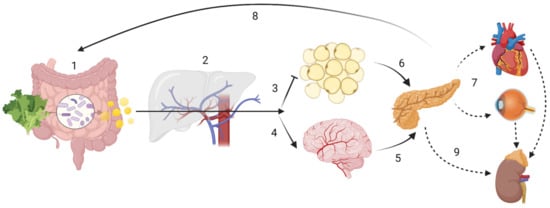
Figure 1. Host-driven variability in SCFA metabolism and distribution may lead to different disease outcomes. ADME (sub-) steps may explain the variability in SCFA effects. The enterotype influences the amount of SCFA produced, while human digestive enzymatic activity may regulate microbial communities; (1) Absorption: SNPs in mucin, MCTs or tight junction function could impair SCFA bioavailability. Butyrate is the main energy source for colonocytes. (2) In the portal circulation SCFA undergo first-pass effects, where a majority of propionate is metabolized via GPR109A, GPR43 and GPR41, having gluconeogenic or lipogenic effects. Distribution In the systemic circulation: although at present at low concentrations, butyrate and propionate are still detectable; acetate is now the most abundant SCFA. (3) Acetate inhibits lipolysis at the adipose tissue level. (4) Acetate can cross the “blood-brain-barrier” (BBB). Metabolism: SCFA have showed to be effective against microglial oxidative stress responses. SCFA may also have cellular signalling properties, as evidenced by its control of centrally released insulin (6) or its impact on the hypothalamic-pituitary-adrenal axis in leptin and cortisol responses, which may ultimately lead into maladaptive health conditions across the body (7). Finally, gluconeogenic, lipogenic and insulinogenic signals impact ghrelin, leptin and peptide YY release, leading to appetite suppression and satiety (8), improved insulin sensitivity and glucose metabolism, as well as reduction of serum lipids. (9) Excretion: in the kidney, SCFA can be re-absorbed by MCT1. Note: the intracellular effect of SCFA e.g., on HDAC or NF-κB are not displayed. Created with BioRender.com.
2. Dietary Fibre and Short Chain Fatty Acids
2.1. Dietary Fibre (DF)
Westernized types of diet are characterized by a relatively low intake in DF, despite attempts to increase its intake since the 1970s. Most European countries have established recommendations on daily intake for DF, e.g., 25–35 g for adults. Concretely, 25–32 g/d for adult women and 30–35 g/d for adult men, while recommendations for children and older adults depend on age, being approximately 3–4 g/MJ [
40]. The Physicians Committee for Responsible Medicine (PCRM) of the US recommends even a considerably higher intake of 40 g/d for an optimal health [
101].
The European Food Security Authority (EFSA) has recommended an adequate intake (AI) of 25 g/d for DF, mostly based on its association with improved bowel function (as per defecation frequency and transit time), and the reduction of gastro-intestinal symptoms such as constipation [
102]. DF refers to total fibre occurring naturally in foods such as fruits, vegetables, pulses and cereal grains [
40,
102]. Grain products are at present the largest source for DF intake worldwide, providing approx. 32% of total dietary fibre intake in the USA and 48% in the Netherlands. Other sources vary widely in European countries, e.g., vegetables (12–21%), potatoes (6–19%) and fruits (8–23%) [
40]. Lack of DF intake has been emphasized as one of the major dietary factors associated with the increased incidence of NCDs [
103,
104,
105,
106]. A recent systematic review and meta-analysis suggested that high DF consumption was associated with a 15–30% decrease in cardiovascular-related mortality, T2D and colorectal cancer, when compared with low-fibre consumption [
107]. Concurring dietary factors such as increased sugar consumption, increased saturated fat consumption and low nutrient density, among others, and their possible relationship to metabolic and neurophysiological disorders, may be present and are expected to play a role [
40,
108]. However, as human lifespan has expanded during the past decades [
109,
110], we expect to face an increase of NCDs, as these are rather associated with age-related chronic inflammation (i.e., inflammageing [
18]). Therefore, it is paramount to fully understand the pathophysiology of NCDs, and how to counteract them with affordable and efficient strategies, including improved dietary patterns and healthy food items [
18,
110,
111,
112,
113,
114,
115,
116,
117]. In this respect, fiber intake could be increased both within a low-fat diet a low-carbohydrate diet. A randomized controlled trial aiming at weight reduction over a period of 12 months assessed sources of DF in a balanced low-fat diet vs. a balanced low-carbohydrate diet. A large proportion of DF for both diets was from non-starchy vegetables. While the low-fat group mainly increased DF intake from whole grains and fruits, the low-carbohydrate one obtained DF rather from vegetables and plant protein sources. This was further reflected in gut microbiota alterations throughout the intervention, and such dietary adaptations may constitute an important factor for precision nutrition [
118].
