Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Guilherme Ramos Meyers | -- | 3851 | 2023-01-04 10:44:32 | | | |
| 2 | Camila Xu | -86 word(s) | 3765 | 2023-01-05 01:42:05 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Meyers, G.R.; Samouda, H.; Bohn, T. Dietary Fibre, Gut Microbiome and Genetic Variability. Encyclopedia. Available online: https://encyclopedia.pub/entry/39722 (accessed on 07 February 2026).
Meyers GR, Samouda H, Bohn T. Dietary Fibre, Gut Microbiome and Genetic Variability. Encyclopedia. Available at: https://encyclopedia.pub/entry/39722. Accessed February 07, 2026.
Meyers, Guilherme Ramos, Hanen Samouda, Torsten Bohn. "Dietary Fibre, Gut Microbiome and Genetic Variability" Encyclopedia, https://encyclopedia.pub/entry/39722 (accessed February 07, 2026).
Meyers, G.R., Samouda, H., & Bohn, T. (2023, January 04). Dietary Fibre, Gut Microbiome and Genetic Variability. In Encyclopedia. https://encyclopedia.pub/entry/39722
Meyers, Guilherme Ramos, et al. "Dietary Fibre, Gut Microbiome and Genetic Variability." Encyclopedia. Web. 04 January, 2023.
Copy Citation
Dietary fibre (DF) and associated compounds are metabolized by the gut microbiota and their resulting metabolites, especially short-chain fatty acids (SCFA), were significantly associated with health beneficial effects. However, SCFA metabolic pathways are not fully understood. As dietary patterns do not affect all individuals equally, the host genetic makeup may play a role in the metabolic fate of these metabolites, in addition to other factors that might influence the microbiota.
nutrigenetics
nutrigenomics
dietary fibre
short chain fatty acids
holobiont
personalized nutrition
1. Introduction
The human organism is composed of eukaryotic cells, as well as of an assembly of microbes collectively termed the microbiota, including archaea, bacteria, fungi and eukaryota. These may outnumber human cells, although a 1:1 ratio seems more likely, according to more recent estimates [1]. Regardless of the quantity of genes within individual microbial cells, the microbiome (the whole genome of the microbiota) encompasses over 1000 microbial species. Thus, the microbiome complements the human genome in functionality, such as enhancing digestion or protecting from pathogenic invasion [2][3]. The largest fraction of microbiota is found in the colon, and is termed, together with a smaller fraction residing in the stomach and small intestine, the gut microbiota [4]. Indeed, evolutionary biology proposes an analogous eukaryon-mitochondrion symbiosis that occurred between multicellular eukaryotes and prokaryotes millions of years ago, the so-called holobiont theory [2].
Evidence is mounting that the gut microbiota (GM) plays a fundamental role in regulating metabolic, immune and endocrine functions, as well as priming the immune response against pathogens. Indeed, GM alterations such as total abundance of or ratios between different species or families have been associated with many different health issues [5], specifically those of non-communicable chronic diseases (NCDs) such as obesity [6], cardiovascular disease and atherosclerosis [7], type 2 diabetes (T2D) [6][8], autoimmune disorders such as rheumatoid arthritis [9], ageing conditions, e.g., osteoporosis and sarcopenia [10], neurodegenerative diseases [11][12] including Parkinson’s [13] and Alzheimer’s disease [14], as well as several types of cancer [15][16]. In addition to GM changes, the majority of these conditions is characterized by a low-grade chronic inflammation [17][18][19][20], concurring with increased levels of oxidative stress [21][22].
Research has highlighted the significant and strong relationship between dietary patterns and the development of NCDs, such as CVD, depression, cognitive decline, multiple sclerosis, Parkinson’s disease, osteoarthritis and gastrointestinal conditions such as irritable bowel syndrome (IBD) [7][23][24][25][26][27][28][29][30][31][32][33][34], with much attention being dedicated to dietary fibre (DF) [35]. Overall, a higher DF intake has been associated with reduced all-cause mortality, e.g., in the Asian population [27], and hypotheses on its role as a health protective factor have been existing for several decades [36]. Studies have demonstrated improved health outcomes with higher fiber intake in conditions ranging from C. difficile infection [37] to paediatric kidney disease [38], showing its wide applicability in health maintenance. Regrettably, in most countries, it appears that DF intake has been on the decline. In Japan, where data are available since the 1950s, a 30% drop in DF intake was observed between the 1950s and 1970s, and then stabilized—though this may be subject to change, as younger generations report far less DF intake than their elders [39]. A review by the Nutrition Society [40], as assessed by national surveys in the UK, revealed a DF intake of approximately 14.8 g/d in adults, men and women, in 1999 [41], and about 13.6 g/d in 2009–2012 [42]. In the USA, DF intakes remained stable from 1999 to 2008, but well below recommendations, at around 15 g/d [43]. Concurrently, the highest consumption of DF in Europe was found in Germany (25 g/d for males and 23 g/d for females), based on a telephone-survey performed in 2005–2006 [40], being in line with EFSA recommendations.
DF may be at the centre of the symbiotic relationship between the GM and the human host [35][44][45][46][47][48][49]. DF is not absorbed or broken down to a significant degree by human digestive enzymes, and can, at least in part, be used as an energy substrate by the GM. Depending on the nature of DF, it is predominantly metabolized into short chain fatty acids (SCFA), including butyrate, acetate, and propionate [44]. Butyrate, acetate and propionate cross the enterocyte layer and are absorbed, while lactate and succinate appear to be intermediate products of DF fermentation [50]. Immediately, butyrate acts as the main energy source for colonocytes and controls maturation of mucosa associated lymphoid tissue (MALT) [51][52][53][54][55][56][57][58][59][60][61][62][63], characterized by a high presence of immune cells such as macrophages, B and T cells and that plays an important role in antigen sensing. Only a fraction of the produced SCFA enter the host’s systemic circulation, with acetate corresponding to around 75% of total peripheral SCFA [64][65]. However, these values have shown a high degree of inter-individual variation, as well as intra-individual variation such as dose–response, time-course and circadian variance [66]. SCFA may act as pleiotropic immunomodulators, i.e., having different functions in different tissues [35][51][67]. SCFA appear to be strong influencers of immune regulation, as seen in studies regarding asthma and atopy in infants, as well as in mice models [68][69][70][71][72], or gastrointestinal health in adults [35][48][51][52][73][74][75]. As described in the following chapters, SCFA production and concentrations were associated with disease risk. In addition to SCFA, DF acts as a vehicle for antioxidants in the upper gastrointestinal tract [76][77], as it is associated with a large number of phenolic compounds [44][78][79][80][81] and other secondary plant metabolites such as carotenoids [44][80]. Especially phenolic compounds may likewise be turned into bioactive metabolites by the GM [77][82], and synergies between these food derived compounds may exist, further highlighting their importance [83][84][85][86][87].
Apart from drugs, age, delivery method, medication intake, alcohol and tobacco consumption, pathogen exposure, besides diet in general, and dietary secondary plant metabolites in particular, are known to be significant modulators of the GM [44][88][89][90][91][92][93][94]. Dietary antioxidants can alter GM composition and thus its products [95]. However, the genetic background also modulates bacterial colonization [3][96]. In particular, genetic variants such as single nucleotide polymorphisms (SNPs), may further explain some of the inter-personal variability observed following fibre intake, such as circulating levels of SCFA [53][72][97][98]. Variations in genes such as GPR41, GPR43 or GPR109A (G-protein coupled receptors for SCFA) [99] could have substantial impact on the immunometabolism of certain tissues in particular, and the organism in general. Furthermore, transporter genes of the SLC16A family (monocarbohydrate transporters), effector genes such as MUC2 (for mucus layer production in the colon) or regulatory genes such as NRF2 (regulating the expression of proteins involved in the bodies’ antioxidant defence mechanism such as superoxide dismutase (SOD)), could have important downstream effects on health outcomes (Figure 1) due to impaired absorption of SCFA or by impacting their functions intracellularly [100].
