Elastomers are a class of polymeric materials that can repeatedly and easily undergo large, reversible deformations with complete recovery. They are usually composed of long-chain molecules, extremely flexible due to their ability to reconfigure themselves and dissipate an applied force. Hydrogels (HGs) are macromolecular structures consisting of polymer networks with the ability to absorb water without any dissolution. By applying sophisticated design and engineering methods, various elastomer–hydrogel systems (EHS) with outstanding performance have been developed in the last decades. These systems composed of elastomers and hydrogels are very attractive due to their high biocompatibility, injectability, controlled porosity and often antimicrobial properties. Moreover, their elastomeric properties and bioadhesiveness are making them suitable for soft tissue engineering.
- elastomers
- hydrogels
- elastomer–hydrogel systems
- injectable biomaterials
- adhesive surfaces
- tissue engineering
1. Introduction
2. Preparation of Elastomer–Hydrogel Systems
Elastomer–hydrogel systems (EHS) are the combination of two or more polymeric materials, commonly of natural and synthetic origin, offering remarkable properties and multifunctionalities through the combination of different structural components (Figure 1). These may be systems where a hydrogel is encapsulated by an elastomer matrix to prevent its dehydration or both components are forming interpenetrating polymer networks (IPN) which can be bonded to each other by covalent bonds or non-covalent interactions such as hydrogen bonding, van der Waals and electrostatic interactions.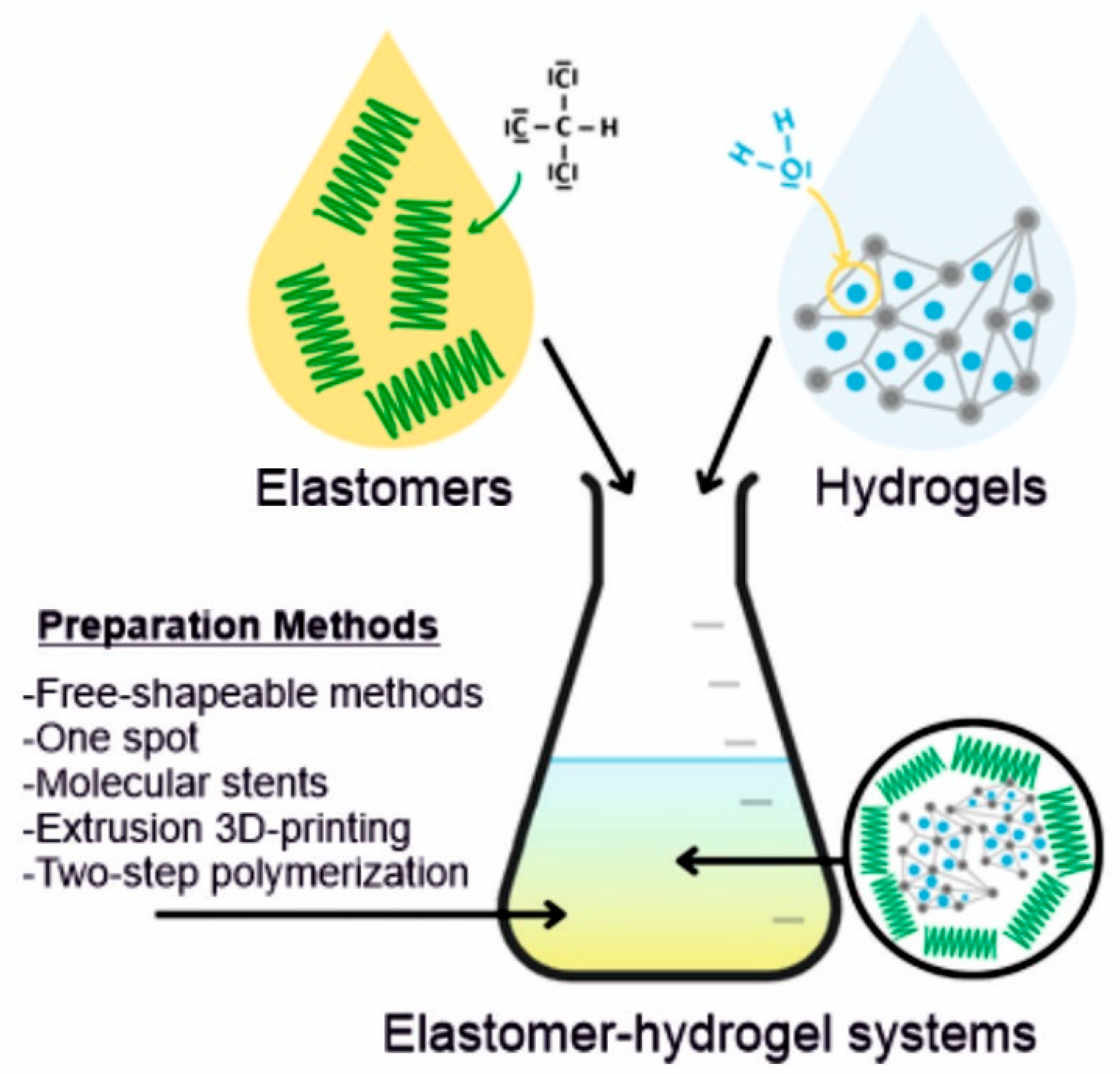
23. Biofunctionalities of Elastomer–Hydrogel Systems
EHS are gaining increased interest for medical applications due to their unique combination of properties, often emulating live organisms’ function and performance. Some of the sophisticated properties found in biomimetic materials will be discussed with emphasis on bioadhesiveness, injectability, antibacterial properties, biodegradability and porosity which are important for tissue engineering (Figure 2).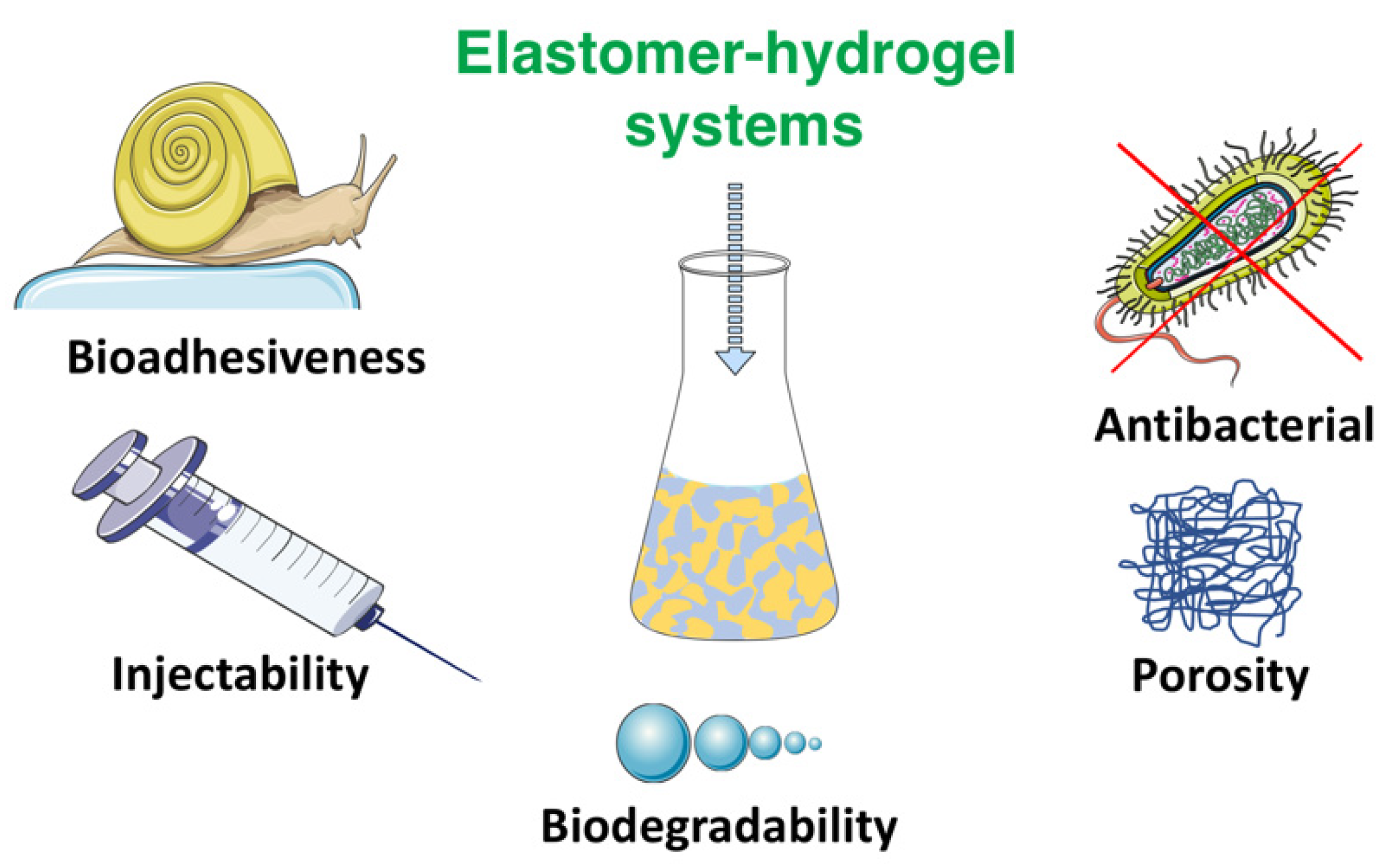
2.1. Bioadhesiveness
3.1. Bioadhesiveness
Most of the medical applications, especially surgical procedures, require tissue adhesives, sealants, and hemostatic agents. Those bioadhesives are mostly a glue to bind the tissues, seal the gaps or cracks and initiate the formation of blood clots, respectively [121,122][42][43]. Synthetic compounds which show adhesive properties such as poly(ethylene glycol) diacrylate (PEGDA) [123][44], N,N-dimethylaminoethyl methacrylate-co-methyl methacrylate (NDMEM) [124][45], gelatin methacrylate (GelMA) [125][46], tannic acid (TA) [126][47], etc., have been successfully introduced by physical or chemical processes into the patches or scaffolds with the development of materials science. This approach has gained successful outcomes in medical applications thanks to the adhesion ability of those materials to various tissues such as soft tissue, bone and skin [127,128,129,130][48][49][50][51]. However, their lack of robust and reversible adhesion abilities limit their application efficiencies. Therefore, inspiration from nature provides enormous information on how to develop materials with versatile adhesion capacities for both wet and dry surfaces. Determination of the key compounds within the various species has opened the way to introducing these compounds into the structured materials for medical applications. Thanks to these compounds, EHS can act fully or partially as bioadhesives, depending on the functional groups introduced that are inspired by nature. EHS can be structured to achieve the desired, controllable and reusable adhesion strength in wet environments. Recently, bioinspired adhesives have attracted great attention due the combination of natural functionality realized through synthetic approaches. For instance, mussels show extremely good adhesion with high binding strength to various surfaces under wet conditions [131,132,133][52][53][54]. It was found that the catechol unit is the main factor that allows mussels to adhere to a variety of surfaces [134,135][55][56]. Materials containing catechol units can be used to create covalent and non-covalent attachments to various substrates for many medical applications, including drug delivery systems and wound healing [47,136,137][57][58][59].2.2. Injectability
