Dietary fibre (DF) and associated compounds are metabolized by the gut microbiota and their resulting metabolites, especially short-chain fatty acids (SCFA), were significantly associated with health beneficial effects. However, SCFA metabolic pathways are not fully understood. As dietary patterns do not affect all individuals equally, the host genetic makeup may play a role in the metabolic fate of these metabolites, in addition to other factors that might influence the microbiota. In this article, we review the metabolic pathways of DF, from intake to the intracellular metabolism of fibre-derived products, and identify possible sources of inter-individual variability related to microbiota composition and genetic variation. Such variability may be indicative of the phenotypic flexibility in response to diet, and may be predictive of long-term adaptations to dietary factors, including maladaptation and tissue damage, which may develop into disease in individuals with specific predispositions, thus allowing for a better prediction of potential health effects following personalized intervention with DF.
- nutrigenetics
- nutrigenomics
- dietary fibre
- short chain fatty acids
- holobiont
- personalized nutrition
1. Introduction
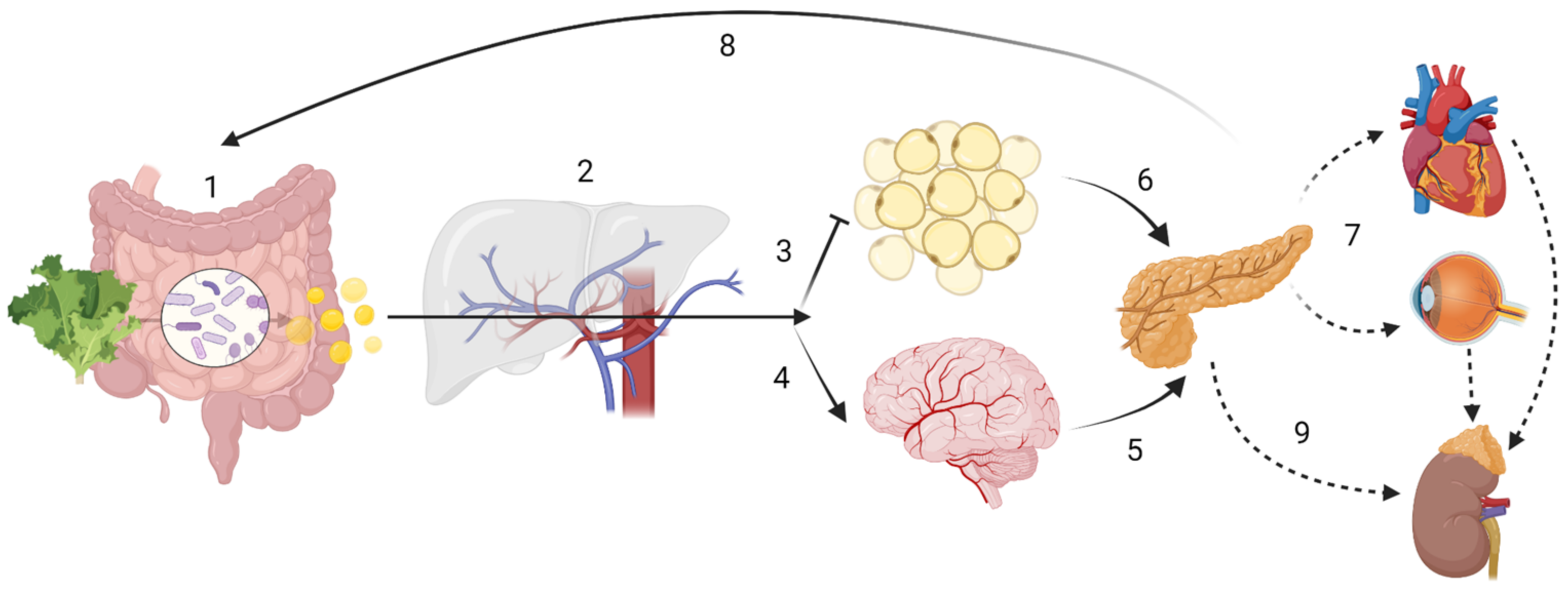
2. Dietary Fibre and Short Chain Fatty Acids
2.1. Dietary Fibre (DF)
2.2. Short Chain Fatty Acids (SCFA)
| SCFA | Study (Sample) | Study Design | Tissues Investigated | End-Point Measured | Observed Effects | Reference | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Human interventional studies | |||||||||||
| C2 | H ( | n | =32) | Case-control | Peripheral blood | Immunopharmacological effects of Ringer’s acetate | Increased polyclonal antibody production and NK cell activity in healthy and cancer subjects | [141] | |||
| C3 | H ( | n | = 6) | Cross-over | Serum and stool | Blood lipids and glucose, stool bulk and microbiota | C3 supplementation lowers blood glucose. Lipid changes not significant; increase in stool bulk and Bifidobacteria after 1 week intervention | [142] | |||
| C4 | H ( | n | = 16) | Cross-over | Sigmoid colon biopsies and plasma | Oxidative stress markers in colon; CRP, calprotectin; histological inflammation | Rectal administration significantly reduced uric acid and increased GSH. No significant changes in other parameters | [143] | |||
| Human Observational studies | |||||||||||
| C2-C6 | H ( | n | = 232) | Observation | Stool | Levels of faecal SCFA and BCFA association with BMI and age | BCFA strongly correlated with age, but not with BMI; BCFA negatively associated with fibre consumption; BMI ≥ 40 showed significantly higher production of SCFA, total BCFA, isobutyrate, isovalerate and caproate SCFA production decreases with age |
[131] | |||
| Animal (interventional) studies | |||||||||||
| C2, C3 | M ( | n | = 15) | Knock-out | Adipose tissue | Effects of GPCR43 activation | Reduction of lipolysis, reduced plasma free fatty acids levels without flushing associated with GPCR109A | [144] | |||
| C2, C3 | M ( | n | = 12) | Case-control | Adipose, gut, vascular and mesenchymal tissues | GPCR41 and GPCR43 mRNA expression | GPCR43 activation promoted adipose differentiation via PPARγ2. No effects on GPCR41 | [145] | |||
| C2, C3, C4 | S ( | n | = 10) | Case-control | Portal and peripheral blood, liver | Food intake following SCFA infusions | Dose-dependent depression in food intake, explained by C3 content in portal vein, which resolved with portal plexus denervation | [146] | |||
| C3 | R ( | n | = 20) P ( | n | = 12, 60) | Case-control | Portal blood and liver | Cholesterol synthesis and distribution | Supplemented C3 likely absorbed in the stomach Dose-dependent hypocholesterolemic effect likely due to redistribution of cholesterol from plasma to liver, as opposed to synthesis inhibition |
[147,148] | [147][148] |
| C3 | R ( | n | = 74, 114) | Case-control | Brain, intracerebral ventricles | Behavioural, electrophysiological, neuropathological, and biochemical effects | C3 intraventricular infusion impaired social behaviours, similar to those seen in human ASD; induced neuroinflammation and oxidative stress; Alteration of brain phospholipid and acylcarnitine1 profiles | [149,150] | [149][150] | ||
| C4 | R ( | n | = 22) | Case-control | Duodenum, jejunum, cecum and distal colon | PYY and proglucagon gene expression in gut epithelial cells | Up-regulation of local peptide YY and proglucagon expression via colonocyte sensing following a RS diet in vivo, proved by in vitro incubation with butyrate | [151] | |||