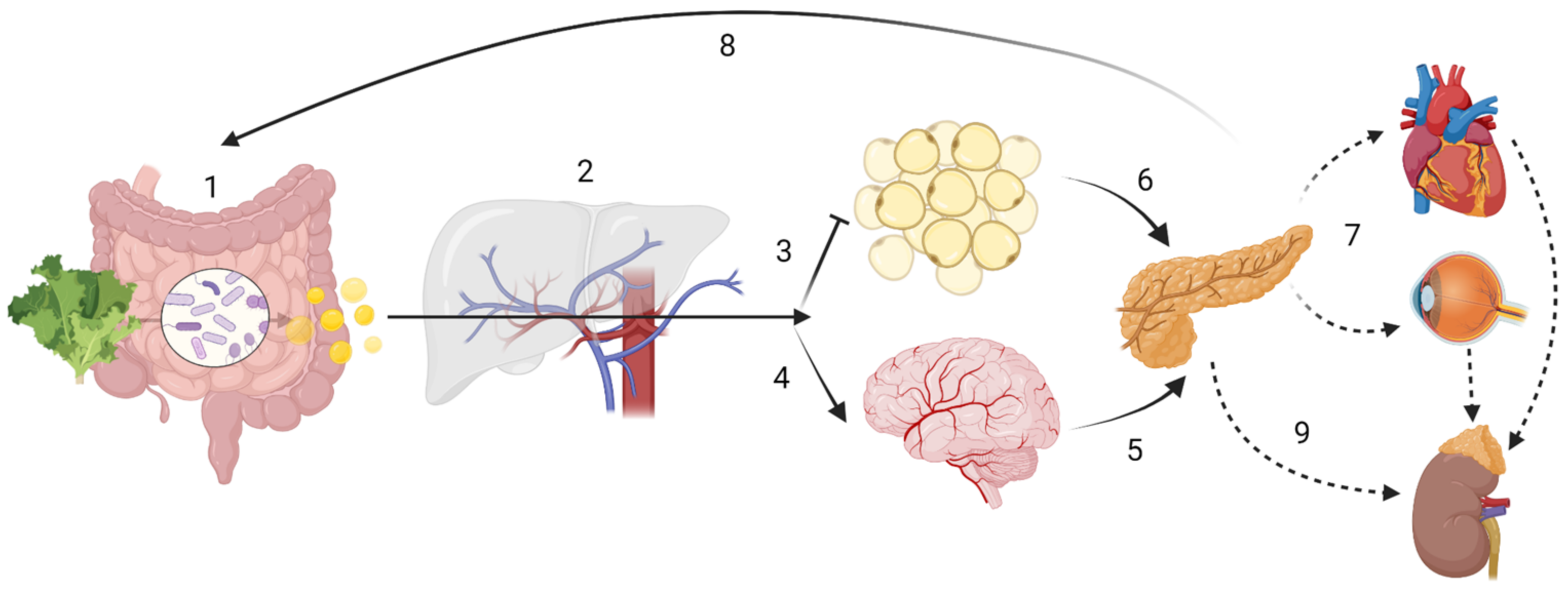
Figure 1. Host-driven variability in SCFA metabolism and distribution may lead to different disease outcomes. ADME (sub-) steps may explain the variability in SCFA effects. The enterotype influences the amount of SCFA produced, while human digestive enzymatic activity may regulate microbial communities; (1) Absorption: SNPs in mucin, MCTs or tight junction function could impair SCFA bioavailability. Butyrate is the main energy source for colonocytes. (2) In the portal circulation SCFA undergo first-pass effects, where a majority of propionate is metabolized via GPR109A, GPR43 and GPR41, having gluconeogenic or lipogenic effects. Distribution In the systemic circulation: although at present at low concentrations, butyrate and propionate are still detectable; acetate is now the most abundant SCFA. (3) Acetate inhibits lipolysis at the adipose tissue level. (4) Acetate can cross the “blood-brain-barrier” (BBB). Metabolism: SCFA have showed to be effective against microglial oxidative stress responses. SCFA may also have cellular signalling properties, as evidenced by its control of centrally released insulin (6) or its impact on the hypothalamic-pituitary-adrenal axis in leptin and cortisol responses, which may ultimately lead into maladaptive health conditions across the body (7). Finally, gluconeogenic, lipogenic and insulinogenic signals impact ghrelin, leptin and peptide YY release, leading to appetite suppression and satiety (8), improved insulin sensitivity and glucose metabolism, as well as reduction of serum lipids. (9) Excretion: in the kidney, SCFA can be re-absorbed by MCT1. Note: the intracellular effect of SCFA e.g., on HDAC or NF-κB are not displayed. Created with BioRender.com.
2. Dietary Fibre and Short Chain Fatty Acids
2.1. Dietary Fibre (DF)
Westernized types of diet are characterized by a relatively low intake in DF, despite attempts to increase its intake since the 1970s. Most European countries have established recommendations on daily intake for DF, e.g., 25–35 g for adults. Concretely, 25–32 g/d for adult women and 30–35 g/d for adult men, while recommendations for children and older adults depend on age, being approximately 3–4 g/MJ [40]. The Physicians Committee for Responsible Medicine (PCRM) of the US recommends even a considerably higher intake of 40 g/d for an optimal health [101].
The European Food Security Authority (EFSA) has recommended an adequate intake (AI) of 25 g/d for DF, mostly based on its association with improved bowel function (as per defecation frequency and transit time), and the reduction of gastro-intestinal symptoms such as constipation [102]. DF refers to total fibre occurring naturally in foods such as fruits, vegetables, pulses and cereal grains [40][102]. Grain products are at present the largest source for DF intake worldwide, providing approx. 32% of total dietary fibre intake in the USA and 48% in the Netherlands. Other sources vary widely in European countries, e.g., vegetables (12–21%), potatoes (6–19%) and fruits (8–23%) [40]. Lack of DF intake has been emphasized as one of the major dietary factors associated with the increased incidence of NCDs [103][104][105][106]. A recent systematic review and meta-analysis suggested that high DF consumption was associated with a 15–30% decrease in cardiovascular-related mortality, T2D and colorectal cancer, when compared with low-fibre consumption [107]. Concurring dietary factors such as increased sugar consumption, increased saturated fat consumption and low nutrient density, among others, and their possible relationship to metabolic and neurophysiological disorders, may be present and are expected to play a role [40][108]. However, as human lifespan has expanded during the past decades [109][110], researchers expect to face an increase of NCDs, as these are rather associated with age-related chronic inflammation (i.e., inflammageing [18]). Therefore, it is paramount to fully understand the pathophysiology of NCDs, and how to counteract them with affordable and efficient strategies, including improved dietary patterns and healthy food items [18][110][111][112][113][114][115][116][117]. In this respect, fiber intake could be increased both within a low-fat diet a low-carbohydrate diet. A randomized controlled trial aiming at weight reduction over a period of 12 months assessed sources of DF in a balanced low-fat diet vs. a balanced low-carbohydrate diet. A large proportion of DF for both diets was from non-starchy vegetables. While the low-fat group mainly increased DF intake from whole grains and fruits, the low-carbohydrate one obtained DF rather from vegetables and plant protein sources. This was further reflected in gut microbiota alterations throughout the intervention, and such dietary adaptations may constitute an important factor for precision nutrition [118].
A variety of definitions has been proposed to classify DF; most were dependent on the methods used to extract DF. This led to difficulties in defining the term, as most non-starch polysaccharides (NSP) were retrieved by such methods, which often did not include resistant (i.e., non-digestible) starches (RS). DF can further be categorized based on its solubility, fermentability or viscosity, which often caused distinctions within the group. While soluble fibres can be fermented to different degrees, and are the main substrate for colonic fermenters (e.g., β-glucans), insoluble fibres mainly serve a stool bulking function (e.g., cellulose). Both types of DF have beneficial health properties, and as such, the dichotomy of soluble-insoluble may no longer play a main role in terms of public health.
To date, definitions have reached a certain consensus [119][120]. DF is composed of carbohydrate polymers with three or more monomeric units (MU), which are neither hydrolysed by human digestive enzymes nor absorbed in the human intestine, and include NSPs from fruits, vegetables, grains and tubers, whether intrinsic or extracted, either chemically, enzymatically, or in physically modified forms. Polymers with more than 10 MU, e.g., cellulose, hemicelluloses, pectins, hydrocolloids (i.e., gums, β-glucans, mucilages); resistant oligosaccharides, e.g., fructo-oligosaccharides (FOS), galacto-saccharides (GOS) with 3–9 MU; and RS with 10 or more MU [40] are included. Furthermore, some constituents produced by micro-organisms (e.g., xanthan) and polysaccharide constituents of crustaceans and fungi (e.g., chitin, chitosan, chondroitin sulphate), are resistant to digestion and are included in the DF definition, according to some national agencies [40]. Furthermore, it has been proposed that proteins resistant to digestion exist, and may reproduce similar effects as DF, namely improved bowel function and improved immunity [121][122][123], but these are typically not included in the DF definition.
Thus, DF is any polymeric carbohydrate not digested in the small intestine. DF generally also includes substances associated with, or linked to plant cell walls, but that are not carbohydrates, such as lignin or polyphenols. Often, these distinctions are not reported in food tables, where only the sum of DF is given. In 2002, the French Agency for Food Security (ANSES), included in its definition all of the above polymeric carbohydrates (MU ≥ 3) as DF, while excluding animal-based sources and lactulose, a non-absorbable sugar, to prevent its incorporation into foods (as it is a strong laxative) as a fibre source [124].