3.2. Injectability
Traditional surgeries are increasingly being replaced by less invasive methods that shorten an overall procedure and the patient’s recovery time. Especially, in tissue engineering, the focus is on to improving the materials’ performance by their injectability [138][60]. The injectable systems can efficiently deliver particles such as drugs (antibiotics, anesthetics), biomolecules (fibrin), fillers (silica nanoparticles) or genes (DNA, siRNA) [139][61]. An attractive model, developed by Li et al., is an injectable probe for measuring oxygen in tissues [140][62]. Hydrogels containing N-isopropylacrylamide copolymer macromers for mesenchymal stem cell (MSC) delivery allow the formation of bone bridges, promoting the viability of MSCs, and can be used to create hard tissues, due to gelatin microparticles (GMP) which are enzymatically digestible porogens and sites for cell attachment [141][63]. Another long-term persistent hydrogel is the photo-crosslinked material composed of a double-network of modified sodium alginate and gelatin created by the Schiff base reaction [142][64]. Collectively, different works have clearly demonstrated the huge potential of injectable materials for biomedical applications. Xu et al., produced an injectable EHS consisting of hyperbranched multi-acrylated poly(ethylene glycol) macromers (HP-PEGs) and thiolated hyaluronic acid (HA-SH) and used it as a stem cell delivery system for diabetic wound healing [143][65]. It is also worth noting that new injectable and photocurable elastomers containing fatty acid derivatives can be successfully used for minimally invasive surgical protocols in the repair of small hernia defects [144][66].2.3. Biodegradation
3.3. Biodegradation
Biodegradable materials are now tending to become the most commonly used materials in medical applications due to their gradual bio-resorption into the human body [145][67]. Biodegradability is one of the key properties for the materials which are used in medical applications. It should be considered that the degradation rate must be consistent with the healing and regeneration process. Various crosslink densities, crosslinking mechanisms and component types were applied to control the degradation rate of such systems [146,147][68][69]. The most commonly used biodegradable materials consist of homo- or copolymers of alpha-hydroxy acids, such as lactic and/or glycolic acids. Biodegradation can be triggered either by water (hydrolytic degradation) and/or enzymes (enzymatic degradation) within the body. The chemical structure of a polymer has the greatest influence on the type of degradation. Other important factors are chemical composition, the type of crosslinking bonds, molecular weight and its distribution, porosity, stereochemistry and chain mobility [148][70]. The elastomeric part of the EHS usually tends towards hydrolytic biodegradation due to its molecular chain structures sensitive to water. The hydrolysis of ester bonds usually leads to the creation of carboxyl and hydroxyl end groups, whereas natural biomaterials tend to degrade enzymatically. The injected and/or implanted EHS can be degraded by oxidative (catalases, horseradish peroxidase and xanthine oxidase) or hydrolytic (protease, hydrolase, phosphatases, lipase and esterase) enzymes when exposed to body fluids and tissues [149,150,151][71][72][73]. Inflammatory cells (e.g., macrophages and leukocytes) create reactive oxygen species such as hydrogen peroxide, superoxide and nitric oxide during the inflammatory response to foreign materials [152][74]. EHS can be cut up by those species which are contributing to material degradation whereas the hydrolytic enzymes hydrolyze the components of the hybrid network to help in the absorption of nutrients and solutes. For instance, a poly(caprolactone) (PCL)/gelatin(Gel) scaffold (sublayer) was electrospun on a dense polyurethane (PU)/propolis(EEP) (top layer) membrane to fabricate a bilayer wound dressing. It was demonstrated that the EHS combining a synthetic polymer with a natural one could enhance the stability of the scaffold. Hydrolytic and enzymatic degradation studies showed that PU/EEP membrane exhibited a slower degradation rate in comparison with a PCL/Gel hybrid structure. In the case of hydrolytic degradation, the total mass loss after 28 days for PU/EEP and PCL/Gel was found to be 1.9 and 76%, respectively [153][75].2.4. Porosity