| C4 | M ( | n | = 16–20) | Case-control | Whole-body autopsy | Insulin sensitivity and energy metabolism, mitochondrial function | C4 supplementation prevented diet-induced insulin resistance and reduced adiposity in high-fat model, without reducing food intake. Attributed to enhanced mitochondrial activity and thermogenesis | [152] | |||
| In Vitro Studies | |||||||||||
| C2-C6 | M ( | n | = 18) | N/A | mouse adipocyte cell line and adipose primary culture | Leptin expression | C2-C6 stimulate leptin expression via GPCR41 Acute administration of C3 increased leptin levels |
[153] | |||
| C2, C4 | R, B | N/A | Anterior pituitary, fat and liver aspirates | Leptin and leptin-receptor protein expression | C2 and C4 enhanced leptin expression in bovine pituitary and fat cells, however C4 inhibited leptin expression in rat anterior pituitary cells; while C4 suppressed leptin receptor expression in both rat and bovine pituitaries; probable species specific nutrient sensing | [154] | |||||
| C2, C3, C4 | R, H | N/A | Colonic stimulation | Effects on colon functions, inc. motility | C3 and C4 induced phasic and tonic contractions of circular muscle via GPCR41 and GPCR43 in mucosae, C2 did not | [155] | |||||
| C2, C3, C4 | M ( | n | = 4) H ( | n | = 3) | N/A | Human blood samples, colon cultures (colo320DM) and mice with colitis | Anti-inflammatory properties of SCFA | All SCFA decreased neutrophil TNF-α release without affecting IL-8; all decreased IL-6 release; all inhibited NF-κB activity in colon cells; C4 > C3 > C2 | [156] | |
| C3 | H ( | n | = 5–9) | N/A | Human umbilical vein endothelial cells (HUVEC) | Expression of endothelial leukocyte adhesion molecules and leukocyte recruitment by cytokine-stimulation | Significant inhibition of TNF-α and NF-κB, reducing expression of VCAM-1 and ICAM-1 in a time- and dose-dependent manner; significantly increased PPARα expression | [157] | |||
| C3 | H ( | n | = 28) | N/A | Omental and subcutaneous adipose tissue | Adipokine expression | Significant leptin induction and secretion; no effect on adiponectin; Reduction of resistin mRNA expression | [158] | |||
| C3 | R, H ( | n | = 1) | N/A | Human blood and rat mesenteric lymph nodes | T and B lymphocyte proliferation and metabolism | Inhibition of lipid synthesis as a possible mechanism leading to reduction of lymphocyte proliferation | [159] | |||
| C3 | R ( | n | = 9) | N/A | Isolated hepatocytes | Hepatic lipidogenesis | Inhibits hepatic cholesterol and fatty acid synthesis in a dose-dependent manner, possibly by competition with C2 | [160] | |||
References
- Sender, R.; Fuchs, S.; Milo, R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol. 2016, 14, e1002533.
- Zilber-Rosenberg, I.; Rosenberg, E. Role of microorganisms in the evolution of animals and plants: The hologenome theory of evolution. FEMS Microbiol. Rev. 2008, 32, 723–735.
- Goodrich, J.K.; Davenport, E.R.; Clark, A.G.; Ley, R.E. The Relationship Between the Human Genome and Microbiome Comes into View. Annu. Rev. Genet. 2017, 51, 413–433.
- Donaldson, G.P.; Lee, S.M.; Mazmanian, S.K. Gut biogeography of the bacterial microbiota. Nat. Rev. Microbiol. 2016, 14, 20–32.
- Sommer, F.; Bäckhed, F. The gut microbiota—Masters of host development and physiology. Nat. Rev. Microbiol. 2013, 11, 227–238.
- Perry, R.J.; Peng, L.; Barry, N.A.; Cline, G.W.; Zhang, D.; Cardone, R.L.; Petersen, K.F.; Kibbey, R.G.; Goodman, A.L.; Shulman, G.I. Acetate mediates a microbiome-brain-β cell axis promoting metabolic syndrome. Nature 2016, 7606, 213–217.
- McRae, M.P. Dietary Fiber is Beneficial for the Prevention of Cardiovascular Disease: An Umbrella Review of Meta-analyses. J. Chiropr. Med. 2017, 16, 289–299.
- Akshintala, V.S.; Talukdar, R.; Singh, V.K.; Goggins, M. The Gut Microbiome in Pancreatic Disease. Clin. Gastroenterol. Hepatol. 2018, 17, 290–295.
- Diamanti, A.P.; Rosado, M.M.; Laganà, B.; D’Amelio, R. Microbiota and chronic inflammatory arthritis: An interwoven link. J. Transl. Med. 2016, 14, 233.
- Kang, L.; Li, P.; Wang, D.; Wang, T.; Hao, D.; Qu, X. Alterations in intestinal microbiota diversity, composition, and function in patients with sarcopenia. Nature 2021, 11, 4628.
- Gentile, F.; Doneddu, P.E.; Riva, N.; Nobile-Orazio, E.; Quattrini, A. Diet, Microbiota and Brain Health: Unraveling the Network Intersecting Metabolism and Neurodegeneration. Int. J. Mol. Sci. 2020, 21, 7471.
- Friedland, R.P. Mechanisms of Molecular Mimicry Involving the Microbiota in Neurodegeneration. J. Alzheimer’s Dis. 2015, 45, 349–352.
- Baldini, F.; Hertel, J.; Sandt, E.; Thinnes, C.C.; Neuberger-Castillo, L.; Pavelka, L.; Betsou, F.; Krüger, R.; Thiele, I. Parkinson’s disease-associated alterations of the gut microbiome predict disease relevant changes in metabolic functions. BMC Biol. 2020, 18, 62.