Within this manuscript, DF is considered as any polymeric compound, which is not digestible by human enzymes and which mainly travels through the gut to reach the colonic milieu, where it is either fermented by colonic bacteria (i.e., broadly, soluble fibres) into smaller molecules such SCFA, or can act as a bulking agent during stool production (i.e., generally insoluble fibres). This broader definition would thus also include non-carbohydrate compounds such as lignin and resistant proteins, as well as compounds associated with plant-based carbohydrates, such as polyphenols. These compounds may also be substrates for bacteria, such as Akkermansia, Lactobacillus and Bifidobacterium, which produce metabolites such as SCFA, which in turn induce various beneficial effects on the host, including reduction in: appetite, insulin resistance, lipid accumulation, and inflammation [100]. However, the effects of phytochemicals are likely to vary according to the composition of the gut microbiota and host genetic polymorphisms, which affect absorption, detoxification, and overall bioactivities [125]. One such example is equol, produced form the isoflavone daidzein, which may bind to β-oestrogen receptors, and has been associated with the incidence of various types of hormone-associated cancers [126]. This is in line with the definition proposed by Jones [127], and may overcome the matter of “functionality” often discussed regarding DF, as previously pointed out [128].
Fibre fermentation relies on its chemical and physical structure, as well as the composition of the colonic microflora. Digestion of DF by the GM may vary or fluctuate depending on which fibres are consumed, and thus the amounts of SCFA produced too. For example, lignin and cellulose are rather lost through the stool, being insoluble bulking fibres; polysaccharides from extremely hard plant tissue areas are also less well digestible because physical encrustation and chemical bonding to lignin can occur [46]. Oligosaccharides, RS and pectins are the DF compounds thought to contribute the most to SCFA production in the colon [35].
2.2. Short Chain Fatty Acids (SCFA)
Recent studies on DF, GM and probiotics have emphasized the role of SCFA. Indeed, SCFA may be a good example of microbiota-derived modulator molecules, i.e., a nutrient that can modulate the host, acting as communicating molecules between the GM and the host [66]. Provided that SCFA metabolism may have a broad range of implications for human health, many studies are being conducted to understand their effects (Table 1). Sakata [66] recently pointed out relevant pitfalls in the study of these molecules. SCFA are defined as volatile fatty acids with a skeleton of six or less carbons in straight (C1, formate; C2, acetate; C3, propionate; C4, butyrate; C5, valerate; C6, caproate), or branched-chain conformation (C4, isobutyrate; C5, isovalerate and 2-methyl-butanoate). Acetate (C2), propionate (C3) and butyrate (C4) amount for 90–95% of total GM SCFA output and are derived from carbohydrate fermentation [129][130]. Until recently, caproate [131] and valerate [132] were considered dietary food components. However, recent studies have demonstrated that these may also be GM products, with caproate being significantly increased in faecal samples of volunteers with severe obesity (BMI ≥ 40) [131].
Branched-chain SCFA (BCFA), mainly isobutyrate, isovalerate and 2-methylbutanoate, contribute to as much as 5% of total SCFA production, and arise from the metabolism of the amino acids valine, leucine, and isoleucine, respectively [129][131]. BCFA levels in faecal samples show an inverse correlation with fibre consumption, especially insoluble fibre [131][133]. BCFA levels in stool have also been related to depression [32][34] and other psychiatric conditions [134], possibly through vagal afferent nerve signalling [135]. Furthermore, BCFA were found to be increased in subjects with hypercholesterolemia compared to normocholesterolemic individuals, with isobutyrate being associated with worse serum lipid profiles [136]. It is likely that such elevated BCFA correspond to high protein intake, such as from meat-based diet and a reduced DF intake, which are likewise associated with negative health outcomes and ageing related health complications [131].
Recently, products of DF fermentation have been termed post-biotics [137]. In human adults, the principal products of DF fermentation are SCFA together with certain gases (CO2, CH4, and H2), which may be taken up by the host, or excreted [50]. Production of SCFA in the colon accompanies the bacterial consumption of ammonia, H2S and BCFA in the synthesis of protein components for the microbial cell. Therefore, the reduction of these metabolites may also be, at least in part, responsible for the health benefits attributed to SCFA [66], as in addition to BCFA also ammonia [138] has been related to negative health outcomes such as neurotoxicity and hepatotoxicity, as well as increased intestinal permeability, loss of tight junction proteins and increase in pro-inflammatory cytokines as found in animal studies [139]. H2S, hydrogen disulphide, may be associated with neurological, cardiovascular and metabolic diseases, when abnormally produced [140].
In this research, SCFA describes, “saturated unbranched alkyl group monocarboxylic acids of 2 to 4 carbon atoms”, referring to acetate (C2), propionate (C3) and butyrate (C4). Researchers will briefly mention valerate (C5) and caproate (C6). It excludes BCFA, as well as succinate and lactate, which are rather intermediate products in GM metabolism, and therefore their concentrations in human serum are related rather to human metabolism, and not influenced considerably by GM or intestinal absorption.
Table 1. Identified effects of SCFA in human interventional, observational, and animal studies.
| SCFA | Study (Sample) | Study Design | Tissues Investigated | End-Point Measured | Observed Effects | Reference |
|---|---|---|---|---|---|---|
| Human interventional studies | ||||||
| C2 | H (n =32) | Case-control | Peripheral blood | Immunopharmacological effects of Ringer’s acetate | Increased polyclonal antibody production and NK cell activity in healthy and cancer subjects | [141] |
| C3 | H (n = 6) | Cross-over | Serum and stool | Blood lipids and glucose, stool bulk and microbiota | C3 supplementation lowers blood glucose. Lipid changes not significant; increase in stool bulk and Bifidobacteria after 1 week intervention | [142] |
| C4 | H (n = 16) | Cross-over | Sigmoid colon biopsies and plasma | Oxidative stress markers in colon; CRP, calprotectin; histological inflammation | Rectal administration significantly reduced uric acid and increased GSH. No significant changes in other parameters | [143] |
| Human Observational studies | ||||||
| C2-C6 | H (n = 232) | Observation | Stool | Levels of faecal SCFA and BCFA association with BMI and age | BCFA strongly correlated with age, but not with BMI; BCFA negatively associated with fibre consumption; BMI ≥ 40 showed significantly higher production of SCFA, total BCFA, isobutyrate, isovalerate and caproate SCFA production decreases with age |
[131] |
| Animal (interventional) studies | ||||||
| C2, C3 | M (n = 15) | Knock-out | Adipose tissue | Effects of GPCR43 activation | Reduction of lipolysis, reduced plasma free fatty acids levels without flushing associated with GPCR109A | [144] |
| C2, C3 | M (n = 12) | Case-control | Adipose, gut, vascular and mesenchymal tissues | GPCR41 and GPCR43 mRNA expression | GPCR43 activation promoted adipose differentiation via PPARγ2. No effects on GPCR41 | [145] |
| C2, C3, C4 | S (n = 10) | Case-control | Portal and peripheral blood, liver | Food intake following SCFA infusions | Dose-dependent depression in food intake, explained by C3 content in portal vein, which resolved with portal plexus denervation | [146] |
| C3 | R (n = 20) P (n = 12, 60) |
Case-control | Portal blood and liver | Cholesterol synthesis and distribution | Supplemented C3 likely absorbed in the stomach Dose-dependent hypocholesterolemic effect likely due to redistribution of cholesterol from plasma to liver, as opposed to synthesis inhibition |
[147][148] |
| C3 | R (n = 74, 114) | Case-control | Brain, intracerebral ventricles | Behavioural, electrophysiological, neuropathological, and biochemical effects | C3 intraventricular infusion impaired social behaviours, similar to those seen in human ASD; induced neuroinflammation and oxidative stress; Alteration of brain phospholipid and acylcarnitine1 profiles | [149][150] |
| C4 | R (n = 22) | Case-control | Duodenum, jejunum, cecum and distal colon | PYY and proglucagon gene expression in gut epithelial cells | Up-regulation of local peptide YY and proglucagon expression via colonocyte sensing following a RS diet in vivo, proved by in vitro incubation with butyrate | [151] |
| C4 | M (n = 16–20) | Case-control | Whole-body autopsy | Insulin sensitivity and energy metabolism, mitochondrial function | C4 supplementation prevented diet-induced insulin resistance and reduced adiposity in high-fat model, without reducing food intake. Attributed to enhanced mitochondrial activity and thermogenesis | [152] |
| In Vitro Studies | ||||||
| C2-C6 | M (n = 18) | N/A | mouse adipocyte cell line and adipose primary culture | Leptin expression | C2-C6 stimulate leptin expression via GPCR41 Acute administration of C3 increased leptin levels |
[153] |
| C2, C4 | R, B | N/A | Anterior pituitary, fat and liver aspirates | Leptin and leptin-receptor protein expression | C2 and C4 enhanced leptin expression in bovine pituitary and fat cells, however C4 inhibited leptin expression in rat anterior pituitary cells; while C4 suppressed leptin receptor expression in both rat and bovine pituitaries; probable species specific nutrient sensing | [154] |
| C2, C3, C4 | R, H | N/A | Colonic stimulation | Effects on colon functions, inc. motility | C3 and C4 induced phasic and tonic contractions of circular muscle via GPCR41 and GPCR43 in mucosae, C2 did not | [155] |
| C2, C3, C4 | M (n= 4) H (n= 3) |
N/A | Human blood samples, colon cultures (colo320DM) and mice with colitis | Anti-inflammatory properties of SCFA | All SCFA decreased neutrophil TNF-α release without affecting IL-8; all decreased IL-6 release; all inhibited NF-κB activity in colon cells; C4 > C3 > C2 | [156] |
| C3 | H (n = 5–9) | N/A | Human umbilical vein endothelial cells (HUVEC) | Expression of endothelial leukocyte adhesion molecules and leukocyte recruitment by cytokine-stimulation | Significant inhibition of TNF-α and NF-κB, reducing expression of VCAM-1 and ICAM-1 in a time- and dose-dependent manner; significantly increased PPARα expression | [157] |
| C3 | H (n = 28) | N/A | Omental and subcutaneous adipose tissue | Adipokine expression | Significant leptin induction and secretion; no effect on adiponectin; Reduction of resistin mRNA expression | [158] |
| C3 | R, H (n = 1) | N/A | Human blood and rat mesenteric lymph nodes | T and B lymphocyte proliferation and metabolism | Inhibition of lipid synthesis as a possible mechanism leading to reduction of lymphocyte proliferation | [159] |
| C3 | R (n = 9) | N/A | Isolated hepatocytes | Hepatic lipidogenesis | Inhibits hepatic cholesterol and fatty acid synthesis in a dose-dependent manner, possibly by competition with C2 | [160] |
ASD, autism spectrum disorder; B, bovine; H, human,; M, mice; P, pigs; R, rat; S, sheep; C2, acetate; C3, propionate; C4, butyrate; C5, valerate; C6, caproate; HUVEC, human umbilical vein endothelial cells; TNF-α, tumour necrosis factor alpha; VCAM-1, vascular cell adhesion molecule-1; ICAM-1, intracellular adhesion molecule-1; RS, resistant starch; GSH, glutathione peroxidase; PYY, peptide YY; SCFA, short chain fatty acids; BCFA, branched-chain fatty acids; BMI, body mass index; GPCR, G-protein coupled receptor; TNF-α, tumour necrosis factor alpha; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells N/A, not applicable.
