1. Possible Curative Efficacy of TQ for the Inflammation and Multiple Organ Failure Associated with COVID-19
Coronavirus-induced cytokine release syndrome (CRS) is a serious condition that leads to multiple organ damage and Acute respiratory distress syndrome (ARDS)
[1]. Once SARS-CoV-2 enters the host cell, it results in disruption of the intracellular environment through redistribution of the ion with activation of inflammation
[2]. Two main substances—nucleotide-binding oligomerization domain (NOD), leucine-rich repeat (LRR9) and pyrin domain-containing protein 3 (NLRP3) and eicosanoids—are well known to play a critical role in inflammation, fever, and pain
[3]. In addition, the disease is associated with increases in the secretion of proinflammatory cytokines: interleukins IL-1β, IL-18, IL-6, and tumor necrosis factor (TNF). Additionally, they have been found in critically ill individuals. This rise in proinflammatory cytokines is associated with the severity of the illness, and it is also a factor in the heightened cytokine storm and tissue inflammation that occur during respiratory illness
[4]. Moreover, additional inflammatory mediators are involved in the pathogenesis of COVID-19, including Chemokines. CCL2 belongs to the group of CC chemokines and is also known as monocyte chemoattractant protein-1 (MCP-1) due to its participation in monocyte recruitment. It can bind to CC chemokine receptor type 2 (CCR2, CD192), triggering various downstream signaling pathways
[5]. One of the most significant pathogenic outcomes of a severe SARS-CoV-2 infection is the infiltration of inflammatory monocytes and macrophages, as well as the dysregulated inflammation brought on by the function of these cells and the produced inflammatory mediators. However, the CCL2/CCR2 chemokine axis is essential for attracting and directing monocytes and macrophages to the lung tissue, according to
[6]. Therefore, utilizing various medications to block this axis may lessen the severity of the condition and regulate excessive inflammation. Additionally, high plasma levels of several inflammatory mediators, including CCL2, granulocyte-macrophage colony-stimulating factor (GM-CSF), CXCL8 (interleukin-8), interferon gamma-induced protein 10 (IP-10), and osteopontin, were found in patients with SARS-CoV-2 infection, supporting the role of monocytes in the immunopathogenesis of COVID-19
[7]. Interestingly, according to studies, SARS-CoV-2 could infect mature cardiomyocytes, as well as those produced from human pluripotent stem cells, causing the release of CCL2 and the subsequent recruitment of monocytes. Monocyte infiltration and increased CCL2 expression were also found in the hearts of hamsters with SARS-CoV-2 infection
[8].
Generally, COVID-19 therapy strategies target the viral replication cycle, which has been determined to be insufficient for increasing host survival and is also required to address the virus-induced cytokine release syndrome (CRS)
[1]. Therefore, a drug that possesses the ability to inhibit both NLRP3 and eicosanoids is an urgent need. In this regard, thymoquinone (TQ) has been approved previously to have a promising anti-inflammatory role
[9][10]. TQ acting as an NLRP3 inhibitor would consequently decrease secreted IL-1β, IL-18, and IL-6 and ameliorate pain and inflammation in COVID-19 patients. In addition to targeting NLRP3, TQ is also able to target the eicosanoid storm
[11], which leads to inhibiting cytokine storm formation and, subsequently, helps prevent inflammation-mediated multiple organ damage in COVID-19 patients (
Figure 1 and
Figure 2) (
Table 1). One of the published clinical studies (NCT04401202) concluded that NSO (TQ) supplementation provides faster recovery of 62% of mild COVID-19 patients on day 14 of the treatment. The normal recovery time was also briefer than the control group. This study suggested that the reduction of COVID-19 symptoms (anosmia, chills, runny nose, and loss of appetite) might be due to the anti-inflammatory properties of NSO
[12]. The potent anti-inflammatory effect of TQ, either in vitro or in vivo, together with its inhibitory effect on cytokine storm formation, highlights the possible curative efficacy of TQ on the inflammation and multiple organ failure associated with COVID-19.
