
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Gokhan Demirci | -- | 2943 | 2022-12-28 10:04:47 | | | |
| 2 | Peter Tang | -4 word(s) | 2939 | 2022-12-28 10:21:35 | | | | |
| 3 | Peter Tang | Meta information modification | 2939 | 2022-12-28 10:22:24 | | |
Video Upload Options
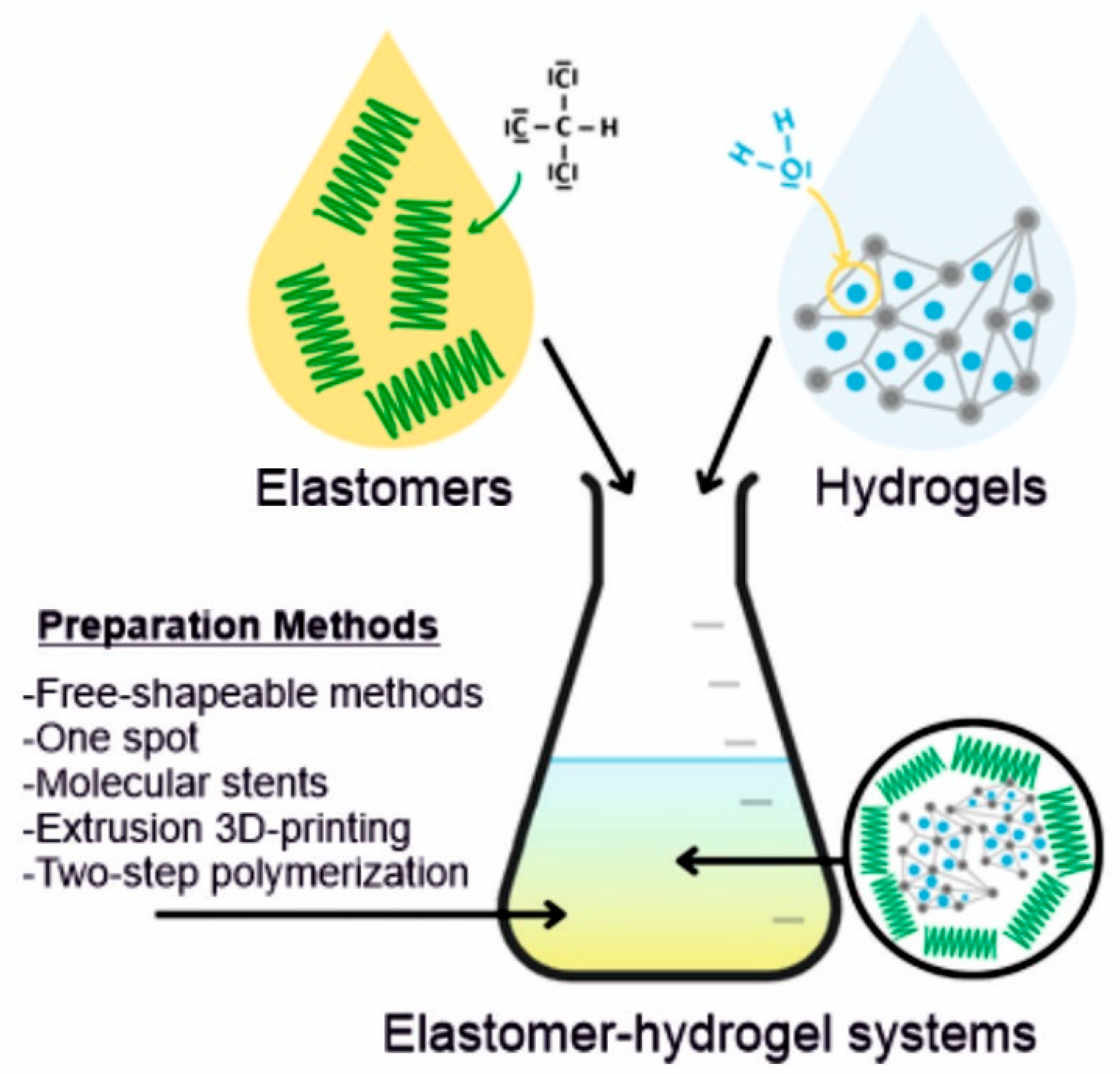
Elastomers are a class of polymeric materials that can repeatedly and easily undergo large, reversible deformations with complete recovery. They are usually composed of long-chain molecules, extremely flexible due to their ability to reconfigure themselves and dissipate an applied force. Hydrogels (HGs) are macromolecular structures consisting of polymer networks with the ability to absorb water without any dissolution. By applying sophisticated design and engineering methods, various elastomer–hydrogel systems (EHS) with outstanding performance have been developed. These systems composed of elastomers and hydrogels are very attractive due to their high biocompatibility, injectability, controlled porosity and often antimicrobial properties. Moreover, their elastomeric properties and bioadhesiveness are making them suitable for soft tissue engineering.