References
- Sender, R.; Fuchs, S.; Milo, R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol. 2016, 14, e1002533.
- Zilber-Rosenberg, I.; Rosenberg, E. Role of microorganisms in the evolution of animals and plants: The hologenome theory of evolution. FEMS Microbiol. Rev. 2008, 32, 723–735.
- Goodrich, J.K.; Davenport, E.R.; Clark, A.G.; Ley, R.E. The Relationship Between the Human Genome and Microbiome Comes into View. Annu. Rev. Genet. 2017, 51, 413–433.
- Donaldson, G.P.; Lee, S.M.; Mazmanian, S.K. Gut biogeography of the bacterial microbiota. Nat. Rev. Microbiol. 2016, 14, 20–32.
- Sommer, F.; Bäckhed, F. The gut microbiota—Masters of host development and physiology. Nat. Rev. Microbiol. 2013, 11, 227–238.
- Perry, R.J.; Peng, L.; Barry, N.A.; Cline, G.W.; Zhang, D.; Cardone, R.L.; Petersen, K.F.; Kibbey, R.G.; Goodman, A.L.; Shulman, G.I. Acetate mediates a microbiome-brain-β cell axis promoting metabolic syndrome. Nature 2016, 7606, 213–217.
- McRae, M.P. Dietary Fiber is Beneficial for the Prevention of Cardiovascular Disease: An Umbrella Review of Meta-analyses. J. Chiropr. Med. 2017, 16, 289–299.
- Akshintala, V.S.; Talukdar, R.; Singh, V.K.; Goggins, M. The Gut Microbiome in Pancreatic Disease. Clin. Gastroenterol. Hepatol. 2018, 17, 290–295.
- Diamanti, A.P.; Rosado, M.M.; Laganà, B.; D’Amelio, R. Microbiota and chronic inflammatory arthritis: An interwoven link. J. Transl. Med. 2016, 14, 233.
- Kang, L.; Li, P.; Wang, D.; Wang, T.; Hao, D.; Qu, X. Alterations in intestinal microbiota diversity, composition, and function in patients with sarcopenia. Nature 2021, 11, 4628.
- Gentile, F.; Doneddu, P.E.; Riva, N.; Nobile-Orazio, E.; Quattrini, A. Diet, Microbiota and Brain Health: Unraveling the Network Intersecting Metabolism and Neurodegeneration. Int. J. Mol. Sci. 2020, 21, 7471.
- Friedland, R.P. Mechanisms of Molecular Mimicry Involving the Microbiota in Neurodegeneration. J. Alzheimer’s Dis. 2015, 45, 349–352.
- Baldini, F.; Hertel, J.; Sandt, E.; Thinnes, C.C.; Neuberger-Castillo, L.; Pavelka, L.; Betsou, F.; Krüger, R.; Thiele, I. Parkinson’s disease-associated alterations of the gut microbiome predict disease relevant changes in metabolic functions. BMC Biol. 2020, 18, 62.
- Goyal, D.; Ali, S.A.; Singh, R.K. Emerging role of gut microbiota in modulation of neuroinflammation and neurodegeneration with emphasis on Alzheimer’s disease. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 106, 9.
- Lazar, V.; Ditu, L.-M.; Pircalabioru, G.G.; Gheorghe, I.; Curutiu, C.; Holban, A.M.; Picu, A.; Petcu, L.; Chifiriuc, M.C. Aspects of Gut Microbiota and Immune System Interactions in Infectious Diseases, Immunopathology, and Cancer. Front. Immunol. 2018, 9, 1830.
- Kunzmann, A.T.; Coleman, H.G.; Huang, W.-Y.; Kitahara, C.M.; Cantwell, M.M.; Berndt, S.I. Dietary fiber intake and risk of colorectal cancer and incident and recurrent adenoma in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. Am. J. Clin. Nutr. 2015, 102, 881–890.
- Kotas, M.E.; Medzhitov, R. Homeostasis, Inflammation, and Disease Susceptibility. Cell 2015, 160, 816–827.
- Cevenini, E.; Monti, D.; Franceschi, C. Inflamm-ageing. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 14–20.
- Hotamisligli, G.S. Inflammation and metabolic disorders. Nature 2006, 444, 860–867.
- Hotamisligil, G.S. Inflammation, metaflammation and immunometabolic disorders. Nature 2017, 542, 177–185.
- Kaulmann, A.; Bohn, T. Carotenoids, inflammation, and oxidative stress—Implications of cellular signaling pathways and relation to chronic disease prevention. Nutr. Res. Rev. 2014, 34, 907–929.
- Menzel, A.; Samouda, H.; Dohet, F.; Loap, S.; Ellulu, M.S.; Bohn, T. Common and Novel Markers for Measuring Inflammation and Oxidative Stress Ex Vivo in Research and Clinical Practice—Which to Use Regarding Disease Outcomes? Antioxidants 2021, 10, 414.
- Pan, A.; Lin, X.; Hemler, E.; Hu, F.B. Diet and Cardiovascular Disease: Advances and Challenges in Population-Based Studies. Cell Metab. 2018, 27, 489–496.
- Christ, A.; Latz, E. The Western lifestyle has lasting effects on metaflammation. Nat. Rev. Immunol. 2019, 19, 267–268.
- Devore, E.E.; Kang, J.H.; Breteler, M.M.B.; Grodstein, F. Dietary intake of berries and flavonoids in relation to cognitive decline. Ann. Neurol. 2012, 72, 135–143.
- Darmadi-Blackberry, I.; Wahlqvist, M.L.; Kouris-Blazos, A.; Steen, B.; Lukito, W.; Horie, Y.; Horie, K. Legumes: The most important dietary predictor of survival in older people of different ethnicities. Asia Pac. J. Clin. Nutr. 2004, 13, 217–220.
- Katagiri, R.; Goto, A.; Sawada, N.; Yamaji, T.; Iwasaki, M.; Noda, M.; Iso, H.; Tsugane, S. Dietary fiber intake and total and cause-specific mortality: The Japan Public Health Center-based prospective study. Am. J. Clin. Nutr. 2020, 111, 1027–1035.
- Szic, K.S.V.; Declerck, K.; Vidaković, M.; Berghe, W.V. From inflammaging to healthy aging by dietary lifestyle choices: Is epigenetics the key to personalized nutrition? Clin. Epigenetics 2015, 7, 33.
- Fitzgerald, K.C.; Tyry, T.; Salter, A.; Cofield, S.S.; Cutter, G.; Fox, R.; Marrie, R.A. Diet quality is associated with disability and symptom severity in multiple sclerosis. Neurology 2018, 90, e1–e11.