3.4. Porosity
The porosity is an important feature in medical applications, especially in scaffolds [154,155][76][77]. The pore architecture and interconnectivity have a beneficial role in proliferation, cell survival and migration to create functional materials, and secrete ECM. Therefore, scaffold porosity is a must for homogenous cell distribution and interconnection throughout engineered tissues [156,157][78][79]. Additionally, pore size can have an effect on the cell growth, vascularization, nutrients and oxygen diffusion, especially in the absence of a functional vascular system [133,134,135,158,159,160][54][55][56][80][81][82]. Various techniques, components and ratios are used to obtain controlled pore size and architecture scaffolds. For instance, Kanimozhi et al. prepared a chitosan/poly(vinyl alcohol)/carboxymethyl cellulose (CP-CMC) porous scaffold by simple freeze drying and salt leaching techniques. Among scaffolds, 1:1 weight ratios showed significantly high porosity as compared to other ratios. The incorporation of CMC enhanced the scaffold porosity from 50 to 90% by increasing the molar ratio of CMC. However, when comparing the freeze-dried scaffolds and salt-leached scaffolds of 1:1 weight ratio, the 50% CP:50% CMC material showed a higher porosity of 90% in salt-leached and 70% in freeze-dried scaffolds, respectively. The reason was explained thus: with the increase of CMC ratio, the actual volume occupied by the molecules decreased [161][83]. In another study, Morris et al. produced porous elastomer–hydrogel scaffolds of chitosan/polyethylene glycol diacrylate (CS/PEGDA) using 3D bioprinting by a stereolithography method to create internal pore and macroscopic shapes. They achieved varied pore sizes by changing the CS molecular weight ratios. For instance, the average pore size of the pure PEGDA scaffolds increased from 24% to 67% by the addition of low molecular weight CS (LMWCS) (MW = 50–190 kDa) into the scaffold with the ratio LMWCS:PEGDA at 1:7.5. These kinds of studies show that controlled pore size and architecture can be achievable for specific needs in medical applications [162][84].2.5. Antibacterial Surfaces
3.5. Antibacterial Surfaces
Antibacterial materials, especially surfaces, are playing an important role in protecting from contamination and eliminating bacteria from skin tissue and the surfaces of medical devices and implants. Bacterial adhesion is the main cause of the creation of 3D biofilm complex structures which infect the surrounding tissues. Therefore, new strategies which eliminate biofilm-based issues are applied. Hence, EHS which contain antibacterial components are being developed. For instance, Piarali et al. fabricated a fiber mesh based on the surface modification of polyhydroxyalkanoate, using an electrospinning technique, for tissue regeneration. Here, basically an EHS was created by a synthetic antimicrobial peptide with anti-biofilm and strong bactericidal properties [149][71]. In another study, Muzammil et al. created elastomer–hydrogel scaffolds containing castor-oil-reinforced chitosan with various hydrophilic polymers. The obtained EHS showed antibacterial and hemostatic activities with good mechanical properties. Therefore, such systems could be good candidates for skin tissue regeneration and wound healing applications [163][85].3. Elastomer–Hydrogel Systems for Soft Tissue Engineering Applications