1. Introduction
2. Preparation of Elastomer–Hydrogel Systems
3. Biofunctionalities of Elastomer–Hydrogel Systems
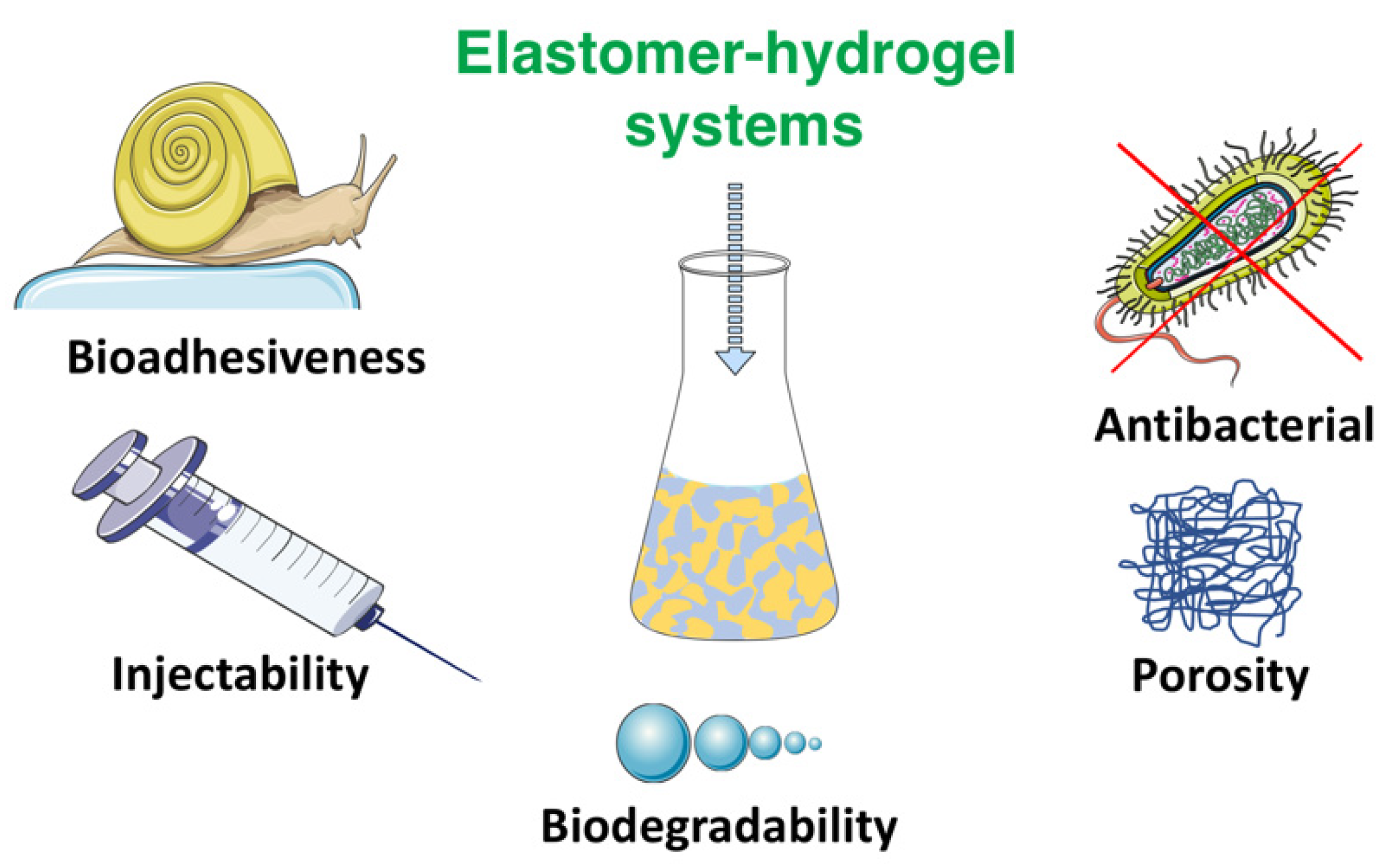
3.1. Bioadhesiveness
3.2. Injectability
3.3. Biodegradation
3.4. Porosity
3.5. Antibacterial Surfaces
4. Elastomer–Hydrogel Systems for Soft Tissue Engineering Applications
References
- Vijayavenkataraman, S.; Yan, W.C.; Lu, W.F.; Wang, C.H.; Fuh, J.Y.H. 3D Bioprinting of Tissues and Organs for Regenerative Medicine. Adv. Drug Deliv. Rev. 2018, 132, 296–332.