- Goyal, D.; Ali, S.A.; Singh, R.K. Emerging role of gut microbiota in modulation of neuroinflammation and neurodegeneration with emphasis on Alzheimer’s disease. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 106, 9.
- Lazar, V.; Ditu, L.-M.; Pircalabioru, G.G.; Gheorghe, I.; Curutiu, C.; Holban, A.M.; Picu, A.; Petcu, L.; Chifiriuc, M.C. Aspects of Gut Microbiota and Immune System Interactions in Infectious Diseases, Immunopathology, and Cancer. Front. Immunol. 2018, 9, 1830.
- Kunzmann, A.T.; Coleman, H.G.; Huang, W.-Y.; Kitahara, C.M.; Cantwell, M.M.; Berndt, S.I. Dietary fiber intake and risk of colorectal cancer and incident and recurrent adenoma in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. Am. J. Clin. Nutr. 2015, 102, 881–890.
- Kotas, M.E.; Medzhitov, R. Homeostasis, Inflammation, and Disease Susceptibility. Cell 2015, 160, 816–827.
- Cevenini, E.; Monti, D.; Franceschi, C. Inflamm-ageing. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 14–20.
- Hotamisligli, G.S. Inflammation and metabolic disorders. Nature 2006, 444, 860–867.
- Hotamisligil, G.S. Inflammation, metaflammation and immunometabolic disorders. Nature 2017, 542, 177–185.
- Kaulmann, A.; Bohn, T. Carotenoids, inflammation, and oxidative stress—Implications of cellular signaling pathways and relation to chronic disease prevention. Nutr. Res. Rev. 2014, 34, 907–929.
- Menzel, A.; Samouda, H.; Dohet, F.; Loap, S.; Ellulu, M.S.; Bohn, T. Common and Novel Markers for Measuring Inflammation and Oxidative Stress Ex Vivo in Research and Clinical Practice—Which to Use Regarding Disease Outcomes? Antioxidants 2021, 10, 414.
- Pan, A.; Lin, X.; Hemler, E.; Hu, F.B. Diet and Cardiovascular Disease: Advances and Challenges in Population-Based Studies. Cell Metab. 2018, 27, 489–496.
- Christ, A.; Latz, E. The Western lifestyle has lasting effects on metaflammation. Nat. Rev. Immunol. 2019, 19, 267–268.
- Devore, E.E.; Kang, J.H.; Breteler, M.M.B.; Grodstein, F. Dietary intake of berries and flavonoids in relation to cognitive decline. Ann. Neurol. 2012, 72, 135–143.
- Darmadi-Blackberry, I.; Wahlqvist, M.L.; Kouris-Blazos, A.; Steen, B.; Lukito, W.; Horie, Y.; Horie, K. Legumes: The most important dietary predictor of survival in older people of different ethnicities. Asia Pac. J. Clin. Nutr. 2004, 13, 217–220.
- Katagiri, R.; Goto, A.; Sawada, N.; Yamaji, T.; Iwasaki, M.; Noda, M.; Iso, H.; Tsugane, S. Dietary fiber intake and total and cause-specific mortality: The Japan Public Health Center-based prospective study. Am. J. Clin. Nutr. 2020, 111, 1027–1035.
- Szic, K.S.V.; Declerck, K.; Vidaković, M.; Berghe, W.V. From inflammaging to healthy aging by dietary lifestyle choices: Is epigenetics the key to personalized nutrition? Clin. Epigenetics 2015, 7, 33.
- Fitzgerald, K.C.; Tyry, T.; Salter, A.; Cofield, S.S.; Cutter, G.; Fox, R.; Marrie, R.A. Diet quality is associated with disability and symptom severity in multiple sclerosis. Neurology 2018, 90, e1–e11.
- Yoo, J.Y.; Kim, S.S. Probiotics and Prebiotics: Present Status and Future Perspectives on Metabolic Disorders. Nutrients 2016, 3, 173.
- Mischley, L.K.; Lau, R.C.; Bennett, R.D. Role of Diet and Nutritional Supplements in Parkinson’s Disease Progression. Oxidative Med. Cell. Longev. 2017, 2017, 6405278.
- Lucas, M.; Chocano-Bedoya, P.; Shulze, M.B.; Mirzaei, F.; O’Reilly, É.J.; Okereke, O.I.; Hu, F.B.; Willett, W.C.; Ascherio, A. Inflammatory dietary pattern and risk of depression among women. Brain Behav. Immun. 2014, 36, 46–53.
- Rayman, M.P. Diet, nutrition and osteoarthritis. BMC Musculoskelet. Disord. 2015, 16, S7.
- Martínez-González, M.A.; Sánchez-Villegas, A. Food patterns and the prevention of depression. Proc. Nutr. Soc. 2016, 75, 139–146.
- Gill, S.K.; Rossi, M.; Bajka, B.; Whelan, K. Dietary fibre in gastrointestinal health and disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 101–116.
- Trowell, H. Definition of dietary fiber and hypotheses that it is a protective factor in certain diseases. Am. J. Clin. Nutr. 1976, 29, 417–427.
- Wu, Z.; Xu, Q.; Wang, Q.; Chen, Y.; Lv, L.; Zheng, B.; Yan, R.; Jiang, H.; Shen, J.; Wang, S.; et al. The impact of dietary fibers on Clostridioides difficile infection in a mouse model. Front. Cell. Infect. Microbiol. 2022, 12, 1028267.
- Snauwaert, E.; Paglialonga, F.; Vande Walle, J.; Wan, M.; Desloovere, A.; Polderman, N.; Renken-Terhaerdt, J.; Shaw, V.; Shroff, R. The benefits of dietary fiber: The gastrointestinal tract and beyond. Pediatr. Nephrol. 2022, 2022, 1–10.
- Nakaji, S.; Sugawara, K.; Saito, D.; Yoshioka, Y.; Macauley, D.; Bradley, T.; Kernohan, G.; Baxter, D. Trends in dietary fiber intake in Japan over the last century. Eur. J. Nutr. 2002, 41, 222–227.
- Stephen, A.M.; Champ, M.M.-J.; Cloran, S.J.; Fleith, M.; Lieshout, L.V.; Mejborn, H.; Burley, V.J. Dietary fibre in Europe: Current state of knowledge on definitions, sources, recommendations, intakes and relationships to health. Nutr. Res. Rev. 2017, 30, 149–190.
- Prynne, C.J.; McCarron, A.; Wadsworth, M.E.; Stephen, A.M. Dietary fibre and phytate—A balancing act: Results from three time points in a British birth cohort. Br. J. Nutr. 2010, 103, 274–280.