The development of advanced systems for tissue engineering applications has been widely studied over the last decades. Specific interactions between the components, the combination of raw material advantages and the molecular organization of these systems dictates the direction of the tissue engineering applications. Different EHS systems which combine different classes of elastomers and hydrogels in one material with large yield formulations and many advantages, such as high interaction with targets to enhance their performance have been effectively developed. EHS play an important role in the success of tissue engineering approaches, as they guide the structure of developing tissues, gaining mechanical and physical stability, and migrating cells or delivering the molecules to transplanted areas. Those highly efficient EHS find applications in soft, bone, skin, neural and cardiac tissue engineering [164,165,166,167][86][87][88][89]. For instance, Fischenich et al. has developed a thermoplastic elastomer (TPE) hydrogel system for soft tissues, especially for articular cartilage, the knee meniscus, etc. The created system was based on a blend of unreacted ω-hydroxy-polystyrene-b-poly(ethylene oxide) (SO) and coupled polystyrene-b-poly(ethylene oxide)-b-polystyrene (SOS). The obtained TPE hydrogel system could be a promising candidate for soft tissue replacement with a comparable equilibrium compressive modulus of approximately 0.5 MPa and shear modulus of 0.2 MPa [168][90]. Lewis et al. reported a thermoplastic elastomer–hydrogel system based on the prefabrication of an efficient nanoscale network architecture using the melt-state ω-hydroxy-polystyrene-b-poly(ethylene oxide) (SO) and polystyrene-b-poly(ethylene oxide)-b-polystyrene (SOS) as amphiphilic block copolymers. They proved by physical and mechanical analysis that the obtained systems have relevant moduli and water compositions, subsecond elastic recovery rates, negligible hysteresis, and unprecedented resistance to fatigue over hundreds of thousands of compression cycles. They suggested that such hydrogels may have tremendous promise beyond the synthetic soft tissue engineering applications for which they have been targeted [169][91]. In another study, Remya et al. synthesized EHS by modifying PCL with different molecular ratios of water soluble polymer PEO using the electrospinning technique to create scaffolds. The weight loss for pure PCL was 8.5% whereas for PCL/PEO blends with 50:50 ratios and differing the molecular weight of the PEO (10 k g/mol vs. 60 k g/mol), the weight loss was 41.7 and 48.7%, respectively after 3 months. The study also showed that the properties of PCL scaffolds such as cell viability, mechanical properties and hydrophilicity were increased by the incorporation of PEO and these materials could be possible candidates for bone tissue engineering applications [169][91].References
- Vijayavenkataraman, S.; Yan, W.C.; Lu, W.F.; Wang, C.H.; Fuh, J.Y.H. 3D Bioprinting of Tissues and Organs for Regenerative Medicine. Adv. Drug Deliv. Rev. 2018, 132, 296–332.
- Teramura, Y.; Ekdahl, K.N.; Barbu, A. A Hybrid of Cells and Pancreatic Islets toward a New Bioartificial Pancreas. Regen. Ther. 2016, 3, 68–74.
- Abbott, R.D.; Kaplan, D.L. Engineering Biomaterials for Enhanced Tissue Regeneration. Curr. Stem Cell Rep. 2016, 2, 140–146.
- Luo, Z.; Weiss, D.E.; Liu, Q.; Tian, B. Biomimetic Approaches toward Smart Bio-Hybrid Systems. Nano Res. 2018, 11, 3009–3030.
- Walia, R.; Akhavan, B.; Kosobrodova, E.; Kondyurin, A.; Oveissi, F.; Naficy, S.; Yeo, G.C.; Hawker, M.; Kaplan, D.L.; Dehghani, F.; et al. Hydrogel−Solid Hybrid Materials for Biomedical Applications Enabled by Surface-Embedded Radicals. Adv. Funct. Mater. 2020, 30, 2004599.
- Ozdil, D.; Aydin, H.M. Polymers for Medical and Tissue Engineering Applications. J. Chem. Technol. Biotechnol. 2014, 89, 1793–1810.
- Li, J.; Osada, Y.; Cooper-White, J. (Eds.) Functional Hydrogels as Biomaterials; Springer Series in Biomaterials Science and Engineering; Springer: Berlin/Heidelberg, Germany, 2018; Volume 12, ISBN 978-3-662-57509-3.
- Król, P. Synthesis Methods, Chemical Structures and Phase Structures of Linear Polyurethanes. Properties and Applications of Linear Polyurethanes in Polyurethane Elastomers, Copolymers and Ionomers. Prog. Mater. Sci. 2007, 52, 915–1015.