- Yoo, J.Y.; Kim, S.S. Probiotics and Prebiotics: Present Status and Future Perspectives on Metabolic Disorders. Nutrients 2016, 3, 173.
- Mischley, L.K.; Lau, R.C.; Bennett, R.D. Role of Diet and Nutritional Supplements in Parkinson’s Disease Progression. Oxidative Med. Cell. Longev. 2017, 2017, 6405278.
- Lucas, M.; Chocano-Bedoya, P.; Shulze, M.B.; Mirzaei, F.; O’Reilly, É.J.; Okereke, O.I.; Hu, F.B.; Willett, W.C.; Ascherio, A. Inflammatory dietary pattern and risk of depression among women. Brain Behav. Immun. 2014, 36, 46–53.
- Rayman, M.P. Diet, nutrition and osteoarthritis. BMC Musculoskelet. Disord. 2015, 16, S7.
- Martínez-González, M.A.; Sánchez-Villegas, A. Food patterns and the prevention of depression. Proc. Nutr. Soc. 2016, 75, 139–146.
- Gill, S.K.; Rossi, M.; Bajka, B.; Whelan, K. Dietary fibre in gastrointestinal health and disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 101–116.
- Trowell, H. Definition of dietary fiber and hypotheses that it is a protective factor in certain diseases. Am. J. Clin. Nutr. 1976, 29, 417–427.
- Wu, Z.; Xu, Q.; Wang, Q.; Chen, Y.; Lv, L.; Zheng, B.; Yan, R.; Jiang, H.; Shen, J.; Wang, S.; et al. The impact of dietary fibers on Clostridioides difficile infection in a mouse model. Front. Cell. Infect. Microbiol. 2022, 12, 1028267.
- Snauwaert, E.; Paglialonga, F.; Vande Walle, J.; Wan, M.; Desloovere, A.; Polderman, N.; Renken-Terhaerdt, J.; Shaw, V.; Shroff, R. The benefits of dietary fiber: The gastrointestinal tract and beyond. Pediatr. Nephrol. 2022, 2022, 1–10.
- Nakaji, S.; Sugawara, K.; Saito, D.; Yoshioka, Y.; Macauley, D.; Bradley, T.; Kernohan, G.; Baxter, D. Trends in dietary fiber intake in Japan over the last century. Eur. J. Nutr. 2002, 41, 222–227.
- Stephen, A.M.; Champ, M.M.-J.; Cloran, S.J.; Fleith, M.; Lieshout, L.V.; Mejborn, H.; Burley, V.J. Dietary fibre in Europe: Current state of knowledge on definitions, sources, recommendations, intakes and relationships to health. Nutr. Res. Rev. 2017, 30, 149–190.
- Prynne, C.J.; McCarron, A.; Wadsworth, M.E.; Stephen, A.M. Dietary fibre and phytate—A balancing act: Results from three time points in a British birth cohort. Br. J. Nutr. 2010, 103, 274–280.
- Public Health England; Food Standards Agency. National Diet and Nutrition Survey: Results from Years 1, 2, 3 and 4 (Combined) of the Rolling Programme (2008/2009–2011/2012); Public Health England: London, UK, 2014.
- King, D.E.; Mainous, A.G., 3rd; Lambourne, C.A. Trends in dietary fiber intake in the United States, 1999–2008. J. Acad. Nutr. Diet. 2012, 112, 642–648.
- Dingeo, G.; Brito, A.; Samouda, H.; Iddir, M.; Frano, M.R.L.; Bohn, T. Phytochemicals as modifiers of gut microbial communities. Food Funct. 2020, 11, 8444–8471.
- Makki, K.; Deehan, E.C.; Walter, J.; Bäckhed, F. The Impact of Dietary Fiber on Gut Microbiota in Host Health and Disease. Cell Host Microbe 2018, 23, 705–716.
- Jha, S.K.; Singh, H.R.; Prakash, P. Dietary Fiber and Human Health: An Introduction; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1–22.
- Desai, M.S.; Seekatz, A.M.; Koropatkin, N.M.; Kamada, N.; Hickey, C.A.; Wolter, M.; Pudlo, N.A.; Kitamoto, S.; Terrapon, N.; Muller, A.; et al. A Dietary Fiber-Deprived Gut Microbiota Degrades the Colonic Mucus Barrier and Enhances Pathogen Susceptibility. Cell 2016, 167, 1339–1353.
- Tan, J.; McKenzie, C.; Vuillermin, P.J.; Goverse, G.; Vinuesa, C.G.; Mebius, R.E.; Macia, L.; Mackay, C.R. Dietary Fiber and Bacterial SCFA Enhance Oral Tolerance and Protect against Food Allergy through Diverse Cellular Pathways. Cell Rep. 2016, 15, 2809–2824.
- Kuo, S.-M. The interplay between fiber and the intestinal microbiome in the inflammatory response. Adv. Nutr. 2013, 4, 16–28.
- Topping, D.L.; Clifton, P.M. Short-Chain Fatty Acids and Human Colonic Function: Roles of Resistant Starch and Nonstarch Polysaccharides. Physiol. Rev. 2001, 81, 1031–1064.
- Gill, P.A.; Zelm, M.C.V.; Muir, J.G.; Gibson, P.R. Review article: Short chain fatty acids as potential therapeutic agents in human gastrointestinal and inflammatory disorders. Aliment. Pharmacol. Ther. 2018, 48, 15–34.
- Louis, P.; Hold, G.L.; Flint, H.J. The gut microbiota, bacterial metabolites and colorectal cancer. Nat. Rev. Microbiol. 2014, 12, 661–672.
- Singh, N.; Gurav, A.; Sivaprakasam, S.; Brady, E.; Padia, R.; Shi, H.; Thangaraju, M.; Prasad, P.D.; Manicassamy, S.; Munn, D.H.; et al. Activation of Gpr109a, Receptor for Niacin and the Commensal Metabolite Butyrate, Suppresses Colonic Inflammation and Carcinogenesis. Immunity 2014, 40, 128–139.
- Chang, P.V.; Hao, L.; Offermanns, S.; Medzhitov, R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc. Natl. Acad. Sci. USA 2014, 111, 2247–2252.
- Furusawa, Y.; Obata, Y.; Fukuda, S.; Endo, T.A.; Nakato, G.; Takahashi, D.; Nakanishi, Y.; Uetake, C.; Kato, K.; Kato, T.; et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013, 504, 446–450.
- Smith, P.M.; Howitt, M.R.; Panikov, N.; Michaud, M.; Gallini, C.A.; Bohlooly, Y.-M.; Glickman, J.N.; Garrett, W.S. The Microbial Metabolites, Short-Chain Fatty Acids, Regulate Colonic Treg Cell Homeostasis. Science 2013, 341, 569–573.
- Zimmerman, M.A.; Singh, N.; Martin, P.M.; Thangaraju, M.; Ganapathy, V.; Waller, J.L.; Shi, H.; Robertson, K.D.; Munn, D.H.; Liu, K. Butyrate suppresses colonic inflammation through HDAC1-dependent Fas upregulation and Fas-mediated apoptosis of T cells. Am. J. Physiol.-Gastrointest. Liver Physiol. 2012, 302, G1405–G1415.
- Donohoe, D.; Garge, N.; Zhang, X.; Sun, W.; O’Connel, T.; Bunger, M.; Bultman, S. The Microbiome and Butyrate Regulate Energy Metabolism and Autophagy in the Mammalian Colon. Cell Metab. 2011, 13, 517–526.
- Usami, M.; Kishimoto, K.; Ohata, A.; Miyoshi, M.; Aoyama, M.; Fueda, Y.; Kotani, J. Butyrate and trichostatin A attenuate nuclear factor κB activation and tumor necrosis factor α secretion and increase prostaglandin E2 secretion in human peripheral blood mononuclear cells. Nutr. Res. 2008, 28, 321–328.
- Hamer, H.M.; Jonkers, D.; Venema, K.; Vanhoutvin, S.; Troost, F.J.; Brummer, R.J. Review article: The role of butyrate on colonic function. Aliment. Pharmacol. Ther. 2007, 27, 104–119.
- Gaudier, E.; Jarry, A.; Blottière, H.M.; Coppet, P.D.; Buisine, M.P.; Aubert, J.P.; Laboisse, C.; Cherbut, C.; Hoebler, C. Butyrate specifically modulates MUC gene expression in intestinal epithelial goblet cells deprived of glucose. Am. J. Physiol. 2004, 287, G1168–G1174.
- Willemsen, L.E.M. Short chain fatty acids stimulate epithelial mucin 2 expression through differential effects on prostaglandin E1 and E2 production by intestinal myofibroblasts. Gut 2003, 52, 1442–1447.
- Pryde, S.E.; Duncan, S.H.; Hold, G.L.; Stewart, C.S.; Flint, H.J. The microbiology of butyrate formation in the human colon. FEMS Microbiol. Lett. 2002, 217, 133–139.
