Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jinap Selamat | -- | 1978 | 2023-01-09 03:30:14 | | | |
| 2 | Catherine Yang | Meta information modification | 1978 | 2023-01-09 04:24:27 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Fakhlaei, R.; Selamat, J.; Khatib, A.; Razis, A.F.A.; Sukor, R.; Ahmad, S.; Babadi, A.A. The Toxic Impact of Honey Adulteration. Encyclopedia. Available online: https://encyclopedia.pub/entry/39881 (accessed on 04 March 2026).
Fakhlaei R, Selamat J, Khatib A, Razis AFA, Sukor R, Ahmad S, et al. The Toxic Impact of Honey Adulteration. Encyclopedia. Available at: https://encyclopedia.pub/entry/39881. Accessed March 04, 2026.
Fakhlaei, Rafieh, Jinap Selamat, Alfi Khatib, Ahmad Faizal Abdull Razis, Rashidah Sukor, Syahida Ahmad, Arman Amani Babadi. "The Toxic Impact of Honey Adulteration" Encyclopedia, https://encyclopedia.pub/entry/39881 (accessed March 04, 2026).
Fakhlaei, R., Selamat, J., Khatib, A., Razis, A.F.A., Sukor, R., Ahmad, S., & Babadi, A.A. (2023, January 09). The Toxic Impact of Honey Adulteration. In Encyclopedia. https://encyclopedia.pub/entry/39881
Fakhlaei, Rafieh, et al. "The Toxic Impact of Honey Adulteration." Encyclopedia. Web. 09 January, 2023.
Copy Citation
Honey is characterized as a natural and raw foodstuff that can be consumed not only as a sweetener but also as medicine due to its therapeutic impact on human health. It is prone to adulterants caused by humans that manipulate the quality of honey. Honey adulterants are any substances that are added to the pure honey.
honey
adulteration
sugar adulterants
toxicity
1. Honey Adulterants
Low-cost sugars and commercial syrups are common substances for honey adulteration. Ismail and Ismail [1] described well-known adulterants from sugar cane and sugar beet such as corn syrup (CS), HFCS, glucose syrup (GS), sucrose syrup (SS), inverted syrup (IS), and high fructose inulin syrup (HFIS). Adulteration of honey by sugars alters the chemical and biochemical properties of honey, such as the enzymatic activity, electrical conductivity, and specific compounds contents [2].
Honey adulterates are selected based on the following three factors: the specific region of origin, the economic benefits and the sugars or sweeteners accessibility. A well-known example is employing rice and wheat syrup extraction as adulterants in Turkey and France [2]. The plant syrups can be harvested from heating vegetable juices or partial enzymatic hydrolysis [3][4][5][6]. According to [7][8], European countries adulterate the honey with HFIS. In this section, common sweeteners that are used in commercial honey adulteration will be introduced and their health impact discussed. The chemical structures of these widely-used sugar adulterants are presented in Figure 1.