A variety of definitions has been proposed to classify DF; most were dependent on the methods used to extract DF. This led to difficulties in defining the term, as most non-starch polysaccharides (NSP) were retrieved by such methods, which often did not include resistant (i.e., non-digestible) starches (RS). DF can further be categorized based on its solubility, fermentability or viscosity, which often caused distinctions within the group. While soluble fibres can be fermented to different degrees, and are the main substrate for colonic fermenters (e.g., β-glucans), insoluble fibres mainly serve a stool bulking function (e.g., cellulose). Both types of DF have beneficial health properties, and as such, the dichotomy of soluble-insoluble may no longer play a main role in terms of public health.
To date, definitions have reached a certain consensus [
119,
120]. DF is composed of carbohydrate polymers with three or more monomeric units (MU), which are neither hydrolysed by human digestive enzymes nor absorbed in the human intestine, and include NSPs from fruits, vegetables, grains and tubers, whether intrinsic or extracted, either chemically, enzymatically, or in physically modified forms. Polymers with more than 10 MU, e.g., cellulose, hemicelluloses, pectins, hydrocolloids (i.e., gums, β-glucans, mucilages); resistant oligosaccharides, e.g., fructo-oligosaccharides (FOS), galacto-saccharides (GOS) with 3–9 MU; and RS with 10 or more MU [
40] are included. Furthermore, some constituents produced by micro-organisms (e.g., xanthan) and polysaccharide constituents of crustaceans and fungi (e.g., chitin, chitosan, chondroitin sulphate), are resistant to digestion and are included in the DF definition, according to some national agencies [
40]. Furthermore, it has been proposed that proteins resistant to digestion exist, and may reproduce similar effects as DF, namely improved bowel function and improved immunity [
121,
122,
123], but these are typically not included in the DF definition.
Thus, DF is any polymeric carbohydrate not digested in the small intestine. DF generally also includes substances associated with, or linked to plant cell walls, but that are not carbohydrates, such as lignin or polyphenols. Often, these distinctions are not reported in food tables, where only the sum of DF is given. In 2002, the French Agency for Food Security (ANSES), included in its definition all of the above polymeric carbohydrates (MU ≥ 3) as DF, while excluding animal-based sources and lactulose, a non-absorbable sugar, to prevent its incorporation into foods (as it is a strong laxative) as a fibre source [
124].
Within this manuscript, DF is considered as any polymeric compound, which is not digestible by human enzymes and which mainly travels through the gut to reach the colonic milieu, where it is either fermented by colonic bacteria (i.e., broadly, soluble fibres) into smaller molecules such SCFA, or can act as a bulking agent during stool production (i.e., generally insoluble fibres). This broader definition would thus also include non-carbohydrate compounds such as lignin and resistant proteins, as well as compounds associated with plant-based carbohydrates, such as polyphenols. These compounds may also be substrates for bacteria, such as
Akkermansia,
Lactobacillus and
Bifidobacterium, which produce metabolites such as SCFA, which in turn induce various beneficial effects on the host, including reduction in: appetite, insulin resistance, lipid accumulation, and inflammation [
100]. However, the effects of phytochemicals are likely to vary according to the composition of the gut microbiota and host genetic polymorphisms, which affect absorption, detoxification, and overall bioactivities [
125]. One such example is equol, produced form the isoflavone daidzein, which may bind to β-oestrogen receptors, and has been associated with the incidence of various types of hormone-associated cancers [
126]. This is in line with the definition proposed by Jones [
127], and may overcome the matter of “functionality” often discussed regarding DF, as previously pointed out [
128].
Fibre fermentation relies on its chemical and physical structure, as well as the composition of the colonic microflora. Digestion of DF by the GM may vary or fluctuate depending on which fibres are consumed, and thus the amounts of SCFA produced too. For example, lignin and cellulose are rather lost through the stool, being insoluble bulking fibres; polysaccharides from extremely hard plant tissue areas are also less well digestible because physical encrustation and chemical bonding to lignin can occur [
46]. Oligosaccharides, RS and pectins are the DF compounds thought to contribute the most to SCFA production in the colon [
35].