- Teramura, Y.; Ekdahl, K.N.; Barbu, A. A Hybrid of Cells and Pancreatic Islets toward a New Bioartificial Pancreas. Regen. Ther. 2016, 3, 68–74.
- Abbott, R.D.; Kaplan, D.L. Engineering Biomaterials for Enhanced Tissue Regeneration. Curr. Stem Cell Rep. 2016, 2, 140–146.
- Luo, Z.; Weiss, D.E.; Liu, Q.; Tian, B. Biomimetic Approaches toward Smart Bio-Hybrid Systems. Nano Res. 2018, 11, 3009–3030.
- Walia, R.; Akhavan, B.; Kosobrodova, E.; Kondyurin, A.; Oveissi, F.; Naficy, S.; Yeo, G.C.; Hawker, M.; Kaplan, D.L.; Dehghani, F.; et al. Hydrogel−Solid Hybrid Materials for Biomedical Applications Enabled by Surface-Embedded Radicals. Adv. Funct. Mater. 2020, 30, 2004599.
- Ozdil, D.; Aydin, H.M. Polymers for Medical and Tissue Engineering Applications. J. Chem. Technol. Biotechnol. 2014, 89, 1793–1810.
- Li, J.; Osada, Y.; Cooper-White, J. (Eds.) Functional Hydrogels as Biomaterials; Springer Series in Biomaterials Science and Engineering; Springer: Berlin/Heidelberg, Germany, 2018; Volume 12, ISBN 978-3-662-57509-3.
- Król, P. Synthesis Methods, Chemical Structures and Phase Structures of Linear Polyurethanes. Properties and Applications of Linear Polyurethanes in Polyurethane Elastomers, Copolymers and Ionomers. Prog. Mater. Sci. 2007, 52, 915–1015.
- Amsden, B. Curable, Biodegradable Elastomers: Emerging Biomaterials for Drug Delivery and Tissue Engineering. Soft Matter 2007, 3, 1335–1348.
- Hassouna, Y.M.; Zamani, S.; Kafienah, W.; Younes, H.M. Synthesis, Characterization & Cytocompatibility of Poly (Diol-Co-Tricarballylate) Based Thermally Crosslinked Elastomers for Drug Delivery & Tissue Engineering Applications. Mater. Sci. Eng. C 2018, 93, 254–264.
- Opris, D.M. Polar Elastomers as Novel Materials for Electromechanical Actuator Applications. Adv. Mater. 2018, 30, 1–23.
- Xu, C.; Huang, Y.; Yepez, G.; Wei, Z.; Liu, F.; Bugarin, A.; Tang, L.; Hong, Y. Development of Dopant-Free Conductive Bioelastomers. Sci. Rep. 2016, 6, 34451.
- Mirfakhrai, T.; Madden, J.D.W.; Baughman, R.H. Polymer Artificial Muscles. Mater. Today 2007, 10, 30–38.
- Jeong, K.H.; Kim, J.; Lee, L.P. Biologically Inspired Artificial Compound Eyes. Science 2006, 312, 557–561.
- Wen, Q.; Mithieux, S.M.; Weiss, A.S. Elastin Biomaterials in Dermal Repair. Trends Biotechnol. 2020, 38, 280–291.