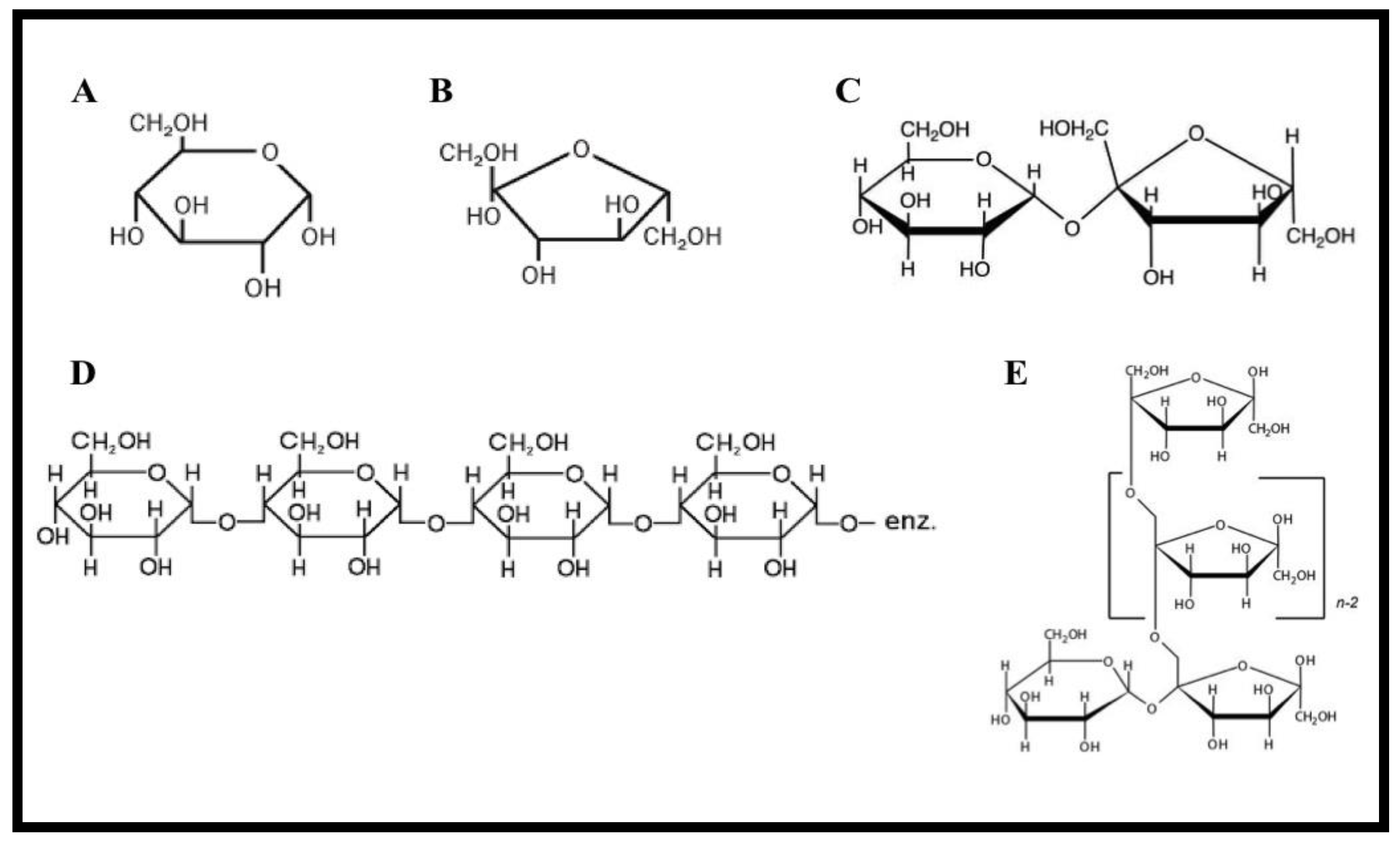
Figure 1. Chemical structures of widely used sugar adulterants in honey. (A) Glucose, (B) Fructose, (C) Sucrose, (D) Rice syrup, and (E) Inulin syrup.
1.1. Cane Sugar
Cane sugar is sucrose consisting of two sugar molecules monosaccharides (glucose and fructose). Glucose and fructose are monosaccharides with an identical chemical formula (C6H12O6) but a different chemical conformation, linked together by a weak glycosidic bond to produce sucrose (C12H22O11), a disaccharide. Generally, cane sugar is obtained by extracting juice from the sugar cane, a perennial C4 grass, followed by purification by chemical and physical means, evaporation to remove the water and separation of the sugar crystal [9]. Cane sugar originates from plants with a C4 metabolic pathway (Hatch-Slack cycle), while nectar originates from the C3 metabolic pathway (Calvin cycle).
In another study [10], cane sugar was used in both direct and indirect adulteration of honey. During direct adulteration, 10, 20, and 40% of syrup were added to the honey sample; for the indirect method, the bees were fed with syrup.
Concerning the toxicity of sugar, the lethal dose (LD50) is a useful tool to measure the short-term toxicity and causes the death of 50% of a test animal population. Hence, the acute oral LD50 of cane sugar in rats is 29,700 mg/kg BW, almost 30 g/kg, which placed this sugar into a particularly safe scale [11].