2.2. Short Chain Fatty Acids (SCFA)
Recent studies on DF, GM and probiotics have emphasized the role of SCFA. Indeed, SCFA may be a good example of microbiota-derived modulator molecules, i.e., a nutrient that can modulate the host, acting as communicating molecules between the GM and the host [
66]. Provided that SCFA metabolism may have a broad range of implications for human health, many studies are being conducted to understand their effects (
Table 1). Sakata [
66] recently pointed out relevant pitfalls in the study of these molecules. SCFA are defined as volatile fatty acids with a skeleton of six or less carbons in straight (C1, formate; C2, acetate; C3, propionate; C4, butyrate; C5, valerate; C6, caproate), or branched-chain conformation (C4, isobutyrate; C5, isovalerate and 2-methyl-butanoate). Acetate (C2), propionate (C3) and butyrate (C4) amount for 90–95% of total GM SCFA output and are derived from carbohydrate fermentation [
129,
130]. Until recently, caproate [
131] and valerate [
132] were considered dietary food components. However, recent studies have demonstrated that these may also be GM products, with caproate being significantly increased in faecal samples of volunteers with severe obesity (BMI ≥ 40) [
131].
Branched-chain SCFA (BCFA), mainly isobutyrate, isovalerate and 2-methylbutanoate, contribute to as much as 5% of total SCFA production, and arise from the metabolism of the amino acids valine, leucine, and isoleucine, respectively [
129,
131]. BCFA levels in faecal samples show an inverse correlation with fibre consumption, especially insoluble fibre [
131,
133]. BCFA levels in stool have also been related to depression [
32,
34] and other psychiatric conditions [
134], possibly through vagal afferent nerve signalling [
135]. Furthermore, BCFA were found to be increased in subjects with hypercholesterolemia compared to normocholesterolemic individuals, with isobutyrate being associated with worse serum lipid profiles [
136]. It is likely that such elevated BCFA correspond to high protein intake, such as from meat-based diet and a reduced DF intake, which are likewise associated with negative health outcomes and ageing related health complications [
131].
Recently, products of DF fermentation have been termed post-biotics [
137]. In human adults, the principal products of DF fermentation are SCFA together with certain gases (CO
2, CH
4, and H
2), which may be taken up by the host, or excreted [
50]. Production of SCFA in the colon accompanies the bacterial consumption of ammonia, H
2S and BCFA in the synthesis of protein components for the microbial cell. Therefore, the reduction of these metabolites may also be, at least in part, responsible for the health benefits attributed to SCFA [
66], as in addition to BCFA also ammonia [
138] has been related to negative health outcomes such as neurotoxicity and hepatotoxicity, as well as increased intestinal permeability, loss of tight junction proteins and increase in pro-inflammatory cytokines as found in animal studies [
139]. H
2S, hydrogen disulphide, may be associated with neurological, cardiovascular and metabolic diseases, when abnormally produced [
140].
In this review, SCFA describes, “saturated unbranched alkyl group monocarboxylic acids of 2 to 4 carbon atoms”, referring to acetate (C2), propionate (C3) and butyrate (C4). We will briefly mention valerate (C5) and caproate (C6). It excludes BCFA, as well as succinate and lactate, which are rather intermediate products in GM metabolism, and therefore their concentrations in human serum are related rather to human metabolism, and not influenced considerably by GM or intestinal absorption.
Table 1. Identified effects of SCFA in human interventional, observational, and animal studies.
| SCFA |
Study (Sample) |
Study Design |
Tissues Investigated |
End-Point Measured |
Observed Effects |
Reference |
| Human interventional studies |
| C2 |
H (n =32) |
Case-control |
Peripheral blood |
Immunopharmacological effects of Ringer’s acetate |
Increased polyclonal antibody production and NK cell activity in healthy and cancer subjects |
[141] |
| C3 |
H (n = 6) |
Cross-over |
Serum and stool |
Blood lipids and glucose, stool bulk and microbiota |
C3 supplementation lowers blood glucose. Lipid changes not significant; increase in stool bulk and Bifidobacteria after 1 week intervention |
[142] |
| C4 |
H (n = 16) |
Cross-over |
Sigmoid colon biopsies and plasma |
Oxidative stress markers in colon; CRP, calprotectin; histological inflammation |
Rectal administration significantly reduced uric acid and increased GSH. No significant changes in other parameters |