- Public Health England; Food Standards Agency. National Diet and Nutrition Survey: Results from Years 1, 2, 3 and 4 (Combined) of the Rolling Programme (2008/2009–2011/2012); Public Health England: London, UK, 2014.
- King, D.E.; Mainous, A.G., 3rd; Lambourne, C.A. Trends in dietary fiber intake in the United States, 1999–2008. J. Acad. Nutr. Diet. 2012, 112, 642–648.
- Dingeo, G.; Brito, A.; Samouda, H.; Iddir, M.; Frano, M.R.L.; Bohn, T. Phytochemicals as modifiers of gut microbial communities. Food Funct. 2020, 11, 8444–8471.
- Makki, K.; Deehan, E.C.; Walter, J.; Bäckhed, F. The Impact of Dietary Fiber on Gut Microbiota in Host Health and Disease. Cell Host Microbe 2018, 23, 705–716.
- Jha, S.K.; Singh, H.R.; Prakash, P. Dietary Fiber and Human Health: An Introduction; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1–22.
- Desai, M.S.; Seekatz, A.M.; Koropatkin, N.M.; Kamada, N.; Hickey, C.A.; Wolter, M.; Pudlo, N.A.; Kitamoto, S.; Terrapon, N.; Muller, A.; et al. A Dietary Fiber-Deprived Gut Microbiota Degrades the Colonic Mucus Barrier and Enhances Pathogen Susceptibility. Cell 2016, 167, 1339–1353.
- Tan, J.; McKenzie, C.; Vuillermin, P.J.; Goverse, G.; Vinuesa, C.G.; Mebius, R.E.; Macia, L.; Mackay, C.R. Dietary Fiber and Bacterial SCFA Enhance Oral Tolerance and Protect against Food Allergy through Diverse Cellular Pathways. Cell Rep. 2016, 15, 2809–2824.
- Kuo, S.-M. The interplay between fiber and the intestinal microbiome in the inflammatory response. Adv. Nutr. 2013, 4, 16–28.
- Topping, D.L.; Clifton, P.M. Short-Chain Fatty Acids and Human Colonic Function: Roles of Resistant Starch and Nonstarch Polysaccharides. Physiol. Rev. 2001, 81, 1031–1064.
- Gill, P.A.; Zelm, M.C.V.; Muir, J.G.; Gibson, P.R. Review article: Short chain fatty acids as potential therapeutic agents in human gastrointestinal and inflammatory disorders. Aliment. Pharmacol. Ther. 2018, 48, 15–34.
- Louis, P.; Hold, G.L.; Flint, H.J. The gut microbiota, bacterial metabolites and colorectal cancer. Nat. Rev. Microbiol. 2014, 12, 661–672.
- Singh, N.; Gurav, A.; Sivaprakasam, S.; Brady, E.; Padia, R.; Shi, H.; Thangaraju, M.; Prasad, P.D.; Manicassamy, S.; Munn, D.H.; et al. Activation of Gpr109a, Receptor for Niacin and the Commensal Metabolite Butyrate, Suppresses Colonic Inflammation and Carcinogenesis. Immunity 2014, 40, 128–139.
- Chang, P.V.; Hao, L.; Offermanns, S.; Medzhitov, R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc. Natl. Acad. Sci. USA 2014, 111, 2247–2252.
- Furusawa, Y.; Obata, Y.; Fukuda, S.; Endo, T.A.; Nakato, G.; Takahashi, D.; Nakanishi, Y.; Uetake, C.; Kato, K.; Kato, T.; et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013, 504, 446–450.
- Smith, P.M.; Howitt, M.R.; Panikov, N.; Michaud, M.; Gallini, C.A.; Bohlooly, Y.-M.; Glickman, J.N.; Garrett, W.S. The Microbial Metabolites, Short-Chain Fatty Acids, Regulate Colonic Treg Cell Homeostasis. Science 2013, 341, 569–573.
- Zimmerman, M.A.; Singh, N.; Martin, P.M.; Thangaraju, M.; Ganapathy, V.; Waller, J.L.; Shi, H.; Robertson, K.D.; Munn, D.H.; Liu, K. Butyrate suppresses colonic inflammation through HDAC1-dependent Fas upregulation and Fas-mediated apoptosis of T cells. Am. J. Physiol.-Gastrointest. Liver Physiol. 2012, 302, G1405–G1415.
- Donohoe, D.; Garge, N.; Zhang, X.; Sun, W.; O’Connel, T.; Bunger, M.; Bultman, S. The Microbiome and Butyrate Regulate Energy Metabolism and Autophagy in the Mammalian Colon. Cell Metab. 2011, 13, 517–526.
- Usami, M.; Kishimoto, K.; Ohata, A.; Miyoshi, M.; Aoyama, M.; Fueda, Y.; Kotani, J. Butyrate and trichostatin A attenuate nuclear factor κB activation and tumor necrosis factor α secretion and increase prostaglandin E2 secretion in human peripheral blood mononuclear cells. Nutr. Res. 2008, 28, 321–328.
- Hamer, H.M.; Jonkers, D.; Venema, K.; Vanhoutvin, S.; Troost, F.J.; Brummer, R.J. Review article: The role of butyrate on colonic function. Aliment. Pharmacol. Ther. 2007, 27, 104–119.
- Gaudier, E.; Jarry, A.; Blottière, H.M.; Coppet, P.D.; Buisine, M.P.; Aubert, J.P.; Laboisse, C.; Cherbut, C.; Hoebler, C. Butyrate specifically modulates MUC gene expression in intestinal epithelial goblet cells deprived of glucose. Am. J. Physiol. 2004, 287, G1168–G1174.
- Willemsen, L.E.M. Short chain fatty acids stimulate epithelial mucin 2 expression through differential effects on prostaglandin E1 and E2 production by intestinal myofibroblasts. Gut 2003, 52, 1442–1447.
- Pryde, S.E.; Duncan, S.H.; Hold, G.L.; Stewart, C.S.; Flint, H.J. The microbiology of butyrate formation in the human colon. FEMS Microbiol. Lett. 2002, 217, 133–139.
- Boets, E.; Gomand, S.V.; Deroover, L.; Preston, T.; Vermeulen, K.; de Preter, V.; Hamer, H.M.; van den Mooter, G.; de Vuyst, L.; Courtin, C.M.; et al. Systemic availability and metabolism of colonic-derived short-chain fatty acids in healthy subjects: A stable isotope study. J. Physiol. 2017, 595, 541–555.