- Christman, K.L.; Vardanian, A.J.; Fang, Q.; Sievers, R.E.; Fok, H.H.; Lee, R.J. Injectable Fibrin Scaffold Improves Cell Transplant Survival, Reduces Infarct Expansion, and Induces Neovasculature Formation in Ischemic Myocardium. J. Am. Coll. Cardiol. 2004, 44, 654–660.
- Chen, Q.Z.; Harding, S.E.; Ali, N.N.; Lyon, A.R.; Boccaccini, A.R. Biomaterials in Cardiac Tissue Engineering: Ten Years of Research Survey. Mater. Sci. Eng. R Rep. 2008, 59, 1–37.
- Zeimaran, E.; Pourshahrestani, S.; Djordjevic, I.; Pingguan-Murphy, B.; Kadri, N.A.; Towler, M.R. Bioactive Glass Reinforced Elastomer Composites for Skeletal Regeneration: A Review. Mater. Sci. Eng. C 2015, 53, 175–188.
- Chen, Q.; Liang, S.; Thouas, G.A. Elastomeric Biomaterials for Tissue Engineering. Prog. Polym. Sci. 2013, 38, 584–671.
- Tian, Y.; Liang, K.; Wang, X.; Ji, Y. Fabrication of Nanocomposite Bioelastomer Porous Scaffold Based on Chitin Nanocrystal Supported Emulsion-Freeze-Casting. ACS Sustain. Chem. Eng. 2017, 5, 3305–3313.
- Hunt, J.A.; Chen, R.; van Veen, T.; Bryan, N. Hydrogels for Tissue Engineering and Regenerative Medicine. J. Mater. Chem. B 2014, 2, 5319–5338.
- Ahadian, S.; Sadeghian, R.B.; Salehi, S.; Ostrovidov, S.; Bae, H.; Ramalingam, M.; Khademhosseini, A. Bioconjugated Hydrogels for Tissue Engineering and Regenerative Medicine. Bioconjug. Chem. 2015, 26, 1984–2001.
- Lutolf, M.P.; Hubbell, J.A. Synthetic Biomaterials as Instructive Extracellular Microenvironments for Morphogenesis in Tissue Engineering. Nat. Biotechnol. 2005, 23, 47–55.
- Sahoo, S.; Chung, C.; Khetan, S.; Burdick, J.A. Hydrolytically Degradable Hyaluronic Acid Hydrogels with Controlled Temporal Structures. Biomacromolecules 2008, 9, 1088–1092.
- Chen, Q.; Chen, H.; Zhu, L.; Zheng, J. Fundamentals of Double Network Hydrogels. J. Mater. Chem. B 2015, 3, 3654–3676.
- Xu, C.; Okpokwasili, C.; Huang, Y.; Shi, X.; Wu, J.; Liao, J.; Tang, L.; Hong, Y. Optimizing Anisotropic Polyurethane Scaffolds to Mechanically Match with Native Myocardium. ACS Biomater. Sci. Eng. 2020, 6, 2757–2769.
- Richbourg, N.R.; Peppas, N.A.; Sikavitsas, V.I. Tuning the Biomimetic Behavior of Scaffolds for Regenerative Medicine through Surface Modifications. J. Tissue Eng. Regen. Med. 2019, 13, 1275–1293.
- Olbrich, J.M.; Tate, P.L.; Corbett, J.T.; Lindsey, J.M.; Nagatomi, S.D.; Shalaby, W.S.W.; Shalaby, S.W. Injectable in Situ Forming Controlled Release Implant Composed of a Poly-Ether-Ester-Carbonate and Applications in the Field of Chemotherapy. J. Biomed. Mater. Res. Part A 2012, 100, 2365–2372.
- Manavitehrani, I.; Fathi, A.; Badr, H.; Daly, S.; Shirazi, A.N.; Dehghani, F. Biomedical Applications of Biodegradable Polyesters. Polymers 2016, 8, 20.
- Ovcharenko, E.; Rezvova, M.; Nikishau, P.; Kostjuk, S.; Glushkova, T.; Antonova, L.; Trebushat, D.; Akentieva, T.; Shishkova, D.; Krivikina, E.; et al. Polyisobutylene-Based Thermoplastic Elastomers for Manufacturing Polymeric Heart Valve Leaflets: In Vitro and In Vivo Results. Appl. Sci. 2019, 9, 4773.