- Amsden, B. Curable, Biodegradable Elastomers: Emerging Biomaterials for Drug Delivery and Tissue Engineering. Soft Matter 2007, 3, 1335–1348.
- Hassouna, Y.M.; Zamani, S.; Kafienah, W.; Younes, H.M. Synthesis, Characterization & Cytocompatibility of Poly (Diol-Co-Tricarballylate) Based Thermally Crosslinked Elastomers for Drug Delivery & Tissue Engineering Applications. Mater. Sci. Eng. C 2018, 93, 254–264.
- Opris, D.M. Polar Elastomers as Novel Materials for Electromechanical Actuator Applications. Adv. Mater. 2018, 30, 1–23.
- Xu, C.; Huang, Y.; Yepez, G.; Wei, Z.; Liu, F.; Bugarin, A.; Tang, L.; Hong, Y. Development of Dopant-Free Conductive Bioelastomers. Sci. Rep. 2016, 6, 34451.
- Mirfakhrai, T.; Madden, J.D.W.; Baughman, R.H. Polymer Artificial Muscles. Mater. Today 2007, 10, 30–38.
- Jeong, K.H.; Kim, J.; Lee, L.P. Biologically Inspired Artificial Compound Eyes. Science 2006, 312, 557–561.
- Wen, Q.; Mithieux, S.M.; Weiss, A.S. Elastin Biomaterials in Dermal Repair. Trends Biotechnol. 2020, 38, 280–291.
- Christman, K.L.; Vardanian, A.J.; Fang, Q.; Sievers, R.E.; Fok, H.H.; Lee, R.J. Injectable Fibrin Scaffold Improves Cell Transplant Survival, Reduces Infarct Expansion, and Induces Neovasculature Formation in Ischemic Myocardium. J. Am. Coll. Cardiol. 2004, 44, 654–660.
- Chen, Q.Z.; Harding, S.E.; Ali, N.N.; Lyon, A.R.; Boccaccini, A.R. Biomaterials in Cardiac Tissue Engineering: Ten Years of Research Survey. Mater. Sci. Eng. R Rep. 2008, 59, 1–37.
- Zeimaran, E.; Pourshahrestani, S.; Djordjevic, I.; Pingguan-Murphy, B.; Kadri, N.A.; Towler, M.R. Bioactive Glass Reinforced Elastomer Composites for Skeletal Regeneration: A Review. Mater. Sci. Eng. C 2015, 53, 175–188.
- Chen, Q.; Liang, S.; Thouas, G.A. Elastomeric Biomaterials for Tissue Engineering. Prog. Polym. Sci. 2013, 38, 584–671.
- Tian, Y.; Liang, K.; Wang, X.; Ji, Y. Fabrication of Nanocomposite Bioelastomer Porous Scaffold Based on Chitin Nanocrystal Supported Emulsion-Freeze-Casting. ACS Sustain. Chem. Eng. 2017, 5, 3305–3313.
- Hunt, J.A.; Chen, R.; van Veen, T.; Bryan, N. Hydrogels for Tissue Engineering and Regenerative Medicine. J. Mater. Chem. B 2014, 2, 5319–5338.
- Ahadian, S.; Sadeghian, R.B.; Salehi, S.; Ostrovidov, S.; Bae, H.; Ramalingam, M.; Khademhosseini, A. Bioconjugated Hydrogels for Tissue Engineering and Regenerative Medicine. Bioconjug. Chem. 2015, 26, 1984–2001.
- Lutolf, M.P.; Hubbell, J.A. Synthetic Biomaterials as Instructive Extracellular Microenvironments for Morphogenesis in Tissue Engineering. Nat. Biotechnol. 2005, 23, 47–55.
- Sahoo, S.; Chung, C.; Khetan, S.; Burdick, J.A. Hydrolytically Degradable Hyaluronic Acid Hydrogels with Controlled Temporal Structures. Biomacromolecules 2008, 9, 1088–1092.
- Chen, Q.; Chen, H.; Zhu, L.; Zheng, J. Fundamentals of Double Network Hydrogels. J. Mater. Chem. B 2015, 3, 3654–3676.
- Xu, C.; Okpokwasili, C.; Huang, Y.; Shi, X.; Wu, J.; Liao, J.; Tang, L.; Hong, Y. Optimizing Anisotropic Polyurethane Scaffolds to Mechanically Match with Native Myocardium. ACS Biomater. Sci. Eng. 2020, 6, 2757–2769.
- Richbourg, N.R.; Peppas, N.A.; Sikavitsas, V.I. Tuning the Biomimetic Behavior of Scaffolds for Regenerative Medicine through Surface Modifications. J. Tissue Eng. Regen. Med. 2019, 13, 1275–1293.
- Olbrich, J.M.; Tate, P.L.; Corbett, J.T.; Lindsey, J.M.; Nagatomi, S.D.; Shalaby, W.S.W.; Shalaby, S.W. Injectable in Situ Forming Controlled Release Implant Composed of a Poly-Ether-Ester-Carbonate and Applications in the Field of Chemotherapy. J. Biomed. Mater. Res. Part A 2012, 100, 2365–2372.
- Manavitehrani, I.; Fathi, A.; Badr, H.; Daly, S.; Shirazi, A.N.; Dehghani, F. Biomedical Applications of Biodegradable Polyesters. Polymers 2016, 8, 20.
- Ovcharenko, E.; Rezvova, M.; Nikishau, P.; Kostjuk, S.; Glushkova, T.; Antonova, L.; Trebushat, D.; Akentieva, T.; Shishkova, D.; Krivikina, E.; et al. Polyisobutylene-Based Thermoplastic Elastomers for Manufacturing Polymeric Heart Valve Leaflets: In Vitro and In Vivo Results. Appl. Sci. 2019, 9, 4773.