1.2. Corn Syrup
Corn syrup, or high fructose corn syrup (HFCS), is a viscous, odorless, and colorless liquid that is much denser than water. Corn syrup is a liquid sweetener derived by cornstarch hydrolysis, which is used as a sweetener in foods [12]. Based on its fructose content, corn syrup is classified as: HFCS-42 (42% fructose), HFCS-55 (55% fructose), HFCS-90 (90% fructose) [13]. The fructose from high fructose corn syrup cannot be used directly to generate energy and has to be stored in the liver as fat or glycogen. Hence, the extreme amount of fructose from HFCS cannot be processed beneficially in the body [14]. The LD50 of rare sugar syrup, which is obtained from HFCSm is 15,000 mg/kg BW for rats with no abnormalities; in humans, the acute non-effect level, which caused diarrhea, was estimated as 0.9 g/Kg BW as a dry solid base [15].
1.3. Palm Sugar
Palm sugar is extracted from the flower buds of the palm. It is a natural sweetener undergoing minimum steps during the chemical-free procedure. One study [16] reported that the major carbohydrates in palm sugar were sucrose, followed by glucose and fructose. The significant advantage of palm sugar is the lack of a blood sugar spiking effect, owing to its low glycemic index (~35). The most popular honey adulterant in India is jaggery syrup, which is prepared by the evaporation of palm tree extraction the evaporation of the sap of palm trees [17]. While sucrose and glucose are the main sugar components of palm sugar, the LD50 for sucrose is 29,700 mg/kg BW and for glucose 25,800 mg/kg BW for a rat [18].
1.4. Invert Sugar
Invert sugar (IS) is produced by cleavage of the sucrose into its monosaccharides building blocks, fructose, and dextrose. The inversion procedure is usually performed by heating the sucrose syrup in the presence of acids, alkali, or invertase [12]. The sugar content of IS originates from beet and cane plants, mimicking the pure honey sugar profile [19]. Invert sugar has been widely used in beverages and food industries for making non-crystallized cream, jams, artificial honey, and liquid sugar [20].
Inverted beet syrup is one of the most well-known adulterants, which can be tailored to mimic the natural sucrose (glucose-fructose) profile of honey and, as beet is a C3 plant, it is usually difficult to detect. In one study, various quantities of inverted beet syrup were added to the pure honey samples of clover, orange, and buckwheat [21]. Invert sugar is a generally accepted safe substance and it does not present toxic effects. It is recommended to take precautions in patients that present diabetes mellitus and also in patients with the rare hereditary problems of fructose intolerance, glucose-galactose malabsorption, or sucrase-isomaltase insufficiency [22].
As inverted sugar is a mixture of fructose and glucose, the LD50 value for this sugar is adopted from its structural sugars; the LD50 for fructose and glucose are 25,800 mg/kg BW and 29,700 mg/kg BW, respectively [23].
1.5. Rice Syrup
Rice syrup (RS), a product of rice polysaccharide hydrolysis, originating from a C3 plant (similar to beet syrup), is one of the most popular honey adulterants in China [12].
Rice syrup contains three sugars: maltotriose (52%), maltose (45%), and glucose (3%). Since maltose is two molecules of glucose and maltotriose is three molecules of glucose, rice syrup acts like 100% glucose inside the body. Honey adulterated with RS has recently emerged on the honey market. Rice syrup is a C3 syrup adulterant that follows a similar Calvin cycle of photosynthesis as natural honey [24]. Thus, rice syrup as a honey adulterant is a critical issue that affects quality assurance and food safety [25].
The unethical substitute brown rice syrup with HFCS in some organic foods has raised researchers’ concerns due to its high arsenic content [26]. Baby formulas that contain organic brown rice syrup (OBRS) have an increased arsenic level, above the drinking water standard as per the research led by [27], and there is no regulation to govern this particular scenario. Rice syrup acts as glucose inside the body; the LD50 of rice syrup is the same as glucose, which is 25,800 mg/kg BW [28].