- Del Bakhshayesh, A.R.; Asadi, N.; Alihemmati, A.; Tayefi Nasrabadi, H.; Montaseri, A.; Davaran, S.; Saghati, S.; Akbarzadeh, A.; Abedelahi, A. An Overview of Advanced Biocompatible and Biomimetic Materials for Creation of Replacement Structures in the Musculoskeletal Systems: Focusing on Cartilage Tissue Engineering. J. Biol. Eng. 2019, 13, 85.
- Zhang, W.; Zhang, Y.S.; Bakht, S.M.; Aleman, J.; Shin, S.R.; Yue, K.; Sica, M.; Ribas, J.; Duchamp, M.; Ju, J.; et al. Elastomeric Free-Form Blood Vessels for Interconnecting Organs on Chip Systems. Lab Chip 2016, 16, 1579–1586.
- Cheung, M.E.; Mellert, L.T.; Firstenberg, M.S. Bedside Procedure: Retained Central Venous Catheter. In Vignettes in Patient Safety; InTech: London, UK, 2018; Volume 2.
- Li, M.; Chen, J.; Shi, M.; Zhang, H.; Ma, P.X.; Guo, B. Electroactive Anti-Oxidant Polyurethane Elastomers with Shape Memory Property as Non-Adherent Wound Dressing to Enhance Wound Healing. Chem. Eng. J. 2019, 375, 121999.
- Sanchez, C.; Julián, B.; Belleville, P.; Popall, M. Applications of Hybrid Organic-Inorganic Nanocomposites. J. Mater. Chem. 2005, 15, 3559–3592.
- Shi, R.; Chen, D.; Liu, Q.; Wu, Y.; Xu, X.; Zhang, L.; Tian, W. Recent Advances in Synthetic Bioelastomers. Int. J. Mol. Sci. 2009, 10, 4223–4256.
- Zhang, Y.; Thakur, V.K.; Li, Y.; Garrison, T.F.; Gao, Z.; Gu, J.; Kessler, M.R. Soybean-Oil-Based Thermosetting Resins with Methacrylated Vanillyl Alcohol as Bio-Based, Low-Viscosity Comonomer. Macromol. Mater. Eng. 2018, 303, 1700278.
- Jia, X.; Kiick, K.L. Hybrid Multicomponent Hydrogels for Tissue Engineering. Macromol. Biosci. 2009, 9, 140–156.
- Liu, Q.; Nian, G.; Yang, C.; Qu, S.; Suo, Z. Bonding Dissimilar Polymer Networks in Various Manufacturing Processes. Nat. Commun. 2018, 9, 846.
- Wirthl, D.; Pichler, R.; Drack, M.; Kettlguber, G.; Moser, R.; Gerstmayr, R.; Hartmann, F.; Bradt, E.; Kaltseis, R.; Siket, C.M.; et al. Instant Tough Bonding of Hydrogels for Soft Machines and Electronics. Sci. Adv. 2017, 3, 1700053.
- Yuk, H.; Zhang, T.; Parada, G.A.; Liu, X.; Zhao, X. Skin-Inspired Hydrogel–Elastomer Hybrids with Robust Interfaces and Functional Microstructures. Nat. Commun. 2016, 7, 12028.
- Mehdizadeh, M.; Yang, J. Design Strategies and Applications of Tissue Bioadhesives. Macromol. Biosci. 2013, 13, 271–288.
- Du, X.; Wu, L.; Yan, H.; Qu, L.; Wang, L.; Wang, X.; Ren, S.; Kong, D.; Wang, L. Multifunctional Hydrogel Patch with Toughness, Tissue Adhesiveness, and Antibacterial Activity for Sutureless Wound Closure. ACS Biomater. Sci. Eng. 2019, 5, 2610–2620.
- Quintanar-Guerrero, D.; Villalobos-García, R.; Alvarez-Colín, E.; Cornejo-Bravo, J.M. In Vitro Evaluation of the Bioadhesive Properties of Hydrophobic Polybasic Gels Containing N,N-Dimethylaminoethyl Methacrylate-Co-Methyl Methacrylate. Biomaterials 2001, 22, 957–961.
- Walker, B.W.; Lara, R.P.; Yu, C.H.; Sani, E.S.; Kimball, W.; Joyce, S.; Annabi, N. Engineering a Naturally-Derived Adhesive and Conductive Cardiopatch. Biomaterials 2019, 207, 89–101.