- Bloemen, J.G.; Venema, K.; van de Poll, M.C.; Olde Damink, S.W.; Buurman, W.A.; Dejong, C.H. Short chain fatty acids exchange across the gut and liver in humans measured at surgery. Clin. Nutr. 2009, 28, 657–661.
- Sakata, T. Pitfalls in short-chain fatty acid research: A methodological review. Anim. Sci. J. 2019, 90, 3–13.
- Reichardt, N.; Vollmer, M.; Holtrop, G.; Farquharson, F.M.; Wefers, D.; Bunzel, M.; Duncan, S.H.; Drew, J.E.; Williams, L.M.; Milligan, G.; et al. Specific substrate-driven changes in human faecal microbiota composition contrast with functional redundancy in short-chain fatty acid production. ISME J. 2018, 12, 610–622.
- Durack, J.; Christophersen, C.T. Human Respiratory and Gut Microbiomes—Do They Really Contribute to Respiratory Health? Front. Pediatrics 2020, 8, 528.
- Levan, S.R.; Stamnes, K.A.; Lin, D.L.; Panzer, A.R.; Fukui, E.; McCauley, K.; Fujimura, K.E.; McKean, M.; Ownby, D.R.; Zoratti, E.M.; et al. Elevated faecal 12,13-diHOME concentration in neonates at high risk for asthma is produced by gut bacteria and impedes immune tolerance. Nat. Microbiol. 2019, 4, 1851–1861.
- Durack, J.; Kimes, N.E.; Lin, D.L.; Rauch, M.; McKean, M.; McCauley, K.; Panzer, A.R.; Mar, J.S.; Cabana, M.D.; Lynch, S.V. Delayed gut microbiota development in high-risk for asthma infants is temporarily modifiable by Lactobacillus supplementation. Nat. Commun. 2018, 9, 707.
- Fujimura, K.E.; Sitarik, A.R.; Havstad, S.; Lin, D.L.; Levan, S.; Fadrosh, D.; Panzer, A.R.; LaMere, B.; Rackaityte, E.; Lukacs, N.W.; et al. Neonatal gut microbiota associates with childhood multi– sensitized atopy and T–cell differentiation. Nat. Med. 2016, 22, 1187–1191.
- Maslowski, K.M.; Vieira, A.T.; Ng, A.; Kranich, J.; Sierro, F.; Di, Y.; Schilter, H.C.; Rolph, M.S.; Mackay, F.; Artis, D.; et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 2009, 461, 1282–1286.
- Skelly, A.N.; Sato, Y.; Kearney, S.; Honda, K. Mining the microbiota for microbial and metabolite-based immunotherapies. Nat. Rev. Immunol. 2019, 19, 305–323.
- Tuck, C.J.; Vanner, S.J. Dietary therapies for functional bowel symptoms: Recent advances, challenges, and future directions. Neurogastroenterol. Motil. 2017, 30, e13238.
- Blacher, E.; Levy, M.; Tatirovsky, E.; Elinav, E. Microbiome-Modulated Metabolites at the Interface of Host Immunity. J. Immunol. 2017, 198, 572–580.
- Saura-Calixto, F. Dietary Fiber as a Carrier of Dietary Antioxidants: An Essential Physiological Function. J. Agric. Food Chem. 2011, 1, 43–49.
- Palafox-Carlos, H.; Ayala-Zavala, J.F.; González-Aguilar, G.A. The Role of Dietary Fiber in the Bioaccessibility and Bioavailability of Fruit and Vegetable Antioxidants. J. Food Sci. 2011, 1, R6–R15.
- Luca, S.V.; Macovei, I.; Bujor, A.; Miron, A.; Skalicka-Woźniak, K.; Aprotosoaie, A.C.; Trifa, A. Bioactivity of dietary polyphenols: The role of metabolites. Food Sci. Nutr. 2020, 60, 626–659.
- Dobson, C.C.; Mottawea, W.; Rodrigue, A.; Pereira, B.L.B.; Hammami, R.; Power, K.A.; Bordenave, N. Impact of molecular interactions with phenolic compounds on food polysaccharides functionality. Adv. Food Nutr. Res. 2019, 90, 135–181.
- Padayachee, A.; Day, L.; Howell, K.; Gidley, M.J. Complexity and health functionality of plant cell wall fibers from fruits and vegetables. Food Sci. Nutr. 2017, 1, 59–81.
- Bohn, T. Dietary factors affecting polyphenol bioavailability. Nutr. Rev. 2014, 72, 429–452.
- Kaulmann, A.; Bohn, T. Bioactivity of Polyphenols: Preventive and Adjuvant Strategies toward Reducing Inflammatory Bowel Diseases—Promises, Perspectives, and Pitfalls. Oxidative Med. Cell. Longev. 2016, 2016, 9346470.
- Çelik, E.E.; Rubio, J.M.A.; Andersen, M.L.; Gökmen, V. Interactions of dietary fiber bound antioxidants with hydroxycinnamic and hydroxybenzoic acids in aqueous and liposome media. Food Chem. 2019, 278, 294–304.
- Bermúdez-Oria, A.; Rodríguez-Gutiérrez, G.; Fernández-Prior, Á.; Vioque, B.; Fernández-Bolaños, J. Strawberry dietary fiber functionalized with phenolic antioxidants from olives. Interactions between polysaccharides and phenolic compounds. Food Chem. 2019, 280, 310–320.
- Çelik, E.E.; Gökmen, V.; Skibsted, L.H. Synergism between Soluble and Dietary Fiber Bound Antioxidants. J. Agric. Food Chem. 2015, 63, 2338–2343.
- Doğan, E.; Gökmen, V. Mechanism of the interaction between insoluble wheat bran and polyphenols leading to increased antioxidant capacity. Food Res. Int. 2015, 69, 189–193.
- Saura-Calixto, F.; Serrano, J.; Goni, I. Intake and bioaccessibility of total polyphenols in a whole diet. Food Chem. 2007, 101, 492–501.
- Johnson, A.J.; Vangay, P.; Al-Ghalith, G.A.; Hillmann, B.M.; Ward, T.L.; Shields-Cutler, R.R.; Kim, A.D.; Shmagel, A.K.; Syed, A.N.; Students, P.M.C.; et al. Daily Sampling Reveals Personalized Diet-Microbiome Associations in Humans. Cell Host Microbe 2019, 25, 789–802.
- Filippis, F.D.; Pellegrini, N.; Vannini, L.; Jeffery, I.B.; Storia, A.L.; Laghi, L.; Serrazanetti, D.I.; Cagno, R.D.; Ferrocino, I.; Lazzi, C.; et al. High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. BMJ Gut 2016, 65, 1812–1821.