1.6. Inulin Syrup
Inulin is naturally occurring polysaccharide, belonging to a class of fructans. These nutritional fibers are a chain of fructose residues linked to glucose at the end of the chain. The linkage arrangements of the fructose molecules determine the fructan type. For example, in the case of inulin, the chain of β2-1 linked fructose has been terminated by glucose. The common source of this polysaccharide are wheat, onion, bananas, garlic, asparagus, sunchoke, and chicory [12]. Furthermore, [26] added different proportions (5, 10, and 20%, w/w) of high fructose inulin syrup to a nectar honey sample to intentionally simulate honey adulteration.
Classical toxicology tests are difficult to apply to inulin, which is a micro ingredient. Although some high dose animal tests have been performed, none have revealed any toxic effects [9]. Thus, the LD50 values for fructose, glucose and sucrose are 25,800 mg/kg BW, 29,700 mg/kg BW and 29,700 mg/kg BW, respectively [29].
2. Adverse Health Impact of Honey Adulteration
The adverse health impacts of consuming adulterated honey on human health are not completely established yet due to an absence of systematic and scientific studies and lack of public awareness. Pure honey showed significantly lower toxicity due to containing simple sugar (glucose and fructose) and other essential nutrients such as proteins, antioxidants, and minerals [30]. While honey has an antibacterial effect, helping to fight common cold and some digestive problems, the mixture of inverted sugar or jaggery can sometimes restrict the antibacterial properties of honey and lead to stomach disorders [31]. Adulteration harms consumers’ health, which may cause increased blood sugar followed by the release of the insulin hormone and type II diabetes, abdominal weight gain, and obesity, a raise in the level of blood lipid, and high blood pressure [32].
Consumption of glucose from sugar-adulterated honey may elevate insulin secretion. Insulin activates the plasma membrane enzyme system with the properties of NADPH-oxidase resulting in not only the production of H2O2 and fructose but also increases uric acid in humans and rodents [2]. Uric acid generation in the body has unfavorable effects, namely, the inability to scavenge lipophilic radicals and breaking the radical chain propagation within the lipid membrane [33]. Under the same circumstances, glucose and fructose of sugar produce ROS through various mechanisms in the body, which is detrimental toward human health and causes chronic diseases such as atherosclerosis, diabetes, obesity, hypertension, coronary artery diseases, and finally heart failure [34]. Glucose that is either naturally produced in the body or added to foodstuff can be detrimental to humans.
Furthermore, the authors of one paper [35] investigated the short-term (two weeks) and long-term (16 weeks) effects of two different brands of honey consumption in the Malaysian market using male Sprague Dawley rats. The finding was illustrated that a total of five rats from both adulterated honey groups showed early mortality and many abnormal signs developed compared to rats fed with natural honey (pineapple honey) as a control. The abnormalities in the adulterated honey group represent significant body weight, fat pads, and BMI, and serum lipid profile (triglycerides, cholesterol, and glucose level) drastically increases. Since glucose and fructose can instantly convert to energy inside the cells, they represent a lower glycemic index compared to sucrose [36]. These results could indicate that long-term consumption of adulterated honey has a harmful impact on human health, requiring local and international authorities’ actions to control and regulate this matter.
The kidney serology and toxicology study of rats that consumed adulterated honey for 16 weeks showed kidney damage due to losing their capability to expel creatinine and urea from the serum [37]. In another in vivo study [36], it was proved that a long-term high sucrose diet may lead to the increment of both urea and creatinine. Meanwhile, Li et al. [38] stated that prolonged consumption of HFCS would lead to rat’s glomerular filtration failure.
According to a recent study by Arise and Malomo [39] on rats, hypercholesterolemia, hypertriglyceridemia, and hyperinsulinemia are caused by prolonged feeding of any sugar syrup such as sucrose or fructose, which leads to animal death. Furthermore, Ajibola et al. [40] reported the synthesis of triacylglycerides in the liver as a result of increasing the gene expressions of lipogenesis enzymes such as acetyl CoA carboxylase and fatty acid synthase.