- Shin, M.; Kim, K.; Shim, W.; Yang, J.W.; Lee, H. Tannic Acid as a Degradable Mucoadhesive Compound. ACS Biomater. Sci. Eng. 2016, 2, 687–696.
- Bouten, P.J.M.; Zonjee, M.; Bender, J.; Yauw, S.T.K.; Van Goor, H.; Van Hest, J.C.M.; Hoogenboom, R. The Chemistry of Tissue Adhesive Materials. Prog. Polym. Sci. 2014, 39, 1375–1405.
- Tian, K.; Bae, J.; Suo, Z.; Vlassak, J.J. Adhesion between Hydrophobic Elastomer and Hydrogel through Hydrophilic Modification and Interfacial Segregation. ACS Appl. Mater. Interfaces 2018, 10, 43252–43261.
- Yang, J.; Bai, R.; Chen, B.; Suo, Z. Hydrogel Adhesion: A Supramolecular Synergy of Chemistry, Topology, and Mechanics. Adv. Funct. Mater. 2020, 30, 1901693.
- Yang, H.; Li, C.; Yang, M.; Pan, Y.; Yin, Q.; Tang, J.; Qi, H.J.; Suo, Z. Printing Hydrogels and Elastomers in Arbitrary Sequence with Strong Adhesion. Adv. Funct. Mater. 2019, 29, 1901721.
- Silverman, H.G.; Roberto, F.F. Understanding Marine Mussel Adhesion. Mar. Biotechnol. 2007, 9, 661–681.
- Waite, J.H. Adhesion a La Moule. Biochemistry 2002, 1180, 1172–1180.
- Lee, H.; Scherer, N.F.; Messersmith, P.B. Single-Molecule Mechanics of Mussel Adhesion. Proc. Natl. Acad. Sci. USA 2006, 103, 12999–13003.
- Ye, N.; Neumeyer, J.L.; Baldessarini, R.J.; Zhen, X.; Zhang, A. Update 1 of: Recent Progress in Development of Dopamine Receptor Subtype-Selective Agents: Potential Therapeutics for Neurological and Psychiatric Disorders. Chem. Rev. 2013, 113, 274–302.
- Ye, Q.; Zhou, F.; Liu, W. Bioinspired Catecholic Chemistry for Surface Modification. Chem. Soc. Rev. 2011, 40, 4244–4258.
- Puertas-Bartolomé, M.; Vázquez-Lasa, B.; San Román, J. Bioactive and Bioadhesive Catechol Conjugated Polymers for Tissue Regeneration. Polymers 2018, 10, 768.
- Krogsgaard, M.; Nue, V.; Birkedal, H. Mussel-Inspired Materials: Self-Healing through Coordination Chemistry. Chem. Eur. J. 2016, 22, 844–857.
- Su, J.; Chen, F.; Cryns, V.L.; Messersmith, P.B. Catechol Polymers for PH-Responsive, Targeted Drug Delivery to Cancer Cells. J. Am. Chem. Soc. 2011, 133, 11850–11853.
- Patenaude, M.; Smeets, N.M.B.; Hoare, T. Designing Injectable, Covalently Cross-Linked Hydrogels for Biomedical Applications. Macromol. Rapid Commun. 2014, 35, 598–617.
- Sivashanmugam, A.; Arun Kumar, R.; Vishnu Priya, M.; Nair, S.V.; Jayakumar, R. An Overview of Injectable Polymeric Hydrogels for Tissue Engineering. Eur. Polym. J. 2015, 72, 543–565.
- Li, C.; Huang, Z.; Gao, N.; Zheng, J.; Guan, J. Injectable, Thermosensitive, Fast Gelation, Bioeliminable, and Oxygen Sensitive Hydrogels. Mater. Sci. Eng. C 2019, 99, 1191–1198.
- Vo, T.N.; Shah, S.R.; Lu, S.; Tatara, A.M.; Lee, E.J.; Roh, T.T.; Tabata, Y.; Mikos, A.G. Injectable Dual-Gelling Cell-Laden Composite Hydrogels for Bone Tissue Engineering. Biomaterials 2016, 83, 1–11.
