J. Idiopathic degenerative thoracic aneurysms are associated with increased aortic medial amyloid. Amyloid 2019, 26, 148–155.
- Migrino, R.Q.; Karamanova, N.; Truran, S.; Serrano, G.E.; Davies, H.A.; Madine, J.; Beach, T.G. Cerebrovascular medin is associated with Alzheimer’s disease and vascular dementia. Alzheimer’s Dement. 2020, 12, e12072.
- Wagner, J.; Degenhardt, K.; Veit, M.; Louros, N.; Konstantoulea, K.; Skodras, A.; Wild, K.; Liu, P.; Obermüller, U.; Bansal, V.; et al. Medin co-aggregates with vascular amyloid-β in Alzheimer’s disease. Nature 2022, 612, 123–131.
- Migrino, R.Q.; Truran, S.; Karamanova, N.; Serrano, G.E.; Madrigal, C.; Davies, H.A.; Madine, J.; Reaven, P.; Beach, T.G. Human cerebral collateral arteriole function in subjects with normal cognition, mild cognitive impairment, and dementia. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H284–H290.
- Olofsson, A.; Borowik, T.; Grobner, G.; Sauer-Eriksson, A.E. Negatively charged phospholipid membranes induce amyloid formation of medin via an alpha-helical intermediate. J. Mol. Biol. 2007, 374, 186–194.
- Ushiki, T. Collagen fibers, reticular fibers and elastic fibers. A comprehensive understanding from a morphological viewpoint. Arch. Histol. Cytol. 2002, 65, 109–126.
- Raspanti, M.; Protasoni, M.; Manelli, A.; Guizzardi, S.; Mantovani, V.; Sala, A. The extracellular matrix of the human aortic wall: Ultrastructural observations by FEG-SEM and by tapping-mode AFM. Micron 2006, 37, 81–86.
- Berquand, A.; Wahart, A.; Henry, A.; Gorisse, L.; Maurice, P.; Blaise, S.; Romier-Crouzet, B.; Pietrement, C.; Bennasroune, A.; Sartelet, H.; et al. Revealing the elasticity of an individual aortic fiber during ageing at nanoscale by in situ atomic force microscopy. Nanoscale 2021, 13, 1124–1133.
- Kuznetsova, T.G.; Starodubtseva, M.N.; Yegorenkov, N.I.; Chizhik, S.A.; Zhdanov, R.I. Atomic force microscopy probing of cell elasticity. Micron 2007, 38, 824–833.
- Le Master, E.; Fancher, I.S.; Lee, J.; Levitan, I. Comparative analysis of endothelial cell and sub-endothelial cell elastic moduli in young and aged mice: Role of CD36. J. Biomech. 2018, 76, 263–268.
- Jannatbabaei, A.; Tafazzoli-Shadpour, M.; Seyedjafari, E. Effects of substrate mechanics on angiogenic capacity and nitric oxide release in human endothelial cells. Ann. N. Y. Acad. Sci. 2020, 1470, 31–43.
- Kolodziejczyk, A.M.; Kucinska, M.; Jakubowska, A.; Sokolowska, P.; Rosowski, M.; Tkacz-Szczesna, B.; Komorowski, P.; Makowski, K.; Walkowiak, B. Endothelial cell aging detection by means of atomic force spectroscopy. J. Mol. Recognit. 2020, 33, e2853.
- Zhu, W.; Kim, B.C.; Wang, M.; Huang, J.; Isak, A.; Bexiga, N.M.; Monticone, R.; Ha, T.; Lakatta, E.G.; An, S.S. TGFβ1 reinforces arterial aging in the vascular smooth muscle cell through a long-range regulation of the cytoskeletal stiffness. Sci. Rep. 2018, 8, 2668.
- Patel, P.D.; Arora, R.R. Pathophysiology, diagnosis, and management of aortic dissection. Ther. Adv. Cardiovasc. Dis. 2008, 2, 439–468.
- Hinterseher, I.; Erdman, R.; Elmore, J.R.; Stahl, E.; Pahl, M.C.; Derr, K.; Golden, A.; Lillvis, J.H.; Cindric, M.C.; Jackson, K.; et al. Novel pathways in the pathobiology of human abdominal aortic aneurysms. Pathobiology 2013, 80, 1–10.
- Westermark, G.T.; Westermark, P. Localized amyloids important in diseases outside the brain--lessons from the islets of Langerhans and the thoracic aorta. FEBS J. 2011, 278, 3918–3929.