In other research [41], rats fed with sugar adulterated honey exhibited a pale reddish color, exceptional kidney size, and observed nodules outside the kidneys. There was also an abnormal difference in the appearance of the liver, with slight discoloration to brown, particularly in the middle of this organ’s surface. In detail, some of the livers showed a roughly brown surface and their sizes were smaller, with whitish micronodules on the entire liver surface. Regardless of weight differentiation, the relative weight of the kidneys and lungs from rats fed with adulterated honey showed a significant increase compared to control rats. Moreover, the heart and brain from the rats fed with adulterated honey exhibited significant decreases [36]. These types of intensive investigations may be harmful toward the human body, and authorities should avoid it in the future.
Natural honey possesses some beneficial effects, such as lower total cholesterol and LDL in healthy overweight human subjects, while consumption of 3 to 20% of dietary fructose caused the elevation of total cholesterol and LDL by 9% and 11%, respectively [36]. In this regard, the addition of sugar to honey could be critical toward human health.
References
- Ismail, M.M.; Ismail, W.I.W. Development of stingless beekeeping projects in Malaysia. In Proceedings of the CSSPO International Conference 2018: Towards Inclusive & Sustainable Agriculture—Harmonizing Environmental, Social and Economic Dimensions: Is it Possible? Sarawak, Malaysia, 9–11 July 2018; p. 5.
- Soares, S.; Amaral, J.S.; Oliveira, M.B.P.; Mafra, I. A comprehensive review on the main honey authentication issues: Production and origin. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1072–1100.
- Guler, A.; Kocaokutgen, H.; Garipoglu, A.V.; Onder, H.; Ekinci, D.; Biyik, S. Detection of adulterated honey produced by honeybee (Apis mellifera L.) colonies fed with different levels of commercial industrial sugar (C3 and C4 plants) syrups by the carbon isotope ratio analysis. Food Chem. 2014, 155, 155–160.
- Wei, G.-X.; Huang, J.-K.; Jun, Y. Honey safety standards and its impacts on China’s honey export. J. Integr. Agric. 2012, 11, 684–693.
- Tosun, M. Detection of adulteration in honey samples added various sugar syrups with 13C/12C isotope ratio analysis method. Food Chem. 2013, 138, 1629–1632.
- Spiteri, M.; Dubin, E.; Cotton, J.; Poirel, M.; Corman, B.; Jamin, E.; Lees, M.; Rutledge, D. Data fusion between high resolution 1 H-NMR and mass spectrometry: A synergetic approach to honey botanical origin characterization. Anal. Bioanal. Chem. 2016, 408, 4389–4401.
- Corradini, C.; Cavazza, A.; Bignardi, C. High-performance anion-exchange chromatography coupled with pulsed electrochemical detection as a powerful tool to evaluate carbohydrates of food interest: Principles and applications. Int. J. Carbohydr. Chem. 2012, 2012, 1–13.
- Low, N.; Hammond, D. Detection of high fructose syrup from inulin in apple juice by capillary gas chromatography with flame ionization detection. Fruit Process. 1996, 4, 135–141.
- Ruiz-Matute, A.I.; Soria, A.C.; Martínez-Castro, I.; Sanz, M.L. A new methodology based on GC-MS to detect honey adulteration with commercial syrups. J. Agric. Food Chem. 2007, 55, 7264–7269.
- Li, S.; Zhang, X.; Shan, Y.; Su, D.; Ma, Q.; Wen, R.; Li, J. Qualitative and quantitative detection of honey adulterated with high-fructose corn syrup and maltose syrup by using near-infrared spectroscopy. Food Chem. 2017, 218, 231–236.
- Cordella, C.; Militao, J.S.; Clément, M.-C.; Drajnudel, P.; Cabrol-Bass, D. Detection and quantification of honey adulteration via direct incorporation of sugar syrups or bee-feeding: Preliminary study using high-performance anion exchange chromatography with pulsed amperometric detection (HPAEC-PAD) and chemometrics. Anal. Chim. Acta 2005, 531, 239–248.