- Yuan, L.; Wu, Y.; Gu, Q.-S.; El-Hamshary, H.; El-Newehy, M.; Mo, X. Injectable Photo Crosslinked Enhanced Double-Network Hydrogels from Modified Sodium Alginate and Gelatin. Int. J. Biol. Macromol. 2017, 96, 569–577.
- Xu, Q.; Sigen, A.; Gao, Y.; Guo, L.; Creagh-Flynn, J.; Zhou, D.; Greiser, U.; Dong, Y.; Wang, F.; Tai, H.; et al. A Hybrid Injectable Hydrogel from Hyperbranched PEG Macromer as a Stem Cell Delivery and Retention Platform for Diabetic Wound Healing. Acta Biomater. 2018, 75, 63–74.
- Skrobot, J.; Zair, L.; Ostrowski, M.; El Fray, M. New Injectable Elastomeric Biomaterials for Hernia Repair and Their Biocompatibility. Biomaterials 2016, 75, 182–192.
- Ulery, B.D.; Nair, L.S.; Laurencin, C.T. Biomedical Applications of Biodegradable Polymers. J. Polym. Sci. Part B Polym. Phys. 2011, 49, 832–864.
- Nair, L.S.; Laurencin, C.T. Biodegradable Polymers as Biomaterials. Prog. Polym. Sci. 2007, 32, 762–798.
- Liu, Q.; Jiang, L.; Shi, R.; Zhang, L. Synthesis, Preparation, In Vitro Degradation, and Application of Novel Degradable Bioelastomers—A Review. Prog. Polym. Sci. 2012, 37, 715–765.
- Gigli, M.; Fabbri, M.; Lotti, N.; Gamberini, R.; Rimini, B.; Munari, A. Poly(Butylene Succinate)-Based Polyesters for Biomedical Applications: A Review in Memory of Our Beloved Colleague and Friend Dr. Lara Finelli. Eur. Polym. J. 2016, 75, 431–460.
- Piarali, S.; Marlinghaus, L.; Viebahn, R.; Lewis, H.; Ryadnov, M.G.; Groll, J.; Salber, J.; Roy, I. Activated Polyhydroxyalkanoate Meshes Prevent Bacterial Adhesion and Biofilm Development in Regenerative Medicine Applications. Front. Bioeng. Biotechnol. 2020, 8, 442.
- Skrobot, J.; Ignaczak, W.; El Fray, M. Hydrolytic and Enzymatic Degradation of Fl Exible Polymer Networks Comprising Fatty Acid Derivatives. Polym. Degrad. Stab. 2015, 120, 368–376.
- Banerjee, A.; Chatterjee, K.; Madras, G. Enzymatic Degradation of Polymers: A Brief Review. Mater. Sci. Technol. 2014, 30, 567–573.
- Santerre, J.P.; Labow, R.S.; Duguay, D.G.; Erfle, D.; Adams, G.A. Biodegradation Evaluation of Polyether and Polyester-Urethanes with Oxidative and Hydrolytic Enzymes. J. Biomed. Mater. Res. 1994, 28, 1187–1199.
- Eskandarinia, A.; Kefayat, A.; Agheb, M.; Rafienia, M.; Amini Baghbadorani, M.; Navid, S.; Ebrahimpour, K.; Khodabakhshi, D.; Ghahremani, F. A Novel Bilayer Wound Dressing Composed of a Dense Polyurethane/Propolis Membrane and a Biodegradable Polycaprolactone/Gelatin Nanofibrous Scaffold. Sci. Rep. 2020, 10, 3063.
- Peppas, N.A.; Hilt, J.Z.; Khademhosseini, A.; Langer, R. Hydrogels in Biology and Medicine: From Molecular Principles to Bionanotechnology. Adv. Mater. 2006, 18, 1345–1360.
- Nichol, J.W.; Khademhosseini, A. Modular Tissue Engineering: Engineering Biological Tissues from the Bottom Up. Soft Matter 2009, 5, 1312–1319.
- Mandal, B.B.; Kundu, S.C. Cell Proliferation and Migration in Silk Fibroin 3D Scaffolds. Biomaterials 2009, 30, 2956–2965.
- Lien, S.M.; Ko, L.Y.; Huang, T.J. Effect of Pore Size on ECM Secretion and Cell Growth in Gelatin Scaffold for Articular Cartilage Tissue Engineering. Acta Biomater. 2009, 5, 670–679.