- Boets, E.; Gomand, S.V.; Deroover, L.; Preston, T.; Vermeulen, K.; de Preter, V.; Hamer, H.M.; van den Mooter, G.; de Vuyst, L.; Courtin, C.M.; et al. Systemic availability and metabolism of colonic-derived short-chain fatty acids in healthy subjects: A stable isotope study. J. Physiol. 2017, 595, 541–555.
- Bloemen, J.G.; Venema, K.; van de Poll, M.C.; Olde Damink, S.W.; Buurman, W.A.; Dejong, C.H. Short chain fatty acids exchange across the gut and liver in humans measured at surgery. Clin. Nutr. 2009, 28, 657–661.
- Sakata, T. Pitfalls in short-chain fatty acid research: A methodological review. Anim. Sci. J. 2019, 90, 3–13.
- Reichardt, N.; Vollmer, M.; Holtrop, G.; Farquharson, F.M.; Wefers, D.; Bunzel, M.; Duncan, S.H.; Drew, J.E.; Williams, L.M.; Milligan, G.; et al. Specific substrate-driven changes in human faecal microbiota composition contrast with functional redundancy in short-chain fatty acid production. ISME J. 2018, 12, 610–622.
- Durack, J.; Christophersen, C.T. Human Respiratory and Gut Microbiomes—Do They Really Contribute to Respiratory Health? Front. Pediatrics 2020, 8, 528.
- Levan, S.R.; Stamnes, K.A.; Lin, D.L.; Panzer, A.R.; Fukui, E.; McCauley, K.; Fujimura, K.E.; McKean, M.; Ownby, D.R.; Zoratti, E.M.; et al. Elevated faecal 12,13-diHOME concentration in neonates at high risk for asthma is produced by gut bacteria and impedes immune tolerance. Nat. Microbiol. 2019, 4, 1851–1861.
- Durack, J.; Kimes, N.E.; Lin, D.L.; Rauch, M.; McKean, M.; McCauley, K.; Panzer, A.R.; Mar, J.S.; Cabana, M.D.; Lynch, S.V. Delayed gut microbiota development in high-risk for asthma infants is temporarily modifiable by Lactobacillus supplementation. Nat. Commun. 2018, 9, 707.
- Fujimura, K.E.; Sitarik, A.R.; Havstad, S.; Lin, D.L.; Levan, S.; Fadrosh, D.; Panzer, A.R.; LaMere, B.; Rackaityte, E.; Lukacs, N.W.; et al. Neonatal gut microbiota associates with childhood multi– sensitized atopy and T–cell differentiation. Nat. Med. 2016, 22, 1187–1191.
- Maslowski, K.M.; Vieira, A.T.; Ng, A.; Kranich, J.; Sierro, F.; Di, Y.; Schilter, H.C.; Rolph, M.S.; Mackay, F.; Artis, D.; et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 2009, 461, 1282–1286.
- Skelly, A.N.; Sato, Y.; Kearney, S.; Honda, K. Mining the microbiota for microbial and metabolite-based immunotherapies. Nat. Rev. Immunol. 2019, 19, 305–323.
- Tuck, C.J.; Vanner, S.J. Dietary therapies for functional bowel symptoms: Recent advances, challenges, and future directions. Neurogastroenterol. Motil. 2017, 30, e13238.
- Blacher, E.; Levy, M.; Tatirovsky, E.; Elinav, E. Microbiome-Modulated Metabolites at the Interface of Host Immunity. J. Immunol. 2017, 198, 572–580.
- Saura-Calixto, F. Dietary Fiber as a Carrier of Dietary Antioxidants: An Essential Physiological Function. J. Agric. Food Chem. 2011, 1, 43–49.
- Palafox-Carlos, H.; Ayala-Zavala, J.F.; González-Aguilar, G.A. The Role of Dietary Fiber in the Bioaccessibility and Bioavailability of Fruit and Vegetable Antioxidants. J. Food Sci. 2011, 1, R6–R15.
- Luca, S.V.; Macovei, I.; Bujor, A.; Miron, A.; Skalicka-Woźniak, K.; Aprotosoaie, A.C.; Trifa, A. Bioactivity of dietary polyphenols: The role of metabolites. Food Sci. Nutr. 2020, 60, 626–659.
- Dobson, C.C.; Mottawea, W.; Rodrigue, A.; Pereira, B.L.B.; Hammami, R.; Power, K.A.; Bordenave, N. Impact of molecular interactions with phenolic compounds on food polysaccharides functionality. Adv. Food Nutr. Res. 2019, 90, 135–181.
- Padayachee, A.; Day, L.; Howell, K.; Gidley, M.J. Complexity and health functionality of plant cell wall fibers from fruits and vegetables. Food Sci. Nutr. 2017, 1, 59–81.
- Bohn, T. Dietary factors affecting polyphenol bioavailability. Nutr. Rev. 2014, 72, 429–452.
- Kaulmann, A.; Bohn, T. Bioactivity of Polyphenols: Preventive and Adjuvant Strategies toward Reducing Inflammatory Bowel Diseases—Promises, Perspectives, and Pitfalls. Oxidative Med. Cell. Longev. 2016, 2016, 9346470.
- Çelik, E.E.; Rubio, J.M.A.; Andersen, M.L.; Gökmen, V. Interactions of dietary fiber bound antioxidants with hydroxycinnamic and hydroxybenzoic acids in aqueous and liposome media. Food Chem. 2019, 278, 294–304.
- Bermúdez-Oria, A.; Rodríguez-Gutiérrez, G.; Fernández-Prior, Á.; Vioque, B.; Fernández-Bolaños, J. Strawberry dietary fiber functionalized with phenolic antioxidants from olives. Interactions between polysaccharides and phenolic compounds. Food Chem. 2019, 280, 310–320.
- Çelik, E.E.; Gökmen, V.; Skibsted, L.H. Synergism between Soluble and Dietary Fiber Bound Antioxidants. J. Agric. Food Chem. 2015, 63, 2338–2343.
- Doğan, E.; Gökmen, V. Mechanism of the interaction between insoluble wheat bran and polyphenols leading to increased antioxidant capacity. Food Res. Int. 2015, 69, 189–193.
- Saura-Calixto, F.; Serrano, J.; Goni, I. Intake and bioaccessibility of total polyphenols in a whole diet. Food Chem. 2007, 101, 492–501.
- Johnson, A.J.; Vangay, P.; Al-Ghalith, G.A.; Hillmann, B.M.; Ward, T.L.; Shields-Cutler, R.R.; Kim, A.D.; Shmagel, A.K.; Syed, A.N.; Students, P.M.C.; et al. Daily Sampling Reveals Personalized Diet-Microbiome Associations in Humans. Cell Host Microbe 2019, 25, 789–802.
- Filippis, F.D.; Pellegrini, N.; Vannini, L.; Jeffery, I.B.; Storia, A.L.; Laghi, L.; Serrazanetti, D.I.; Cagno, R.D.; Ferrocino, I.; Lazzi, C.; et al. High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. BMJ Gut 2016, 65, 1812–1821.
- Martínez, G.P.; Bäuerl, C.; Collado, M.C. Understanding gut microbiota in elderly’s health will enable intervention through probiotics. Benef. Microbes 2014, 3, 235–246.
- Wu, G.D.; Chen, J.; Hoffmann, C.; Bittinger, K.; Chen, Y.-Y.; Keilbaugh, S.A.; Bewtra, M.; Knights, D.; Walters, W.A.; Knight, R.; et al. Linking Long-Term Dietary Patterns with Gut Microbial Enterotypes. Science 2011, 334, 105–109.
- Markiewicz, L.H.; Honke, J.; Haros, M.; Swiaztecka, D.; Wróblewska, B. Diet shapes the ability of human intestinal microbiota to degrade phytate—In vitro studies. J. Appl. Microbiol. 2013, 115, 247–259.
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563.
- Merra, G.; Noce, A.; Marrone, G.; Cintoni, M.; Tarsitano, M.G.; Capacci, A.; de Lorenzo, A. Influence of Mediterranean Diet on Human Gut Microbiota. Nutrients 2020, 13, 7.
- Riaz Rajoka, M.S.; Thirumdas, R.; Mehwish, H.M.; Umair, M.; Khurshid, M.; Hayat, H.F.; Phimolsiripol, Y.; Pallarés, N.; Martí-Quijal, F.J.; Barba, F.J. Role of Food Antioxidants in Modulating Gut Microbial Communities: Novel Understandings in Intestinal Oxidative Stress Damage and Their Impact on Host Health. Antioxidants 2021, 10, 1563.
- Esworthy, R.S.; Smith, D.D.; Chu, F.-F. A Strong Impact of Genetic Background on Gut Microflora in Mice. Int. J. Inflamm. 2010, 2010, 986046.
- Nøhr, M.K.; Egerod, K.L.; Christiansen, S.H.; Gille, A.; Offermanns, S.; Schwartz, T.W.; Møller, M. Expression of the short chain fatty acid receptor GPR41/FFAR3 in autonomic and somatic sensory ganglia. Neuroscience 2015, 290, 126–137.