[143] |
| Human Observational studies |
| C2-C6 |
H (n = 232) |
Observation |
Stool |
Levels of faecal SCFA and BCFA association with BMI and age |
BCFA strongly correlated with age, but not with BMI;
BCFA negatively associated with fibre consumption;
BMI ≥ 40 showed significantly higher production of SCFA, total BCFA, isobutyrate, isovalerate and caproate
SCFA production decreases with age |
[131] |
| Animal (interventional) studies |
| C2, C3 |
M (n = 15) |
Knock-out |
Adipose tissue |
Effects of GPCR43 activation |
Reduction of lipolysis, reduced plasma free fatty acids levels without flushing associated with GPCR109A |
[144] |
| C2, C3 |
M (n = 12) |
Case-control |
Adipose, gut, vascular and mesenchymal tissues |
GPCR41 and GPCR43 mRNA expression |
GPCR43 activation promoted adipose differentiation via PPARγ2. No effects on GPCR41 |
[145] |
| C2, C3, C4 |
S (n = 10) |
Case-control |
Portal and peripheral blood, liver |
Food intake following SCFA infusions |
Dose-dependent depression in food intake, explained by C3 content in portal vein, which resolved with portal plexus denervation |
[146] |
| C3 |
R (n = 20)
P (n = 12, 60) |
Case-control |
Portal blood and liver |
Cholesterol synthesis and distribution |
Supplemented C3 likely absorbed in the stomach
Dose-dependent hypocholesterolemic effect likely due to redistribution of cholesterol from plasma to liver, as opposed to synthesis inhibition |
[147,148] |
| C3 |
R (n = 74, 114) |
Case-control |
Brain, intracerebral ventricles |
Behavioural, electrophysiological, neuropathological, and biochemical effects |
C3 intraventricular infusion impaired social behaviours, similar to those seen in human ASD; induced neuroinflammation and oxidative stress; Alteration of brain phospholipid and acylcarnitine1 profiles |
[149,150] |
| C4 |
R (n = 22) |
Case-control |
Duodenum, jejunum, cecum and distal colon |
PYY and proglucagon gene expression in gut epithelial cells |
Up-regulation of local peptide YY and proglucagon expression via colonocyte sensing following a RS diet in vivo, proved by in vitro incubation with butyrate |
[151] |
| C4 |
M (n = 16–20) |
Case-control |
Whole-body autopsy |
Insulin sensitivity and energy metabolism, mitochondrial function |
C4 supplementation prevented diet-induced insulin resistance and reduced adiposity in high-fat model, without reducing food intake. Attributed to enhanced mitochondrial activity and thermogenesis |
[152] |
| In Vitro Studies |
| C2-C6 |
M (n = 18) |
N/A |
mouse adipocyte cell line and adipose primary culture |
Leptin expression |
C2-C6 stimulate leptin expression via GPCR41
Acute administration of C3 increased leptin levels |
[153] |
| C2, C4 |
R, B |
N/A |
Anterior pituitary, fat and liver aspirates |
Leptin and leptin-receptor protein expression |
C2 and C4 enhanced leptin expression in bovine pituitary and fat cells, however C4 inhibited leptin expression in rat anterior pituitary cells; while C4 suppressed leptin receptor expression in both rat and bovine pituitaries; probable species specific nutrient sensing |
[154] |
| C2, C3, C4 |
R, H |
N/A |
Colonic stimulation |
Effects on colon functions, inc. motility |
C3 and C4 induced phasic and tonic contractions of circular muscle via GPCR41 and GPCR43 in mucosae, C2 did not |
[155] |
| C2, C3, C4 |
M (n= 4)
H (n= 3) |
N/A |
Human blood samples, colon cultures (colo320DM) and mice with colitis |
Anti-inflammatory properties of SCFA |
All SCFA decreased neutrophil TNF-α release without affecting IL-8; all decreased IL-6 release; all inhibited NF-κB activity in colon cells; C4 > C3 > C2 |
[156] |
| C3 |
H (n = 5–9) |
N/A |
Human umbilical vein endothelial cells (HUVEC) |
Expression of endothelial leukocyte adhesion molecules and leukocyte recruitment by cytokine-stimulation |
Significant inhibition of TNF-α and NF-κB, reducing expression of VCAM-1 and ICAM-1 in a time- and dose-dependent manner; significantly increased PPARα expression |
[157] |
| C3 |
H (n = 28) |
N/A |
Omental and subcutaneous adipose tissue |
Adipokine expression |
Significant leptin induction and secretion; no effect on adiponectin; Reduction of resistin mRNA expression |
[158] |
| C3 |
R, H (n = 1) |
N/A |
Human blood and rat mesenteric lymph nodes |
T and B lymphocyte proliferation and metabolism |
Inhibition of lipid synthesis as a possible mechanism leading to reduction of lymphocyte proliferation |
[159] |
| C3 |
R (n = 9) |
N/A |
Isolated hepatocytes |
Hepatic lipidogenesis |
Inhibits hepatic cholesterol and fatty acid synthesis in a dose-dependent manner, possibly by competition with C2 |
[160] |