- Griffon, D.J.; Sedighi, M.R.; Schaeffer, D.V.; Eurell, J.A.; Johnson, A.L. Chitosan Scaffolds: Interconnective Pore Size and Cartilage Engineering. Acta Biomater. 2006, 2, 313–320.
- Kim, H.J.; Kim, U.J.; Vunjak-Novakovic, G.; Min, B.H.; Kaplan, D.L. Influence of Macroporous Protein Scaffolds on Bone Tissue Engineering from Bone Marrow Stem Cells. Biomaterials 2005, 26, 4442–4452.
- Dutta Roy, T.; Simon, J.L.; Ricci, J.L.; Rekow, E.D.; Thompson, V.P.; Parsons, J.R. Performance of Degradable Composite Bone Repair Products Made via Three-Dimensional Fabrication Techniques. J. Biomed. Mater. Res. Part A 2003, 66, 283–291.
- Kanimozhi, K.; Basha, S.K.; Kumari, V.S.; Kaviyarasu, K. Development of Biomimetic Hybrid Porous Scaffold of Chitosan/Polyvinyl Alcohol/Carboxymethyl Cellulose by Freeze-Dried and Salt Leached Technique. J. Nanosci. Nanotechnol. 2017, 18, 4916–4922.
- Morris, V.B.; Nimbalkar, S.; Younesi, M.; McClellan, P.; Akkus, O. Mechanical Properties, Cytocompatibility and Manufacturability of Chitosan:PEGDA Hybrid-Gel Scaffolds by Stereolithography. Ann. Biomed. Eng. 2017, 45, 286–296.
- Muzammil, K.M.; Mukherjee, D.; Azamthulla, M.; Teja, B.V.; Kaamnoore, D.; Anbu, J.; Srinivasan, B.; Jeevan Kasture, G. Castor Oil Reinforced Polymer Hybrids for Skin Tissue Augmentation. Int. J. Polym. Mater. Polym. Biomater. 2020, 70, 530–544.
- Yousefzade, O.; Katsarava, R.; Puiggalí, J. Biomimetic Hybrid Systems for Tissue Engineering. Biomimetics 2020, 5, 49.
- Allison, S.; Ahumada, M.; Andronic, C.; McNeill, B.; Variola, F.; Griffith, M.; Ruel, M.; Hamel, V.; Liang, W.; Suuronen, E.J.; et al. Electroconductive Nanoengineered Biomimetic Hybrid Fibers for Cardiac Tissue Engineering. J. Mater. Chem. B 2017, 5, 2402–2406.
- Setayeshmehr, M.; Esfandiari, E.; Rafieinia, M.; Hashemibeni, B.; Taheri-Kafrani, A.; Samadikuchaksaraei, A.; Kaplan, D.L.; Moroni, L.; Joghataei, M.T. Hybrid and Composite Scaffolds Based on Extracellular Matrices for Cartilage Tissue Engineering. Tissue Eng. Part B Rev. 2019, 25, 202–224.
- Neves, S.C.; Moroni, L.; Barrias, C.C.; Granja, P.L. Leveling Up Hydrogels: Hybrid Systems in Tissue Engineering. Trends Biotechnol. 2020, 38, 292–315.
- Fischenich, K.M.; Lewis, J.T.; Bailey, T.S.; Haut Donahue, T.L. Mechanical Viability of a Thermoplastic Elastomer Hydrogel as a Soft Tissue Replacement Material. J. Mech. Behav. Biomed. Mater. 2018, 79, 341–347.
- Lewis, J.T.; Fischenich, K.M.; Haut Donahue, T.L.; Bailey, T.S. Nanostructure-Driven Replication of Soft Tissue Biomechanics in a Thermoplastic Elastomer Hydrogel. ACS Biomater. Sci. Eng. 2018, 4, 3854–3863.
- Remya, K.R.; Chandran, S.; Mani, S.; John, A.; Ramesh, P. Hybrid Polycaprolactone/Polyethylene Oxide Scaffolds with Tunable Fiber Surface Morphology, Improved Hydrophilicity and Biodegradability for Bone Tissue Engineering Applications. J. Biomater. Sci. Polym. Ed. 2018, 29, 1444–1462.