- Martínez, G.P.; Bäuerl, C.; Collado, M.C. Understanding gut microbiota in elderly’s health will enable intervention through probiotics. Benef. Microbes 2014, 3, 235–246.
- Wu, G.D.; Chen, J.; Hoffmann, C.; Bittinger, K.; Chen, Y.-Y.; Keilbaugh, S.A.; Bewtra, M.; Knights, D.; Walters, W.A.; Knight, R.; et al. Linking Long-Term Dietary Patterns with Gut Microbial Enterotypes. Science 2011, 334, 105–109.
- Markiewicz, L.H.; Honke, J.; Haros, M.; Swiaztecka, D.; Wróblewska, B. Diet shapes the ability of human intestinal microbiota to degrade phytate—In vitro studies. J. Appl. Microbiol. 2013, 115, 247–259.
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563.
- Merra, G.; Noce, A.; Marrone, G.; Cintoni, M.; Tarsitano, M.G.; Capacci, A.; de Lorenzo, A. Influence of Mediterranean Diet on Human Gut Microbiota. Nutrients 2020, 13, 7.
- Riaz Rajoka, M.S.; Thirumdas, R.; Mehwish, H.M.; Umair, M.; Khurshid, M.; Hayat, H.F.; Phimolsiripol, Y.; Pallarés, N.; Martí-Quijal, F.J.; Barba, F.J. Role of Food Antioxidants in Modulating Gut Microbial Communities: Novel Understandings in Intestinal Oxidative Stress Damage and Their Impact on Host Health. Antioxidants 2021, 10, 1563.
- Esworthy, R.S.; Smith, D.D.; Chu, F.-F. A Strong Impact of Genetic Background on Gut Microflora in Mice. Int. J. Inflamm. 2010, 2010, 986046.
- Nøhr, M.K.; Egerod, K.L.; Christiansen, S.H.; Gille, A.; Offermanns, S.; Schwartz, T.W.; Møller, M. Expression of the short chain fatty acid receptor GPR41/FFAR3 in autonomic and somatic sensory ganglia. Neuroscience 2015, 290, 126–137.
- Poul, E.L.; Loison, C.; Struyf, S.; Springael, J.-Y.; Lannoy, V.; Decobecq, M.-E.; Brezillon, S.; Dupriez, V.; Vassart, G.; Damme, J.V.; et al. Functional Characterization of Human Receptors for Short Chain Fatty Acids and Their Role in Polymorphonuclear Cell Activation. J. Biol. Chem. 2003, 278, 25481–25489.
- Hudson, B.D.; Murdoch, H.; Milligan, G. Minireview: The Effects of Species Ortholog and SNP Variation on Receptors for Free Fatty Acids. Mol. Endocrinol. 2013, 27, 1177–1187.
- Van der Hee, B.; Wells, J.M. Microbial Regulation of Host Physiology by Short-chain Fatty Acids. Trends Microbiol. 2021, 29, 700–712.
- Medicine, P.C.F.R. Dietary Fibre Recommendations. Available online: https://www.pcrm.org/good-nutrition/nutrition-information/fiber (accessed on 22 October 2021).
- EFSA Panel on Dietetic Products, Nutrition and Allergies. Scientific Opinion on Dietary Reference Values for carbohydrates and dietary fibre. EFSA J. 2010, 8, 1462.
- Alkerwi, A.A.; Sauvageot, N.; Donneau, A.-F.; Lair, M.-L.; Couffignal, S.; Beissel, J.; Delagardelle, C.; Wagener, Y.; Albert, A.; Guillaume, M. First nationwide survey on cardiovascular risk factors in Grand-Duchy of Luxembourg (ORISCAV-LUX). BMC Public Health 2010, 10, 468.
- Alkerwi, A.A.; Donneau, A.-F.; Sauvageot, N.; Lair, M.-L.; Albert, A.; Guillaume, M. Dietary, behavioural and socio-economic determinants of the metabolic syndrome among adults in Luxembourg: Findings from the ORISCAV-LUX study. Public Health Nutr. 2012, 15, 849–859.
- Alkerwi, A.A.; Pastore, J.; Sauvageot, N.; Coroller, G.L.; Bocquet, V.; d’Incau, M.; Aguayo, G.; Appenzeller, B.; Bejko, D.; Bohn, T.; et al. Challenges and benefits of integrating diverse sampling strategies in the observation of cardiovascular risk factors (ORISCAV-LUX 2) study. BMC Med. Res. Metholody 2019, 19, 27.
- Vangay, P.; Johnson, A.J.; Ward, T.L.; Al-Ghalith, G.A.; Shields-Cutler, R.R.; Hillmann, B.M.; Lucas, S.K.; Beura, L.K.; Thompson, E.A.; Till, L.M.; et al. U.S. immigration westernizes the human gut microbiome. Cell 2018, 175, 962–972.
- Reynolds, A.; Mann, J.; Cummings, J.; Winter, N.; Mete, E.; Te Morenga, L. Carbohydrate quality and human health: A series of systematic reviews and meta-analyses. Lancet 2019, 393, 434–445.
- Schättin, A.; Gennaro, F.; Egloff, M.; Vogt, S.; de Bruin, E.D. Physical Activity, Nutrition, Cognition, Neurophysiology, and Short-Time Synaptic Plasticity in Healthy Older Adults: A Cross-Sectional Study. Front. Aging Neurosci. 2018, 10, 242.
- Chesnais, J.-C. The Inversion of the Age Pyramid and the Future Population Decline in France: Implications and Policy Responses; United Nations: New York, NY, USA, 2000.
- Biagi, E.; Candela, M.; Turroni, S.; Garagnani, P.; Franceschi, C.; Brigidi, P. Ageing and gut microbes: Perspectives for health maintenance and longevity. Pharmacol. Res. 2013, 1, 11–20.
- Franceschi, C.; Capri, M.; Monti, D.; Giunta, S.; Olivieri, F.; Sevini, F.; Panourgia, M.P.; Invidia, L.; Celani, L.; Scurti, M.; et al. Inflammaging and anti-inflammaging: A systemic perspective on aging and longevity emerged from studies in humans. Mech. Ageing Dev. 2006, 128, 92–105.
- Franceschi, C.; Olivieri, F.; Marchegiani, F.; Cardelli, M.; Cavallone, L.; Capri, M.; Salvioli, S.; Valensin, S.; Benedictis, G.D.; Iorio, A.D.; et al. Genes involved in immune response/inflammation, IGF1/insulin pathway and response to oxidative stress play a major role in the genetics of human longevity: The lesson of centenarians. Mech. Ageing Dev. 2005, 126, 351–361.