- Del Bakhshayesh, A.R.; Asadi, N.; Alihemmati, A.; Tayefi Nasrabadi, H.; Montaseri, A.; Davaran, S.; Saghati, S.; Akbarzadeh, A.; Abedelahi, A. An Overview of Advanced Biocompatible and Biomimetic Materials for Creation of Replacement Structures in the Musculoskeletal Systems: Focusing on Cartilage Tissue Engineering. J. Biol. Eng. 2019, 13, 85.
- Zhang, W.; Zhang, Y.S.; Bakht, S.M.; Aleman, J.; Shin, S.R.; Yue, K.; Sica, M.; Ribas, J.; Duchamp, M.; Ju, J.; et al. Elastomeric Free-Form Blood Vessels for Interconnecting Organs on Chip Systems. Lab Chip 2016, 16, 1579–1586.
- Cheung, M.E.; Mellert, L.T.; Firstenberg, M.S. Bedside Procedure: Retained Central Venous Catheter. In Vignettes in Patient Safety; InTech: London, UK, 2018; Volume 2.
- Li, M.; Chen, J.; Shi, M.; Zhang, H.; Ma, P.X.; Guo, B. Electroactive Anti-Oxidant Polyurethane Elastomers with Shape Memory Property as Non-Adherent Wound Dressing to Enhance Wound Healing. Chem. Eng. J. 2019, 375, 121999.
- Sanchez, C.; Julián, B.; Belleville, P.; Popall, M. Applications of Hybrid Organic-Inorganic Nanocomposites. J. Mater. Chem. 2005, 15, 3559–3592.
- Shi, R.; Chen, D.; Liu, Q.; Wu, Y.; Xu, X.; Zhang, L.; Tian, W. Recent Advances in Synthetic Bioelastomers. Int. J. Mol. Sci. 2009, 10, 4223–4256.
- Zhang, Y.; Thakur, V.K.; Li, Y.; Garrison, T.F.; Gao, Z.; Gu, J.; Kessler, M.R. Soybean-Oil-Based Thermosetting Resins with Methacrylated Vanillyl Alcohol as Bio-Based, Low-Viscosity Comonomer. Macromol. Mater. Eng. 2018, 303, 1700278.
- Jia, X.; Kiick, K.L. Hybrid Multicomponent Hydrogels for Tissue Engineering. Macromol. Biosci. 2009, 9, 140–156.
- Liu, Q.; Nian, G.; Yang, C.; Qu, S.; Suo, Z. Bonding Dissimilar Polymer Networks in Various Manufacturing Processes. Nat. Commun. 2018, 9, 846.
- Wirthl, D.; Pichler, R.; Drack, M.; Kettlguber, G.; Moser, R.; Gerstmayr, R.; Hartmann, F.; Bradt, E.; Kaltseis, R.; Siket, C.M.; et al. Instant Tough Bonding of Hydrogels for Soft Machines and Electronics. Sci. Adv. 2017, 3, 1700053.
- Yuk, H.; Zhang, T.; Parada, G.A.; Liu, X.; Zhao, X. Skin-Inspired Hydrogel–Elastomer Hybrids with Robust Interfaces and Functional Microstructures. Nat. Commun. 2016, 7, 12028.
- Mehdizadeh, M.; Yang, J. Design Strategies and Applications of Tissue Bioadhesives. Macromol. Biosci. 2013, 13, 271–288.
- Du, X.; Wu, L.; Yan, H.; Qu, L.; Wang, L.; Wang, X.; Ren, S.; Kong, D.; Wang, L. Multifunctional Hydrogel Patch with Toughness, Tissue Adhesiveness, and Antibacterial Activity for Sutureless Wound Closure. ACS Biomater. Sci. Eng. 2019, 5, 2610–2620.
- Quintanar-Guerrero, D.; Villalobos-García, R.; Alvarez-Colín, E.; Cornejo-Bravo, J.M. In Vitro Evaluation of the Bioadhesive Properties of Hydrophobic Polybasic Gels Containing N,N-Dimethylaminoethyl Methacrylate-Co-Methyl Methacrylate. Biomaterials 2001, 22, 957–961.
- Walker, B.W.; Lara, R.P.; Yu, C.H.; Sani, E.S.; Kimball, W.; Joyce, S.; Annabi, N. Engineering a Naturally-Derived Adhesive and Conductive Cardiopatch. Biomaterials 2019, 207, 89–101.
- Shin, M.; Kim, K.; Shim, W.; Yang, J.W.; Lee, H. Tannic Acid as a Degradable Mucoadhesive Compound. ACS Biomater. Sci. Eng. 2016, 2, 687–696.
- Bouten, P.J.M.; Zonjee, M.; Bender, J.; Yauw, S.T.K.; Van Goor, H.; Van Hest, J.C.M.; Hoogenboom, R. The Chemistry of Tissue Adhesive Materials. Prog. Polym. Sci. 2014, 39, 1375–1405.
- Tian, K.; Bae, J.; Suo, Z.; Vlassak, J.J. Adhesion between Hydrophobic Elastomer and Hydrogel through Hydrophilic Modification and Interfacial Segregation. ACS Appl. Mater. Interfaces 2018, 10, 43252–43261.
- Yang, J.; Bai, R.; Chen, B.; Suo, Z. Hydrogel Adhesion: A Supramolecular Synergy of Chemistry, Topology, and Mechanics. Adv. Funct. Mater. 2020, 30, 1901693.