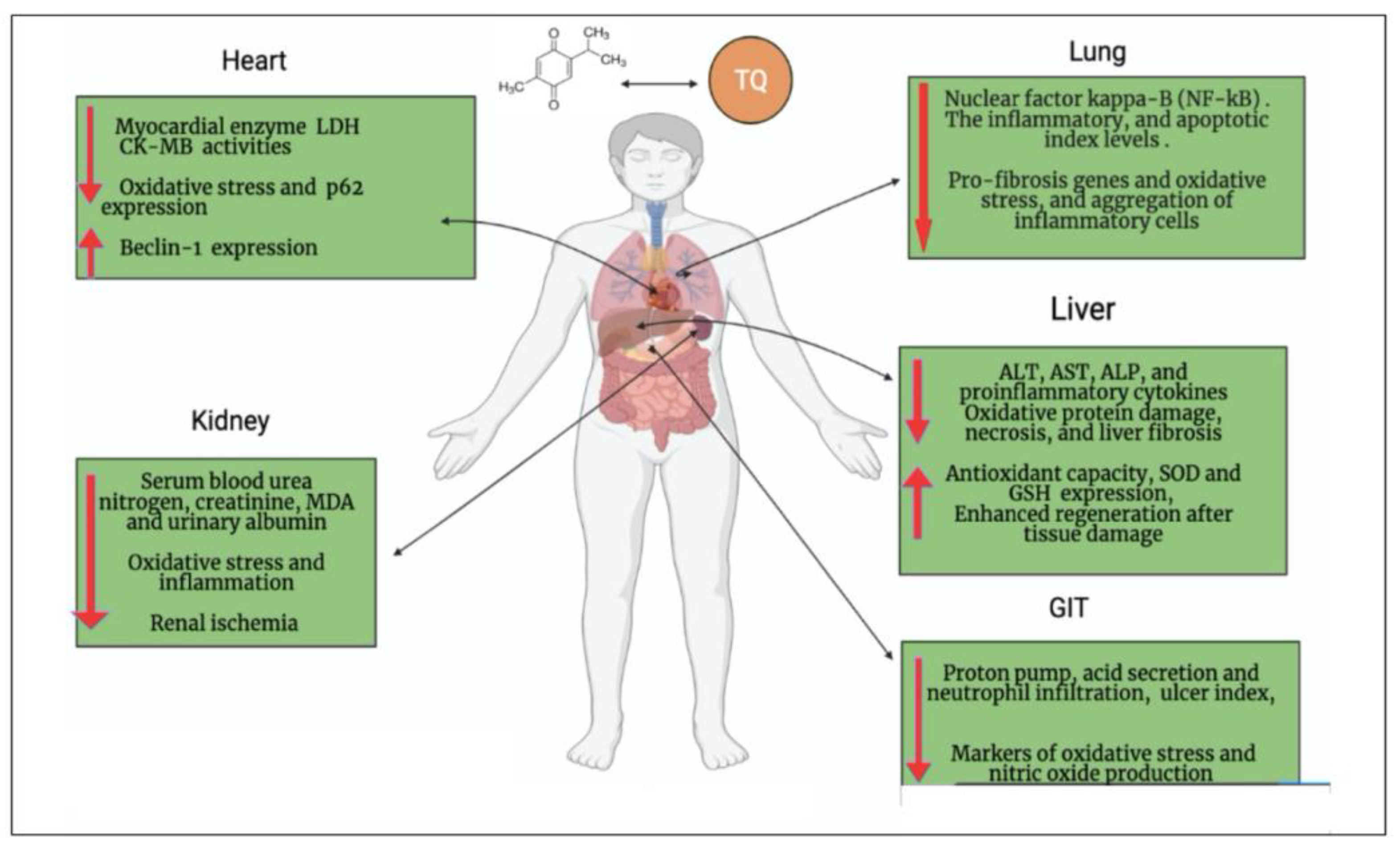
Figure 1. The general biological effect of thymoquinone (TQ). Figure generated using BioRender.
Figure 2. Possible curative efficacy of thymoquinone (TQ) on the complications associated with COVID-19 infection. Figure generated using BioRender.