- Poul, E.L.; Loison, C.; Struyf, S.; Springael, J.-Y.; Lannoy, V.; Decobecq, M.-E.; Brezillon, S.; Dupriez, V.; Vassart, G.; Damme, J.V.; et al. Functional Characterization of Human Receptors for Short Chain Fatty Acids and Their Role in Polymorphonuclear Cell Activation. J. Biol. Chem. 2003, 278, 25481–25489.
- Hudson, B.D.; Murdoch, H.; Milligan, G. Minireview: The Effects of Species Ortholog and SNP Variation on Receptors for Free Fatty Acids. Mol. Endocrinol. 2013, 27, 1177–1187.
- Van der Hee, B.; Wells, J.M. Microbial Regulation of Host Physiology by Short-chain Fatty Acids. Trends Microbiol. 2021, 29, 700–712.
- Medicine, P.C.F.R. Dietary Fibre Recommendations. Available online: https://www.pcrm.org/good-nutrition/nutrition-information/fiber (accessed on 22 October 2021).
- EFSA Panel on Dietetic Products, Nutrition and Allergies. Scientific Opinion on Dietary Reference Values for carbohydrates and dietary fibre. EFSA J. 2010, 8, 1462.
- Alkerwi, A.A.; Sauvageot, N.; Donneau, A.-F.; Lair, M.-L.; Couffignal, S.; Beissel, J.; Delagardelle, C.; Wagener, Y.; Albert, A.; Guillaume, M. First nationwide survey on cardiovascular risk factors in Grand-Duchy of Luxembourg (ORISCAV-LUX). BMC Public Health 2010, 10, 468.
- Alkerwi, A.A.; Donneau, A.-F.; Sauvageot, N.; Lair, M.-L.; Albert, A.; Guillaume, M. Dietary, behavioural and socio-economic determinants of the metabolic syndrome among adults in Luxembourg: Findings from the ORISCAV-LUX study. Public Health Nutr. 2012, 15, 849–859.
- Alkerwi, A.A.; Pastore, J.; Sauvageot, N.; Coroller, G.L.; Bocquet, V.; d’Incau, M.; Aguayo, G.; Appenzeller, B.; Bejko, D.; Bohn, T.; et al. Challenges and benefits of integrating diverse sampling strategies in the observation of cardiovascular risk factors (ORISCAV-LUX 2) study. BMC Med. Res. Metholody 2019, 19, 27.
- Vangay, P.; Johnson, A.J.; Ward, T.L.; Al-Ghalith, G.A.; Shields-Cutler, R.R.; Hillmann, B.M.; Lucas, S.K.; Beura, L.K.; Thompson, E.A.; Till, L.M.; et al. U.S. immigration westernizes the human gut microbiome. Cell 2018, 175, 962–972.
- Reynolds, A.; Mann, J.; Cummings, J.; Winter, N.; Mete, E.; Te Morenga, L. Carbohydrate quality and human health: A series of systematic reviews and meta-analyses. Lancet 2019, 393, 434–445.
- Schättin, A.; Gennaro, F.; Egloff, M.; Vogt, S.; de Bruin, E.D. Physical Activity, Nutrition, Cognition, Neurophysiology, and Short-Time Synaptic Plasticity in Healthy Older Adults: A Cross-Sectional Study. Front. Aging Neurosci. 2018, 10, 242.
- Chesnais, J.-C. The Inversion of the Age Pyramid and the Future Population Decline in France: Implications and Policy Responses; United Nations: New York, NY, USA, 2000.
- Biagi, E.; Candela, M.; Turroni, S.; Garagnani, P.; Franceschi, C.; Brigidi, P. Ageing and gut microbes: Perspectives for health maintenance and longevity. Pharmacol. Res. 2013, 1, 11–20.
- Franceschi, C.; Capri, M.; Monti, D.; Giunta, S.; Olivieri, F.; Sevini, F.; Panourgia, M.P.; Invidia, L.; Celani, L.; Scurti, M.; et al. Inflammaging and anti-inflammaging: A systemic perspective on aging and longevity emerged from studies in humans. Mech. Ageing Dev. 2006, 128, 92–105.
- Franceschi, C.; Olivieri, F.; Marchegiani, F.; Cardelli, M.; Cavallone, L.; Capri, M.; Salvioli, S.; Valensin, S.; Benedictis, G.D.; Iorio, A.D.; et al. Genes involved in immune response/inflammation, IGF1/insulin pathway and response to oxidative stress play a major role in the genetics of human longevity: The lesson of centenarians. Mech. Ageing Dev. 2005, 126, 351–361.
- Dinan, T.G.; Cryan, J.F. Gut instincts: Microbiota as a key regulator of brain development, ageing and neurodegeneration. J. Physiol. 2016, 595, 489–494.
- Biagi, E.; Nylund, L.; Candela, M.; Ostan, R.; Bucci, L.; Pini, E.; Nikkïla, J.; Monti, D.; Satokari, R.; Franceschi, C.; et al. Through Ageing, and Beyond: Gut Microbiota and Inflammatory Status in Seniors and Centenarians. PLoS ONE 2010, 5, e10667.
- Biagi, E.; Candela, M.; Fairweather-Tait, S.; Franceschi, C.; Brigidi, P. Ageing of the human metaorganism: The microbial counterpart. Age 2012, 34, 247–267.
- Tiihonen, K.; Ouwehand, A.C.; Rautonen, N. Human intestinal microbiota and healthy ageing. Ageing Res. Rev. 2010, 2, 107–116.
- Duncan, S.H.; Flint, H.J. Probiotics and prebiotics and health in ageing populations. Maturitas 2013, 1, 44–50.
- Offringa, L.C.; Hartle, J.C.; Rigdon, J.; Gardner, C.D. Changes in Quantity and Sources of Dietary Fiber from Adopting Healthy Low-Fat vs. Healthy Low-Carb Weight Loss Diets: Secondary Analysis of DIETFITS Weight Loss Diet Study. Nutrients 2021, 13, 3625.
- Mccleary, B.V. Total Dietary Fiber (CODEX Definition) in Foods and Food Ingredients by a Rapid Enzymatic-Gravimetric Method and Liquid Chromatography: Collaborative Study, First Action 2017.16. J. AOAC Int. 2019, 102, 196–207.
- Codex Alimentarius Commission. Report of the 30th Session of the Codex Committee on Nutrition and Foods for Special Dietary Uses; No. ALINORM 02/32/26; FAO: Rome, Italy; WHO: Geneva, Switzerland, 2009.
- Kato, N.; Iwami, K. Resistant Protein; Its Existence and Function Beneficial to Health. J. Nutr. Sci. Vitaminol. 2002, 48, 1–5.
- Wang, Z.; Liang, M.; Li, H.; Cai, L.; Yang, L. Rice Protein Exerts Anti-Inflammatory Effect in Growing and Adult Rats via Suppressing NF-κB Pathway. Int. J. Mol. Sci. 2019, 20, 6164.
- Yang, L.; Chen, J.; Xu, T.; Qiu, W.; Zhang, Y.; Zhang, L.; Xu, F.; Liu, H. Rice Protein Extracted by Different Methods Affects Cholesterol Metabolism in Rats Due to Its Lower Digestibility. Int. J. Mol. Sci. 2011, 12, 7594–7608.
- Agence Française de Sécurité Sanitaire des Aliments (AFFSA). Dietary Fibre: Definitions, Analysis and Nutrition Claims; Agence Française de Sécurité Sanitaire des Aliments (AFFSA): Paris, France, 2002.
- Martel, J.; Ojcius, D.M.; Ko, Y.-F.; Young, J.D. Phytochemicals as Prebiotics and Biological Stress Inducers. Trends Biochem. Sci. 2020, 45, 462–471.
- Fatima, A.; Khan, M.S.; Ahmad, M.W. Therapeutic Potential of Equol: A Comprehensive Review. Curr. Pharm. Des. 2020, 26, 5837–5843.
- Jones, J.M. CODEX-aligned dietary fiber definitions help to bridge the ‘fiber gap’. Nutr. J. 2014, 13, 34.
- Williams, B.A.; Mikkelsen, D.; Flanagan, B.M.; Gidley, M.J. “Dietary fibre”: Moving beyond the “soluble/insoluble” classification for monogastric nutrition, with an emphasis on humans and pigs. J. Anim. Sci. Biotechnol. 2019, 10, 45.
- Macfarlane, G.T.; Macfarlane, S. Bacteria, Colonic Fermentation, and Gastrointestinal Health. J. AOAC Int. 2012, 95, 50–60.
- Cummings, J.H.; Gibson, G.R.; Macfarlane, G.T. Quantitative estimates of fermentation in the hind gut of man. Acta Vet. Scand. Suppl. 1989, 86, 76–82.