- Yang, H.; Li, C.; Yang, M.; Pan, Y.; Yin, Q.; Tang, J.; Qi, H.J.; Suo, Z. Printing Hydrogels and Elastomers in Arbitrary Sequence with Strong Adhesion. Adv. Funct. Mater. 2019, 29, 1901721.
- Silverman, H.G.; Roberto, F.F. Understanding Marine Mussel Adhesion. Mar. Biotechnol. 2007, 9, 661–681.
- Waite, J.H. Adhesion a La Moule. Biochemistry 2002, 1180, 1172–1180.
- Lee, H.; Scherer, N.F.; Messersmith, P.B. Single-Molecule Mechanics of Mussel Adhesion. Proc. Natl. Acad. Sci. USA 2006, 103, 12999–13003.
- Ye, N.; Neumeyer, J.L.; Baldessarini, R.J.; Zhen, X.; Zhang, A. Update 1 of: Recent Progress in Development of Dopamine Receptor Subtype-Selective Agents: Potential Therapeutics for Neurological and Psychiatric Disorders. Chem. Rev. 2013, 113, 274–302.
- Ye, Q.; Zhou, F.; Liu, W. Bioinspired Catecholic Chemistry for Surface Modification. Chem. Soc. Rev. 2011, 40, 4244–4258.
- Puertas-Bartolomé, M.; Vázquez-Lasa, B.; San Román, J. Bioactive and Bioadhesive Catechol Conjugated Polymers for Tissue Regeneration. Polymers 2018, 10, 768.
- Krogsgaard, M.; Nue, V.; Birkedal, H. Mussel-Inspired Materials: Self-Healing through Coordination Chemistry. Chem. Eur. J. 2016, 22, 844–857.
- Su, J.; Chen, F.; Cryns, V.L.; Messersmith, P.B. Catechol Polymers for PH-Responsive, Targeted Drug Delivery to Cancer Cells. J. Am. Chem. Soc. 2011, 133, 11850–11853.
- Patenaude, M.; Smeets, N.M.B.; Hoare, T. Designing Injectable, Covalently Cross-Linked Hydrogels for Biomedical Applications. Macromol. Rapid Commun. 2014, 35, 598–617.
- Sivashanmugam, A.; Arun Kumar, R.; Vishnu Priya, M.; Nair, S.V.; Jayakumar, R. An Overview of Injectable Polymeric Hydrogels for Tissue Engineering. Eur. Polym. J. 2015, 72, 543–565.
- Li, C.; Huang, Z.; Gao, N.; Zheng, J.; Guan, J. Injectable, Thermosensitive, Fast Gelation, Bioeliminable, and Oxygen Sensitive Hydrogels. Mater. Sci. Eng. C 2019, 99, 1191–1198.
- Vo, T.N.; Shah, S.R.; Lu, S.; Tatara, A.M.; Lee, E.J.; Roh, T.T.; Tabata, Y.; Mikos, A.G. Injectable Dual-Gelling Cell-Laden Composite Hydrogels for Bone Tissue Engineering. Biomaterials 2016, 83, 1–11.
- Yuan, L.; Wu, Y.; Gu, Q.-S.; El-Hamshary, H.; El-Newehy, M.; Mo, X. Injectable Photo Crosslinked Enhanced Double-Network Hydrogels from Modified Sodium Alginate and Gelatin. Int. J. Biol. Macromol. 2017, 96, 569–577.
- Xu, Q.; Sigen, A.; Gao, Y.; Guo, L.; Creagh-Flynn, J.; Zhou, D.; Greiser, U.; Dong, Y.; Wang, F.; Tai, H.; et al. A Hybrid Injectable Hydrogel from Hyperbranched PEG Macromer as a Stem Cell Delivery and Retention Platform for Diabetic Wound Healing. Acta Biomater. 2018, 75, 63–74.
- Skrobot, J.; Zair, L.; Ostrowski, M.; El Fray, M. New Injectable Elastomeric Biomaterials for Hernia Repair and Their Biocompatibility. Biomaterials 2016, 75, 182–192.
- Ulery, B.D.; Nair, L.S.; Laurencin, C.T. Biomedical Applications of Biodegradable Polymers. J. Polym. Sci. Part B Polym. Phys. 2011, 49, 832–864.
- Nair, L.S.; Laurencin, C.T. Biodegradable Polymers as Biomaterials. Prog. Polym. Sci. 2007, 32, 762–798.
- Liu, Q.; Jiang, L.; Shi, R.; Zhang, L. Synthesis, Preparation, In Vitro Degradation, and Application of Novel Degradable Bioelastomers—A Review. Prog. Polym. Sci. 2012, 37, 715–765.
- Gigli, M.; Fabbri, M.; Lotti, N.; Gamberini, R.; Rimini, B.; Munari, A. Poly(Butylene Succinate)-Based Polyesters for Biomedical Applications: A Review in Memory of Our Beloved Colleague and Friend Dr. Lara Finelli. Eur. Polym. J. 2016, 75, 431–460.
- Piarali, S.; Marlinghaus, L.; Viebahn, R.; Lewis, H.; Ryadnov, M.G.; Groll, J.; Salber, J.; Roy, I. Activated Polyhydroxyalkanoate Meshes Prevent Bacterial Adhesion and Biofilm Development in Regenerative Medicine Applications. Front. Bioeng. Biotechnol. 2020, 8, 442.
- Skrobot, J.; Ignaczak, W.; El Fray, M. Hydrolytic and Enzymatic Degradation of Fl Exible Polymer Networks Comprising Fatty Acid Derivatives. Polym. Degrad. Stab. 2015, 120, 368–376.
- Banerjee, A.; Chatterjee, K.; Madras, G. Enzymatic Degradation of Polymers: A Brief Review. Mater. Sci. Technol. 2014, 30, 567–573.