- European Chemical Agency. Evaluation of New Scientific Evidence Concerning the Restrictions Contained in Annex XVII to Regulation (EC) No. 1907/2006 (REACH): Review of New Available Information for Di-’isononyl’Phthalate (DIN); European Chemical Agency: Helsinki, Finland, 2010.
- Olivares-Pérez, A.; Mejias-Brizuela, N.; Grande-Grande, A.; Fuentes-Tapia, I. Corn syrup holograms. Optik 2012, 123, 447–450.
- Zábrodská, B.; Vorlová, L. Adulteration of honey and available methods for detection—A review. Acta Vet. Brno 2015, 83, 85–102.
- Malik, V.S.; Popkin, B.M.; Bray, G.A.; Després, J.-P.; Willett, W.C.; Hu, F.B. Sugar-sweetened beverages and risk of metabolic syndrome and type 2 diabetes: A meta-analysis. Diabetes Care 2010, 33, 2477–2483.
- Yamada, T.; Iida, T.; Takamine, S.; Hayashi, N.; Okuma, K. Safety evaluation of rare sugar syrup: Single-dose oral toxicity in rats, reverse mutation assay, chromosome aberration assay, and acute non-effect level for diarrhea of a single dose in humans. Shokuhin Eiseigaku Zasshi. J. Food Hyg. Soc. Jpn. 2015, 56, 211–216.
- Luis, G.; Rubio, C.; Gutiérrez, A.; Hernández, C.; González-Weller, D.; Revert, C.; Castilla, A.; Abreu, P.; Hardisson, A. Palm tree syrup; nutritional composition of a natural edulcorant. Nutr. Hosp. 2012, 27, 548–552.
- Mishra, S.; Kamboj, U.; Kaur, H.; Kapur, P. Detection of jaggery syrup in honey using near-infrared spectroscopy. Int. J. Food Sci. Nutr. 2010, 61, 306–315.
- Gehlawat, J. New Technology for Invert Sugar and High Fructose Syrups from Sugarcane. Indian J. Chem. Technol. 2001, 8, 28–32.
- Se, K.W.; Ghoshal, S.K.; Wahab, R.A.; Ibrahim, R.K.R.; Lani, M.N. A simple approach for rapid detection and quantification of adulterants in stingless bees (Heterotrigona itama) honey. Food Res. Int. 2018, 105, 453–460.
- Veana, F.; Flores-Gallegos, A.C.; Gonzalez-Montemayor, A.M.; Michel-Michel, M.; Lopez-Lopez, L.; Aguilar-Zarate, P.; Ascacio-Valdés, J.A.; Rodríguez-Herrera, R. Invertase: An Enzyme with Importance in Confectionery Food Industry. In Enzymes in Food Technology; Springer: Singapore, 2018; pp. 187–212.
- Paradkar, M.; Irudayaraj, J. Discrimination and classification of beet and cane inverts in honey by FT-Raman spectroscopy. Food Chem. 2002, 76, 231–239.
- White, J.S.; Foreyt, J.P.; Melanson, K.J.; Angelopoulos, T.J. High-fructose corn syrup: Controversies and common sense. Am. J. Lifestyle Med. 2010, 4, 515–520.
- Wu, L.; Du, B.; Vander Heyden, Y.; Chen, L.; Zhao, L.; Wang, M.; Xue, X. Recent advancements in detecting sugar-based adulterants in honey—A challenge. TrAC Trends Anal. Chem. 2017, 86, 25–38.
- Du, B.; Wu, L.; Xue, X.; Chen, L.; Li, Y.; Zhao, J.; Cao, W. Rapid screening of multiclass syrup adulterants in honey by ultrahigh-performance liquid chromatography/quadrupole time of flight mass spectrometry. J. Agric. Food Chem. 2015, 63, 6614–6623.
- Siddiqui, A.J.; Musharraf, S.G.; Choudhary, M.I. Application of analytical methods in authentication and adulteration of honey. Food Chem. 2017, 217, 687–698.
- Lai, T.N.H.; André, C.; Rogez, H.; Mignolet, E.; Nguyen, T.B.T.; Larondelle, Y. Nutritional composition and antioxidant properties of the sim fruit (Rhodomyrtus tomentosa). Food Chem. 2015, 168, 410–416.