- Rios-Covian, D.; González, S.; Nogacka, A.M.; Arboleya, S.; Salazar, N.; Gueimonde, M.; de Los Reyes-Gavilán, C.G. An Overview on Fecal Branched Short-Chain Fatty Acids Along Human Life and as Related with Body Mass Index: Associated Dietary and Anthropometric Factors. Front. Microbiol. 2020, 11, 973.
- McDonald, J.A.K.; Mullish, B.H.; Pechlivanis, A.; Liu, Z.; Brignardello, J.; Kao, D.; Holmes, E.; Li, J.V.; Clarke, T.B.; Thursz, M.R.; et al. Inhibiting Growth of Clostridioides difficile by Restoring Valerate, Produced by the Intestinal Microbiota. Gastroenterology 2018, 155, 1495–1507.e1415.
- François, I.E.J.A.; Lescroart, O.; Veraverbeke, W.S.; Marzorati, M.; Possemiers, S.; Hamer, H.; Windey, K.; Welling, G.W.; Delcour, J.A.; Courtin, C.M.; et al. Effects of Wheat Bran Extract Containing Arabinoxylan Oligosaccharides on Gastrointestinal Parameters in Healthy Preadolescent Children. J. Pediatric Gastroenterol. Nutr. 2014, 58, 647–653.
- Breit, S.; Kupferberg, A.; Rogler, G.; Hasler, G. Vagus Nerve as Modulator of the Gut-Brain Axis in Psychiatric and Inflammatory Disorders. Front. Psychiatry 2018, 9, 44.
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids from Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020, 11, 25.
- Granado-Serrano, A.B.; Martín-Garí, M.; Sánchez, V.; Riart Solans, M.; Berdún, R.; Ludwig, I.A.; Rubió, L.; Vilaprinyó, E.; Portero-Otín, M.; Serrano, J.C.E. Faecal bacterial and short-chain fatty acids signature in hypercholesterolemia. Sci. Rep. 2019, 9, 1772.
- Nataraj, B.H.; Ali, S.A.; Behare, P.V.; Yadav, H. Postbiotics-parabiotics: The new horizons in microbial biotherapy and functional foods. Microb. Cell Factories 2020, 19, 168.
- Dasarathy, S.; Mookerjee, R.P.; Rackayova, V.; Rangroo Thrane, V.; Vairappan, B.; Ott, P.; Rose, C.F. Ammonia toxicity: From head to toe? Metab. Brain Dis. 2017, 32, 529–538.
- Ding, L.; Huang, Z.; Lu, Y.; Liang, L.; Li, N.; Xu, Z.; Zhang, J.; Shi, H.; Hong, M. Toxic effects of ammonia on intestinal health and microbiota in red-eared slider (Trachemys scripta elegans). Chemosphere 2021, 280, 130630.
- Di Masi, A.; Ascenzi, P. H2S: A “double face” molecule in health and disease. BioFactors 2013, 39, 186–196.
- Ishizaka, S.; Kikuchi, E.; Tsujii, T. Effects of acetate on human immune system. Immunopharmacol. Immunotoxicol. 1993, 15, 151–162.
- Todesco, T.; Rao, A.V.; Bosello, O.; Jenkins, D.J. Propionate lowers blood glucose and alters lipid metabolism in healthy subjects. Am. J. Clin. Nutr. 1991, 54, 860–865.
- Hamer, H.M.; Jonkers, D.M.; Bast, A.; Vanhoutvin, S.A.; Fischer, M.A.; Kodde, A.; Troost, F.J.; Venema, K.; Brummer, R.J. Butyrate modulates oxidative stress in the colonic mucosa of healthy humans. Clin. Nutr. 2009, 28, 88–93.
- Ge, H.; Li, X.; Weiszmann, J.; Wang, P.; Baribault, H.; Chen, J.L.; Tian, H.; Li, Y. Activation of G protein-coupled receptor 43 in adipocytes leads to inhibition of lipolysis and suppression of plasma free fatty acids. Endocrinology 2008, 149, 4519–4526.
- Hong, Y.-H.; Nishimura, Y.; Hishikawa, D.; Tsuzuki, H.; Miyahara, H.; Gotoh, C.; Choi, K.-C.; Feng, D.D.; Chen, C.; Lee, H.-G.; et al. Acetate and Propionate Short Chain Fatty Acids Stimulate Adipogenesis via GPCR43. Endocrinology 2005, 146, 5092–5099.
- Anil, M.H.; Forbes, J.M. Feeding in sheep during intraportal infusions of short-chain fatty acids and the effect of liver denervation. J. Physiol. 1980, 298, 407–414.
- Thacker, P.A.; Bell, J.M.; Classen, H.L.; Campbell, G.L.; Rossnagel, B.G. The nutritive value of hulless barley for swine. Anim. Feed Sci. Technol. 1988, 19, 191–196.
- Illman, R.J.; Topping, D.L.; McLntosh, G.H.; Trimble, R.P.; Storer, G.B.; Taylor, M.N.; Cheng, B.Q. Hypocholesterolaemic Effects of Dietary Propionate: Studies in Whole Animals and Perfused Rat Liver. Ann. Nutr. Metab. 1988, 32, 97–107.
- MacFabe, D.F.; Cain, D.P.; Rodriguez-Capote, K.; Franklin, A.E.; Hoffman, J.E.; Boon, F.; Taylor, A.R.; Kavaliers, M.; Ossenkopp, K.P. Neurobiological effects of intraventricular propionic acid in rats: Possible role of short chain fatty acids on the pathogenesis and characteristics of autism spectrum disorders. Behav. Brain Res. 2007, 176, 149–169.
- Shultz, S.R.; MacFabe, D.F.; Ossenkopp, K.P.; Scratch, S.; Whelan, J.; Taylor, R.; Cain, D.P. Intracerebroventricular injection of propionic acid, an enteric bacterial metabolic end-product, impairs social behavior in the rat: Implications for an animal model of autism. Neuropharmacology 2008, 54, 901–911.
- Zhou, J.; Hegsted, M.; McCutcheon, K.L.; Keenan, M.J.; Xi, X.; Raggio, A.M.; Martin, R.J. Peptide YY and Proglucagon mRNA Expression Patterns and Regulation in the Gut. Obesity 2006, 14, 683–689.
- Gao, Z.; Yin, J.; Zhang, J.; Ward, R.E.; Martin, R.J.; Lefevre, M.; Cefalu, W.T.; Ye, J. Butyrate Improves Insulin Sensitivity and Increases Energy Expenditure in Mice. Diabetes 2009, 58, 1509–1517.
- Xiong, Y.; Miyamoto, N.; Shibata, K.; Valasek, M.A.; Motoike, T.; Kedzierski, R.M.; Yanagisawa, M. Short-chain fatty acids stimulate leptin production in adipocytes through the G protein-coupled receptor GPR41. Proc. Natl. Acad. Sci. USA 2004, 101, 1045–1050.
- Yonekura, S.; Senoo, T.; Kobayashi, Y.; Yonezawa, T.; Katoh, K.; Obara, Y. Effects of acetate and butyrate on the expression of leptin and short-form leptin receptor in bovine and rat anterior pituitary cells. Gen. Comp. Endocrinol. 2003, 133, 165–172.
- Tazoe, H.; Otomo, Y.; Kaji, I.; Tanaka, R.; Karaki, S.; Kuwahara, A. Roles of short-chain fatty acids receptors, GPR41 and GPR43 on colonic functions. J. Physiol. Pharmacol. 2008, 59, 251–262.
- Tedelind, S.; Westberg, F.; Kjerrulf, M.; Vidal, A. Anti-inflammatory properties of the short-chain fatty acids acetate and propionate: A study with relevance to inflammatory bowel disease. World J. Gastroenterol. 2007, 13, 2826–2832.
- Zapolska-Downar, D.; Naruszewicz, M. Propionate reduces the cytokine-induced VCAM-1 and ICAM-1 expression by inhibiting nuclear factor-kappa B (NF-kappaB) activation. J. Physiol. Pharmacol. 2009, 60, 123–131.
- Al-Lahham, S.H.; Roelofsen, H.; Priebe, M.; Weening, D.; Dijkstra, M.; Hoek, A.; Rezaee, F.; Venema, K.; Vonk, R.J. Regulation of adipokine production in human adipose tissue by propionic acid. Eur. J. Clin. Investig. 2010, 40, 401–407.
- Curi, R.; Bond, J.A.; Calder, P.C.; Newsholme, E.A. Propionate regulates lymphocyte proliferation and metabolism. Gen. Pharmacol. 1993, 24, 591–597.
- Wright, R.S.; Anderson, J.W.; Bridges, S.R. Propionate Inhibits Hepatocyte Lipid Synthesis. Proc. Soc. Exp. Biol. Med. 1990, 195, 26–29.
More
Information
Subjects:
Nutrition & Dietetics
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
595
Revisions:
2 times
(View History)
Update Date:
05 Jan 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