2. Oxidative Stress Associated with COVID-19 and the Antioxidant Effect of TQ
It is well established that COVID-19 infection causes oxidative damage as a result of the oxidative stress the virus causes. Briefly, the basic mechanisms that regulate mitochondrial respiration and metabolism are disrupted as a result of interactions between some components of the reactive oxygen species (ROS) pathway and the proteins of the virus that infect cells
[13]. According to reports, the severity of the condition was correlated with an increase in oxidative stress rates
[14]. Therefore, it is advised to administer antioxidant supplements to lessen oxidative stress and the severity of the illness
[14][15][16]. According to this trend, previous research showed TQ’s antioxidant effects
[17][18] (
Figure 1 and
Figure 2) (
Table 1). It was reported that TQ stimulates the expression of several detoxifying enzymes, including glutathione reductase, superoxide dismutase 1 (SOD1), catalase, and glutathione peroxidase 2 (GPX)
[17][18][19]. A considerable rise in the level of antioxidant enzymes was observed in rats treated with TQ at a dose of 50 mg/kg body weight. The combination of honey and NSO reduced COVID-19 symptoms, viral clearance, and mortality among COVID-19 patients, according to published results of a clinical trial (NCT04347382). In addition to existing COVID-19 therapies, this paper also promoted the usage of honey and NS
[20]. According to this study, the combination of honey and NS provided its anti-COVID-19 activity due to their antioxidative/antiviral/immunostimulant chemical constituents (phenolic compounds, flavonoids, and zinc) that attack the multiple sites (lowering the expression of ACE-2 receptor, RdRp, Mpro protease, etc.) of SARS-CoV-2
[20]. Additionally, it has been suggested that N. sativa components may help cure COVID-19 by preventing the virus from entering the body, boosting the zinc immunological response against SARS-CoV-2, and preventing viral multiplication
[21].
3. Cardiopulmonary Protective Effect of TQ
Cardiac injury is one of the fatal complications of COVID-19
[22]. Direct myocardial injury, systemic inflammation and a cytokine storm, downregulation of ACE2 receptors, abnormal myocardial oxygen demand-supply, plaque rupture with subsequent coronary thrombosis, side effects of several COVID-19 treatment options, electrolyte imbalances, and endothelial damage are some of the potential COVID-19 mechanisms that could result in CVD
[23].
In a cohort investigation of subsequent autopsy cases conducted in Hamburg in April 2020,
[24] detected the SARS-CoV-2 genome in the cardiac tissue in 24 out of 39 autopsies (61.6%). In addition to the virus’s presence in the myocardial tissue and the progeny it produced, Linder et al.
[24] also noted that the viral genome is not directly localized in the cardiomyocytes, but rather in the macrophages or interstitial cells that make up the cardiac tissue. Another study
[25] proved that patients with cardiac injury had higher mortality when infected with COVID-19 than those without cardiac injury (42 of 82 [51.2%] vs. 15 of 334 [4.5%], respectively;
p < 0.001). In a Cox regression model, patients with vs. those without cardiac injury were at a higher risk of death, both during the time from symptom onset (hazard ratio, 4.26 [95% CI, 1.92–9.49]) and from admission to end point (hazard ratio, 3.41 [95% CI, 1.62–7.16]). In addition, increasing the level of plasma troponin-T (c-Tnt) is one of the most important markers of cardiac damage
[26]. Patients with severe COVID-19 instances had greater plasma levels of c-Tnt, required more mechanical breathing, were more prone to malignant arrhythmias, and required glucocorticoid medication
[22]. Furthermore, cardiac damage in severe cases of COVID-19 is characterized by increasing and decreasing the expression of P62 and beclin1 in plasma, respectively
[27]. Interestingly, TQ induces a cardioprotective effect through four main scenarios: (i) it significantly decreases
cardiac troponin T (TnT) levels and markedly reduces cardiac tissue-inflammatory cell infiltration
[28]; (ii) it decreases the expression of P62 and increases the expression of beclin1
[22]; (iii) it restores cardiomyocyte injury enzymes, leading to the repair of injured cardiomyocytes
[29]; and (iv) it enhances the production of endogenous antioxidants and attenuates oxidative stress, which results in maintaining the structural integrity of myocardial muscle
[17] (
Table 1). Additionally, it is believed that TQ’s potential for preventing CVD is a result of its ability to stimulate endothelial cells’ production of NO and endothelium-derived hyperpolarizing factor (EDHF); decrease the endothelial production of vasoconstrictive factors, such as thromboxane A2; and also have an antioxidant effect on vascular SMCs. Therefore, TQ’s effects on the endothelium and SMCs may improve vascular health in COVID-19 patients and may even lessen the disease’s morbidity and mortality. Numerous studies have also demonstrated the effectiveness of TQ and NS seeds in preventing the production of thrombi
[30]. It is a well-known fact that thrombus formation causes multiple organ collapse and fatality among COVID-19 patients. Therefore, NS may be used as a therapeutic formulation, including its nano-formulations, to treat COVID-19, and it may also be used as a supportive therapy with anti-COVID-19 medicines
[30].