- Dinan, T.G.; Cryan, J.F. Gut instincts: Microbiota as a key regulator of brain development, ageing and neurodegeneration. J. Physiol. 2016, 595, 489–494.
- Biagi, E.; Nylund, L.; Candela, M.; Ostan, R.; Bucci, L.; Pini, E.; Nikkïla, J.; Monti, D.; Satokari, R.; Franceschi, C.; et al. Through Ageing, and Beyond: Gut Microbiota and Inflammatory Status in Seniors and Centenarians. PLoS ONE 2010, 5, e10667.
- Biagi, E.; Candela, M.; Fairweather-Tait, S.; Franceschi, C.; Brigidi, P. Ageing of the human metaorganism: The microbial counterpart. Age 2012, 34, 247–267.
- Tiihonen, K.; Ouwehand, A.C.; Rautonen, N. Human intestinal microbiota and healthy ageing. Ageing Res. Rev. 2010, 2, 107–116.
- Duncan, S.H.; Flint, H.J. Probiotics and prebiotics and health in ageing populations. Maturitas 2013, 1, 44–50.
- Offringa, L.C.; Hartle, J.C.; Rigdon, J.; Gardner, C.D. Changes in Quantity and Sources of Dietary Fiber from Adopting Healthy Low-Fat vs. Healthy Low-Carb Weight Loss Diets: Secondary Analysis of DIETFITS Weight Loss Diet Study. Nutrients 2021, 13, 3625.
- Mccleary, B.V. Total Dietary Fiber (CODEX Definition) in Foods and Food Ingredients by a Rapid Enzymatic-Gravimetric Method and Liquid Chromatography: Collaborative Study, First Action 2017.16. J. AOAC Int. 2019, 102, 196–207.
- Codex Alimentarius Commission. Report of the 30th Session of the Codex Committee on Nutrition and Foods for Special Dietary Uses; No. ALINORM 02/32/26; FAO: Rome, Italy; WHO: Geneva, Switzerland, 2009.
- Kato, N.; Iwami, K. Resistant Protein; Its Existence and Function Beneficial to Health. J. Nutr. Sci. Vitaminol. 2002, 48, 1–5.
- Wang, Z.; Liang, M.; Li, H.; Cai, L.; Yang, L. Rice Protein Exerts Anti-Inflammatory Effect in Growing and Adult Rats via Suppressing NF-κB Pathway. Int. J. Mol. Sci. 2019, 20, 6164.
- Yang, L.; Chen, J.; Xu, T.; Qiu, W.; Zhang, Y.; Zhang, L.; Xu, F.; Liu, H. Rice Protein Extracted by Different Methods Affects Cholesterol Metabolism in Rats Due to Its Lower Digestibility. Int. J. Mol. Sci. 2011, 12, 7594–7608.
- Agence Française de Sécurité Sanitaire des Aliments (AFFSA). Dietary Fibre: Definitions, Analysis and Nutrition Claims; Agence Française de Sécurité Sanitaire des Aliments (AFFSA): Paris, France, 2002.
- Martel, J.; Ojcius, D.M.; Ko, Y.-F.; Young, J.D. Phytochemicals as Prebiotics and Biological Stress Inducers. Trends Biochem. Sci. 2020, 45, 462–471.
- Fatima, A.; Khan, M.S.; Ahmad, M.W. Therapeutic Potential of Equol: A Comprehensive Review. Curr. Pharm. Des. 2020, 26, 5837–5843.
- Jones, J.M. CODEX-aligned dietary fiber definitions help to bridge the ‘fiber gap’. Nutr. J. 2014, 13, 34.
- Williams, B.A.; Mikkelsen, D.; Flanagan, B.M.; Gidley, M.J. “Dietary fibre”: Moving beyond the “soluble/insoluble” classification for monogastric nutrition, with an emphasis on humans and pigs. J. Anim. Sci. Biotechnol. 2019, 10, 45.
- Macfarlane, G.T.; Macfarlane, S. Bacteria, Colonic Fermentation, and Gastrointestinal Health. J. AOAC Int. 2012, 95, 50–60.
- Cummings, J.H.; Gibson, G.R.; Macfarlane, G.T. Quantitative estimates of fermentation in the hind gut of man. Acta Vet. Scand. Suppl. 1989, 86, 76–82.
- Rios-Covian, D.; González, S.; Nogacka, A.M.; Arboleya, S.; Salazar, N.; Gueimonde, M.; de Los Reyes-Gavilán, C.G. An Overview on Fecal Branched Short-Chain Fatty Acids Along Human Life and as Related with Body Mass Index: Associated Dietary and Anthropometric Factors. Front. Microbiol. 2020, 11, 973.
- McDonald, J.A.K.; Mullish, B.H.; Pechlivanis, A.; Liu, Z.; Brignardello, J.; Kao, D.; Holmes, E.; Li, J.V.; Clarke, T.B.; Thursz, M.R.; et al. Inhibiting Growth of Clostridioides difficile by Restoring Valerate, Produced by the Intestinal Microbiota. Gastroenterology 2018, 155, 1495–1507.e1415.
- François, I.E.J.A.; Lescroart, O.; Veraverbeke, W.S.; Marzorati, M.; Possemiers, S.; Hamer, H.; Windey, K.; Welling, G.W.; Delcour, J.A.; Courtin, C.M.; et al. Effects of Wheat Bran Extract Containing Arabinoxylan Oligosaccharides on Gastrointestinal Parameters in Healthy Preadolescent Children. J. Pediatric Gastroenterol. Nutr. 2014, 58, 647–653.
- Breit, S.; Kupferberg, A.; Rogler, G.; Hasler, G. Vagus Nerve as Modulator of the Gut-Brain Axis in Psychiatric and Inflammatory Disorders. Front. Psychiatry 2018, 9, 44.
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids from Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020, 11, 25.
- Granado-Serrano, A.B.; Martín-Garí, M.; Sánchez, V.; Riart Solans, M.; Berdún, R.; Ludwig, I.A.; Rubió, L.; Vilaprinyó, E.; Portero-Otín, M.; Serrano, J.C.E. Faecal bacterial and short-chain fatty acids signature in hypercholesterolemia. Sci. Rep. 2019, 9, 1772.
- Nataraj, B.H.; Ali, S.A.; Behare, P.V.; Yadav, H. Postbiotics-parabiotics: The new horizons in microbial biotherapy and functional foods. Microb. Cell Factories 2020, 19, 168.