- Santerre, J.P.; Labow, R.S.; Duguay, D.G.; Erfle, D.; Adams, G.A. Biodegradation Evaluation of Polyether and Polyester-Urethanes with Oxidative and Hydrolytic Enzymes. J. Biomed. Mater. Res. 1994, 28, 1187–1199.
- Eskandarinia, A.; Kefayat, A.; Agheb, M.; Rafienia, M.; Amini Baghbadorani, M.; Navid, S.; Ebrahimpour, K.; Khodabakhshi, D.; Ghahremani, F. A Novel Bilayer Wound Dressing Composed of a Dense Polyurethane/Propolis Membrane and a Biodegradable Polycaprolactone/Gelatin Nanofibrous Scaffold. Sci. Rep. 2020, 10, 3063.
- Peppas, N.A.; Hilt, J.Z.; Khademhosseini, A.; Langer, R. Hydrogels in Biology and Medicine: From Molecular Principles to Bionanotechnology. Adv. Mater. 2006, 18, 1345–1360.
- Nichol, J.W.; Khademhosseini, A. Modular Tissue Engineering: Engineering Biological Tissues from the Bottom Up. Soft Matter 2009, 5, 1312–1319.
- Mandal, B.B.; Kundu, S.C. Cell Proliferation and Migration in Silk Fibroin 3D Scaffolds. Biomaterials 2009, 30, 2956–2965.
- Lien, S.M.; Ko, L.Y.; Huang, T.J. Effect of Pore Size on ECM Secretion and Cell Growth in Gelatin Scaffold for Articular Cartilage Tissue Engineering. Acta Biomater. 2009, 5, 670–679.
- Griffon, D.J.; Sedighi, M.R.; Schaeffer, D.V.; Eurell, J.A.; Johnson, A.L. Chitosan Scaffolds: Interconnective Pore Size and Cartilage Engineering. Acta Biomater. 2006, 2, 313–320.
- Kim, H.J.; Kim, U.J.; Vunjak-Novakovic, G.; Min, B.H.; Kaplan, D.L. Influence of Macroporous Protein Scaffolds on Bone Tissue Engineering from Bone Marrow Stem Cells. Biomaterials 2005, 26, 4442–4452.
- Dutta Roy, T.; Simon, J.L.; Ricci, J.L.; Rekow, E.D.; Thompson, V.P.; Parsons, J.R. Performance of Degradable Composite Bone Repair Products Made via Three-Dimensional Fabrication Techniques. J. Biomed. Mater. Res. Part A 2003, 66, 283–291.
- Kanimozhi, K.; Basha, S.K.; Kumari, V.S.; Kaviyarasu, K. Development of Biomimetic Hybrid Porous Scaffold of Chitosan/Polyvinyl Alcohol/Carboxymethyl Cellulose by Freeze-Dried and Salt Leached Technique. J. Nanosci. Nanotechnol. 2017, 18, 4916–4922.
- Morris, V.B.; Nimbalkar, S.; Younesi, M.; McClellan, P.; Akkus, O. Mechanical Properties, Cytocompatibility and Manufacturability of Chitosan:PEGDA Hybrid-Gel Scaffolds by Stereolithography. Ann. Biomed. Eng. 2017, 45, 286–296.
- Muzammil, K.M.; Mukherjee, D.; Azamthulla, M.; Teja, B.V.; Kaamnoore, D.; Anbu, J.; Srinivasan, B.; Jeevan Kasture, G. Castor Oil Reinforced Polymer Hybrids for Skin Tissue Augmentation. Int. J. Polym. Mater. Polym. Biomater. 2020, 70, 530–544.
- Yousefzade, O.; Katsarava, R.; Puiggalí, J. Biomimetic Hybrid Systems for Tissue Engineering. Biomimetics 2020, 5, 49.
- Allison, S.; Ahumada, M.; Andronic, C.; McNeill, B.; Variola, F.; Griffith, M.; Ruel, M.; Hamel, V.; Liang, W.; Suuronen, E.J.; et al. Electroconductive Nanoengineered Biomimetic Hybrid Fibers for Cardiac Tissue Engineering. J. Mater. Chem. B 2017, 5, 2402–2406.
- Setayeshmehr, M.; Esfandiari, E.; Rafieinia, M.; Hashemibeni, B.; Taheri-Kafrani, A.; Samadikuchaksaraei, A.; Kaplan, D.L.; Moroni, L.; Joghataei, M.T. Hybrid and Composite Scaffolds Based on Extracellular Matrices for Cartilage Tissue Engineering. Tissue Eng. Part B Rev. 2019, 25, 202–224.
- Neves, S.C.; Moroni, L.; Barrias, C.C.; Granja, P.L. Leveling Up Hydrogels: Hybrid Systems in Tissue Engineering. Trends Biotechnol. 2020, 38, 292–315.
- Fischenich, K.M.; Lewis, J.T.; Bailey, T.S.; Haut Donahue, T.L. Mechanical Viability of a Thermoplastic Elastomer Hydrogel as a Soft Tissue Replacement Material. J. Mech. Behav. Biomed. Mater. 2018, 79, 341–347.
- Lewis, J.T.; Fischenich, K.M.; Haut Donahue, T.L.; Bailey, T.S. Nanostructure-Driven Replication of Soft Tissue Biomechanics in a Thermoplastic Elastomer Hydrogel. ACS Biomater. Sci. Eng. 2018, 4, 3854–3863.
- Remya, K.R.; Chandran, S.; Mani, S.; John, A.; Ramesh, P. Hybrid Polycaprolactone/Polyethylene Oxide Scaffolds with Tunable Fiber Surface Morphology, Improved Hydrophilicity and Biodegradability for Bone Tissue Engineering Applications. J. Biomater. Sci. Polym. Ed. 2018, 29, 1444–1462.