- Chen, H.Z.; Wang, F.; Gao, P.; Pei, J.F.; Liu, Y.; Xu, T.T.; Tang, X.; Fu, W.Y.; Lu, J.; Yan, Y.F.; et al. Age-Associated Sirtuin 1 Reduction in Vascular Smooth Muscle Links Vascular Senescence and Inflammation to Abdominal Aortic Aneurysm. Circ. Res. 2016, 119, 1076–1088.
- Hu, R.; Wang, M.Q.; Ni, S.H.; Wang, M.; Liu, L.Y.; You, H.Y.; Wu, X.H.; Wang, Y.J.; Lu, L.; Wei, L.B. Salidroside ameliorates endothelial inflammation and oxidative stress by regulating the AMPK/NF-kappaB/NLRP3 signaling pathway in AGEs-induced HUVECs. Eur. J. Pharmacol. 2020, 867, 172797.
- Muniz Castro, H.M.; Bhattacharjee, M.B.; Chaudhry, I.A.; Chuang, A.Z.; Mankiewicz, K.A.; Adesina, O.O. Diagnosis of giant cell arteritis using clinical, laboratory, and histopathological findings in patients undergoing temporal artery biopsy. Clin. Neurol. Neurosurg. 2022, 221, 107377.
- Sharma, A.; Mohammad, A.J.; Turesson, C. Incidence and prevalence of giant cell arteritis and polymyalgia rheumatica: A systematic literature review. Semin. Arthritis Rheum. 2020, 50, 1040–1048.
- Weyand, C.M.; Goronzy, J.J. Medium- and large-vessel vasculitis. N. Engl. J. Med. 2003, 349, 160–169.
- Peng, S.; Glennert, J.; Westermark, P. Medin-amyloid: A recently characterized age-associated arterial amyloid form affects mainly arteries in the upper part of the body. Amyloid 2005, 12, 96–102.
- Wang, F.; Cao, Y.; Ma, L.; Pei, H.; Rausch, W.D.; Li, H. Dysfunction of Cerebrovascular Endothelial Cells: Prelude to Vascular Dementia. Front. Aging Neurosci. 2018, 10, 376.
- Ott, A.; Breteler, M.M.; van Harskamp, F.; Claus, J.J.; van der Cammen, T.J.; Grobbee, D.E.; Hofman, A. Prevalence of Alzheimer’s disease and vascular dementia: Association with education. The Rotterdam study. BMJ 1995, 310, 970–973.
- Caruso, P.; Signori, R.; Moretti, R. Small vessel disease to subcortical dementia: A dynamic model, which interfaces aging, cholinergic dysregulation and the neurovascular unit. Vasc. Health Risk. Manag. 2019, 15, 259–281.
- Guo, S.; Deng, W.; Xing, C.; Zhou, Y.; Ning, M.; Lo, E.H. Effects of aging, hypertension and diabetes on the mouse brain and heart vasculomes. Neurobiol. Dis. 2019, 126, 117–123.
- Knopman, D.S.; Parisi, J.E.; Boeve, B.F.; Cha, R.H.; Apaydin, H.; Salviati, A.; Edland, S.D.; Rocca, W.A. Vascular dementia in a population-based autopsy study. Arch. Neurol. 2003, 60, 569–575.
- McAleese, K.E.; Alafuzoff, I.; Charidimou, A.; De Reuck, J.; Grinberg, L.T.; Hainsworth, A.H.; Hortobagyi, T.; Ince, P.; Jellinger, K.; Gao, J.; et al. Post-mortem assessment in vascular dementia: Advances and aspirations. BMC Med. 2016, 14, 129.
- Bryant, A.G.; Hu, M.; Carlyle, B.C.; Arnold, S.E.; Frosch, M.P.; Das, S.; Hyman, B.T.; Bennett, R.E. Cerebrovascular Senescence Is Associated With Tau Pathology in Alzheimer’s Disease. Front. Neurol. 2020, 11, 575953.
- Ungvari, Z.; Tarantini, S.; Sorond, F.; Merkely, B.; Csiszar, A. Mechanisms of Vascular Aging, A Geroscience Perspective: JACC Focus Seminar. J. Am. Coll. Cardiol. 2020, 75, 931–941.
- Whitehead, M.; Ahmad, S.; Shanahan, C. BS47 Role of vascular smooth muscle cell-derived exosomes in age-related vascular amyloidosis. Heart 2019, 105, A169–A170.