Breathlessness, pneumonia, and lung fibrosis are the main respiratory symptoms related to COVID-19 infection
[31]. Acute SARS-CoV-2 infection results in denudation of airway epithelial cells, with the accumulation of debris, which leads to obstructed airway functions and, subsequently, acute lung injury (ALI), as well as the more severe form, acute respiratory distress syndrome (ARDS)
[31]. The ameliorating effect of TQ on respiratory disease and its promising effect on lung protection have been studied (
Figure 1 and
Figure 2) (
Table 1). In this regard, TQ decreases lung damage induced by long-lasting exposure to toluene in rats
[32]. It also inhibits pulmonary fibrosis induced by bleomycin, lipopolysaccharide (LPS), and cyclophosphamide
[33] through inhibition of activated NF-kβ in lung tissues, downregulated pro-fibrosis genes, decreased oxidative stress, and significantly reduced PGE2, TGF-β1, and INF-γ
[34]. TQ (50 mg/kg b.w.) treatment significantly (
p < 0.05) decreased the level of inflammatory cytokines, such as TNF-α, IL-1β, IL-6, and ICAM1, resulting from Benzopyrene toxication
[35].
N. sativa oil exhibits airway anti-inflammatory and immune-regulatory effects, which may support its use for treatment of allergic asthma. Peripheral blood eosinophil count, IgG1 and IgG2a levels, cytokine profiles (IL-2, IL-12, IL-10, and IFN-γ levels), and inflammatory cells counts in lung tissue were significantly decreased by the plant oil in a mouse model of allergic asthma. The plant showed comparable immunomodulatory properties with dexamethasone, except that the plant had a greater effect on IFN-γ levels. Moreover, it was reported that
N. sativa oil in the dose of (500 mg soft-gel capsules) one capsule orally, twice daily for 10 days, plus standard of care treatment has potential outcomes on patients with mild COVID-19
[36]. Another clinical study (IRCT20180712040449N2) was conducted in Iran, utilizing NS seed powder and a mixture of different herbs. This treatment significantly reduced the hospital dyspnea, accelerated recovery time, and lowered the COVID-19 symptoms. This study implicitly indicates that the chemical constituents of NS (TQ, hederagenin, THQ, nigelledine, and α-hederin) are anti-COVID-19 compounds.
4. Neuroprotective Effect of TQ can Overcome the Neurologic/Cognitive Manifestations Associated with COVID-19
Patients with COVID-19 infection suffer from different signs, such as headache, memory loss, mood changes, vision changes, hearing loss, impaired mobility, limb numbness, tremor, fatigue, and myalgia
[37]. This is along with cases of encephalitis, necrotizing hemorrhagic encephalopathy, stroke, and epileptic seizures
[38]. It is well known that neuroinflammation, induction of inflammation, and oxidative stress response are the main factors involved in the pathogenesis of almost all neurodegenerative diseases
[39]. Subsequently, the administration of natural neuroprotective agents may reduce both neuroinflammation and oxidative stress, which may help in the recovery of COVID-19 patients
[40]. In this regard, two previous studies
[41] in rats exposed to lipopolysaccharides-induced neuroinflammation reported the effect of TQ in the inhibition of inflammatory mediators (TNF-a, IL-6, and IL-1beta) and their messenger RNA (mRNA) levels in BV2 microglia. Also, TQ may have the ability to ameliorate motor impairment and memory loss associated with COVID-19 infection, as it successfully inhibits rotenone-induced Parkinson’s disease symptoms in animal models through stopped motor defects
[42] and prevented neurotoxicity induced by amyloid protein (Ab1-42) in hippocampal and cortical neurons via ameliorating oxidative stress and improving the level of lipid peroxide changes in the hippocampal region, SOD, and acetylcholine esterase (AChE) activities
[43]. The ameliorative effect of TQ on the inflammatory mediator, antioxidant enzymes, and neurotoxicity indicate the possible defensive effect of this natural compound against neurological complications associated with COVID-19 (
Figure 1 and
Figure 2) (
Table 1).
5. Hepatorenal Protective Effect of TQ against COVID-19
With the increasing number of COVID-19 infected patients, several studies reported that the liver is the most frequently affected organ after lung damage. Liver injury is a serious, fatal complication of COVID-19
[44]. The mechanism of hepatic injury in COVID-19 is not completely known. However, the injury may be caused directly by the invasion of the virus in the liver tissue, or it may be indirect (drug induced or the effect of inflammatory mediators)
[45], and the last one is more prominent. Moreover, patients suffering from parasitic infections, including malaria, Schistosoma, and Fasciola with increasing liver fibrosis and liver injury, are more susceptible to COVID-19 liver complications. A previous study has shown that schistosomiasis and helminth infection may increase the rate of unfavorable COVID-19 pandemic outcomes
[46].