- Dasarathy, S.; Mookerjee, R.P.; Rackayova, V.; Rangroo Thrane, V.; Vairappan, B.; Ott, P.; Rose, C.F. Ammonia toxicity: From head to toe? Metab. Brain Dis. 2017, 32, 529–538.
- Ding, L.; Huang, Z.; Lu, Y.; Liang, L.; Li, N.; Xu, Z.; Zhang, J.; Shi, H.; Hong, M. Toxic effects of ammonia on intestinal health and microbiota in red-eared slider (Trachemys scripta elegans). Chemosphere 2021, 280, 130630.
- Di Masi, A.; Ascenzi, P. H2S: A “double face” molecule in health and disease. BioFactors 2013, 39, 186–196.
- Ishizaka, S.; Kikuchi, E.; Tsujii, T. Effects of acetate on human immune system. Immunopharmacol. Immunotoxicol. 1993, 15, 151–162.
- Todesco, T.; Rao, A.V.; Bosello, O.; Jenkins, D.J. Propionate lowers blood glucose and alters lipid metabolism in healthy subjects. Am. J. Clin. Nutr. 1991, 54, 860–865.
- Hamer, H.M.; Jonkers, D.M.; Bast, A.; Vanhoutvin, S.A.; Fischer, M.A.; Kodde, A.; Troost, F.J.; Venema, K.; Brummer, R.J. Butyrate modulates oxidative stress in the colonic mucosa of healthy humans. Clin. Nutr. 2009, 28, 88–93.
- Ge, H.; Li, X.; Weiszmann, J.; Wang, P.; Baribault, H.; Chen, J.L.; Tian, H.; Li, Y. Activation of G protein-coupled receptor 43 in adipocytes leads to inhibition of lipolysis and suppression of plasma free fatty acids. Endocrinology 2008, 149, 4519–4526.
- Hong, Y.-H.; Nishimura, Y.; Hishikawa, D.; Tsuzuki, H.; Miyahara, H.; Gotoh, C.; Choi, K.-C.; Feng, D.D.; Chen, C.; Lee, H.-G.; et al. Acetate and Propionate Short Chain Fatty Acids Stimulate Adipogenesis via GPCR43. Endocrinology 2005, 146, 5092–5099.
- Anil, M.H.; Forbes, J.M. Feeding in sheep during intraportal infusions of short-chain fatty acids and the effect of liver denervation. J. Physiol. 1980, 298, 407–414.
- Thacker, P.A.; Bell, J.M.; Classen, H.L.; Campbell, G.L.; Rossnagel, B.G. The nutritive value of hulless barley for swine. Anim. Feed Sci. Technol. 1988, 19, 191–196.
- Illman, R.J.; Topping, D.L.; McLntosh, G.H.; Trimble, R.P.; Storer, G.B.; Taylor, M.N.; Cheng, B.Q. Hypocholesterolaemic Effects of Dietary Propionate: Studies in Whole Animals and Perfused Rat Liver. Ann. Nutr. Metab. 1988, 32, 97–107.
- MacFabe, D.F.; Cain, D.P.; Rodriguez-Capote, K.; Franklin, A.E.; Hoffman, J.E.; Boon, F.; Taylor, A.R.; Kavaliers, M.; Ossenkopp, K.P. Neurobiological effects of intraventricular propionic acid in rats: Possible role of short chain fatty acids on the pathogenesis and characteristics of autism spectrum disorders. Behav. Brain Res. 2007, 176, 149–169.
- Shultz, S.R.; MacFabe, D.F.; Ossenkopp, K.P.; Scratch, S.; Whelan, J.; Taylor, R.; Cain, D.P. Intracerebroventricular injection of propionic acid, an enteric bacterial metabolic end-product, impairs social behavior in the rat: Implications for an animal model of autism. Neuropharmacology 2008, 54, 901–911.
- Zhou, J.; Hegsted, M.; McCutcheon, K.L.; Keenan, M.J.; Xi, X.; Raggio, A.M.; Martin, R.J. Peptide YY and Proglucagon mRNA Expression Patterns and Regulation in the Gut. Obesity 2006, 14, 683–689.
- Gao, Z.; Yin, J.; Zhang, J.; Ward, R.E.; Martin, R.J.; Lefevre, M.; Cefalu, W.T.; Ye, J. Butyrate Improves Insulin Sensitivity and Increases Energy Expenditure in Mice. Diabetes 2009, 58, 1509–1517.
- Xiong, Y.; Miyamoto, N.; Shibata, K.; Valasek, M.A.; Motoike, T.; Kedzierski, R.M.; Yanagisawa, M. Short-chain fatty acids stimulate leptin production in adipocytes through the G protein-coupled receptor GPR41. Proc. Natl. Acad. Sci. USA 2004, 101, 1045–1050.
- Yonekura, S.; Senoo, T.; Kobayashi, Y.; Yonezawa, T.; Katoh, K.; Obara, Y. Effects of acetate and butyrate on the expression of leptin and short-form leptin receptor in bovine and rat anterior pituitary cells. Gen. Comp. Endocrinol. 2003, 133, 165–172.
- Tazoe, H.; Otomo, Y.; Kaji, I.; Tanaka, R.; Karaki, S.; Kuwahara, A. Roles of short-chain fatty acids receptors, GPR41 and GPR43 on colonic functions. J. Physiol. Pharmacol. 2008, 59, 251–262.
- Tedelind, S.; Westberg, F.; Kjerrulf, M.; Vidal, A. Anti-inflammatory properties of the short-chain fatty acids acetate and propionate: A study with relevance to inflammatory bowel disease. World J. Gastroenterol. 2007, 13, 2826–2832.
- Zapolska-Downar, D.; Naruszewicz, M. Propionate reduces the cytokine-induced VCAM-1 and ICAM-1 expression by inhibiting nuclear factor-kappa B (NF-kappaB) activation. J. Physiol. Pharmacol. 2009, 60, 123–131.
- Al-Lahham, S.H.; Roelofsen, H.; Priebe, M.; Weening, D.; Dijkstra, M.; Hoek, A.; Rezaee, F.; Venema, K.; Vonk, R.J. Regulation of adipokine production in human adipose tissue by propionic acid. Eur. J. Clin. Investig. 2010, 40, 401–407.
- Curi, R.; Bond, J.A.; Calder, P.C.; Newsholme, E.A. Propionate regulates lymphocyte proliferation and metabolism. Gen. Pharmacol. 1993, 24, 591–597.
- Wright, R.S.; Anderson, J.W.; Bridges, S.R. Propionate Inhibits Hepatocyte Lipid Synthesis. Proc. Soc. Exp. Biol. Med. 1990, 195, 26–29.