- Jackson, B.P.; Taylor, V.F.; Karagas, M.R.; Punshon, T.; Cottingham, K.L. Arsenic, organic foods, and brown rice syrup. Environ. Health Perspect. 2012, 120, 623–626.
- Drabińska, N.; Jarocka-Cyrta, E.; Markiewicz, L.H.; Krupa-Kozak, U. The effect of oligofructose-enriched inulin on faecal bacterial counts and microbiota-associated characteristics in celiac disease children following a gluten-free diet: Results of a randomized, placebo-controlled trial. Nutrients 2018, 10, 201.
- Peng, J.; Xie, W.; Jiang, J.; Zhao, Z.; Zhou, F.; Liu, F. Fast Quantification of Honey Adulteration with Laser-Induced Breakdown Spectroscopy and Chemometric Methods. Foods 2020, 9, 341.
- Bogdanov, S.; Jurendic, T.; Sieber, R.; Gallmann, P. Honey for nutrition and health: A review. J. Am. Coll. Nutr. 2008, 27, 677–689.
- Awasthi, S.; Jain, K.; Das, A.; Alam, R.; Surti, G.; Kishan, N. Analysis of food quality and food adulterants from different departmental & local grocery stores by qualitative analysis for food safety. IOSR JESTFT 2014, 8, 22–26.
- Afroz, R.; Tanvir, E.; Paul, S.; Bhoumik, N.C.; Gan, S.H.; Khalil, M.I. DNA damage inhibition properties of sundarban honey and its phenolic composition. J. Food Biochem. 2016, 40, 436–445.
- Muraoka-Cook, R.; Shin, I.; Yi, J.; Easterly, E.; Barcellos-Hoff, M.; Yingling, J.; Zent, R.; Arteaga, C. Activated type I TGF β receptor kinase enhances the survival of mammary epithelial cells and accelerates tumor progression. Oncogene 2006, 25, 3408–3423.
- Samat, S.; Enchang, F.K.; Abd Razak, A.; Hussein, F.N.; Ismail, W.I.W. Adulterated honey consumption can induce obesity, increase blood glucose level and demonstrate toxicity effects. Sains Malays. 2018, 47, 353–365.
- Samat, S.; Kanyan Enchang, F.; Nor Hussein, F.; Wan Ismail, W.I. Four-week consumption of Malaysian honey reduces excess weight gain and improves obesity-related parameters in high fat diet induced obese rats. Evid. Based Complement. Altern. Med 2017, 2017.
- Al-Waili, N.; Salom, K.; Al-Ghamdi, A.; Ansari, M.J. Antibiotic, pesticide, and microbial contaminants of honey: Human health hazards. Sci. World J. 2012, 2012.
- Li, L.; Zhao, Z.; Xia, J.; Xin, L.; Chen, Y.; Yang, S.; Li, K. A long-term high-fat/high-sucrose diet promotes kidney lipid deposition and causes apoptosis and glomerular hypertrophy in bama minipigs. PLoS ONE 2015, 10, e0142884.
- Arise, R.; Malomo, S. Effects of ivermectin and albendazole on some liver and kidney function indices in rats. Afr. J. Biochem. Res. 2009, 3, 190–197.
- Ajibola, A.; Chamunorwa, J.P.; Erlwanger, K.H. Dietary supplementation with natural honey promotes growth and health of male and female rats compared to cane syrup. Sci. Res. Essays 2013, 8, 543–553.
- Jensen, T.; Abdelmalek, M.F.; Sullivan, S.; Nadeau, K.J.; Green, M.; Roncal, C.; Nakagawa, T.; Kuwabara, M.; Sato, Y.; Kang, D.-H. Fructose and sugar: A major mediator of non-alcoholic fatty liver disease. J. Hepatol. 2018, 68, 1063–1075.
More
Information
Subjects:
Food Science & Technology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
3.7K
Revisions:
2 times
(View History)
Update Date:
09 Jan 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