Helminth infections are typically connected with Th2-mediated immune responses
[46]. Commonly, schistosomiasis infection leads to downregulation of the inflammation associated with Th2 immune response and subsequently lowers immunity to COVID-19, with increased susceptibility and higher incidence of COVID-19 in schistosomiasis-endemic areas of Africa
[46]. Therefore, a treatment that ameliorates liver fibrosis of different origins and improves hepatotoxicity is required. In previous years, several studies reported the hepatoprotective effect of TQ, especially on liver toxicity and fibrosis, and their consequences
[47][48]. Administration of TQ protects against hepatotoxicity associated with chemotherapy by reducing liver injury markers (SGOT: serum glutamic-oxaloacetic transaminase, SGPT: Serum glutamic pyruvic transaminase, GGT: gamma-glutamyl transferase) and tumor marker (alphafetoprotein) expression
[49]. The hepatoprotective role of TQ may be attributed to its strong antioxidant property. Furthermore, TQ maintains the normal level of intracellular enzymes (reduced glutathione) and keeps the integrity of the membrane by reducing the leakage of AST and ALT
[50]. TQ also reduced the damage to a liver cell and accumulation of extracellular matrix proteins, such as collagen, tenascins, laminins, and elastin. TQ was found to overcome liver fibrosis by reducing the mRNA levels of α-smooth muscle actin (α-SMA), collagen-I, and tissue inhibitor of metalloproteinase-1 (TIMP-1)
[48]. Moreover, another study attributed the activity of TQ to improving liver function and the immunological system of infected mice, and partly to its antioxidant effects
[50] (
Figure 1 and
Figure 2) (
Table 1). Subsequently, TQ can overcome hepatic injury associated with COVID-19.
Kidney injury is another COVID-19-related serious complication
[51]. Certain chemotherapeutic regimens used for the treatment of COVID-19 result in nephrotoxicity
[52]. TQ shows protective effects on the kidneys against mercuric chloride-induced renal damage
[53]. TQ enhanced kidney function indicators, including blood urea nitrogen and creatinine, in addition to ameliorating antioxidant enzymes (GSH level and activities of GSHPx and CAT) in the renal cortex with inhibited lipid peroxidation
[54]. Moreover, TQ shows reno-protective effects in sepsis-induced acute kidney injury (AKI). AKI is mediated by dysregulated activation of inflammasomes and proinflammatory cytokines that can be ameliorated by anti-inflammatory properties of TQ
[55], where TQ decreases apoptosis of kidney cells and alleviates AKI. TQ supplementation improved the sloughing off of epithelial cells, contraction of glomeruli, and necrosis of renal tubules induced by cypermethrin in the kidneys of mice
[56]. Finally, TQ reverses increased NFκB expression in the kidney of septic mice
[55]. Collectively, by controlling pyroptosis, proinflammatory cytokines, and apoptosis-related expression, TQ lessens sepsis-induced AKI and ameliorates kidney damage following COVID-19 infection
[55] (
Figure 1 and
Figure 2) (
Table 1).
6. Gastrointestinal Dysfunction Associated with COVID-19 and the Gastroprotective Effect of TQ
It has been reported that patients with COVID-19 experience diarrhea, as well as nausea/vomiting and abdominal pain
[57]. In addition to acting as a gastroprotective agent, TQ also acts as a proton pump inhibitor and increases mucin secretion
[58] (See
Figure 1 and
Figure 2 for examples) (
Table 1). As a possible complication of COVID-19, commensal bacteria in the gastrointestinal tract (GIT) may cause secondary bacterial infection. As a result of this pattern, the administration of TQ, which has a broad spectrum of antibacterial efficacy, especially against Gram positive cocci (
Staphylococcus aureus ATCC 25923 and
Staphylococcus epidermidis CIP 106510), is advised. It is possible that the antibacterial effect of TQ is due to: (a) increased ROS enervation, which is responsible for cell death caused by oxidative stress; (b) TQ inhibits biofilm formation and, as a result, inhibits its binding and matrix formation, resulting in changes in the phenotype of the organisms due to changes in growth rate and gene transcription; or (c) TQ has specific selective cytotoxicity towards bacterial cells without causing membrane damage to normal cells
[59].
Table 1. The beneficial effects of TQ against COVID-19 pathophysiological effects.