You're using an outdated browser. Please upgrade to a modern browser for the best experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Daniel Rodriguez | -- | 2092 | 2022-12-19 23:20:15 | | | |
| 2 | Amina Yu | + 12 word(s) | 2104 | 2022-12-20 04:34:14 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Rodriguez, D.; Elagizi, A.; Milani, R.V.; Lavie, C.J. Omega-3 Polyunsaturated Fatty Acids on Cardiovascular Health. Encyclopedia. Available online: https://encyclopedia.pub/entry/38978 (accessed on 25 December 2025).
Rodriguez D, Elagizi A, Milani RV, Lavie CJ. Omega-3 Polyunsaturated Fatty Acids on Cardiovascular Health. Encyclopedia. Available at: https://encyclopedia.pub/entry/38978. Accessed December 25, 2025.
Rodriguez, Daniel, Andrew Elagizi, Richard V. Milani, Carl J. Lavie. "Omega-3 Polyunsaturated Fatty Acids on Cardiovascular Health" Encyclopedia, https://encyclopedia.pub/entry/38978 (accessed December 25, 2025).
Rodriguez, D., Elagizi, A., Milani, R.V., & Lavie, C.J. (2022, December 20). Omega-3 Polyunsaturated Fatty Acids on Cardiovascular Health. In Encyclopedia. https://encyclopedia.pub/entry/38978
Rodriguez, Daniel, et al. "Omega-3 Polyunsaturated Fatty Acids on Cardiovascular Health." Encyclopedia. Web. 20 December, 2022.
Copy Citation
High consumption of polyunsaturated fatty acids (PU FAs), specifically omega-3 FAs (Ω3FAs) such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), results in low plasma cholesterol levels and minimal coronary heart disease (CHD). Furthermore, as elevated triglycerides (TGs) appear to be a causal factor for atherosclerotic cardiovascular diseases (CVD)(ASCVD) and possibly for premature all-cause mortality, more so when they are associated with genetic variants, PUFAs can reduce TG levels by decreasing lipoproteins with high amounts of TGs, such as very-low-density lipoproteins, intermediate-density lipoproteins, chylomicrons, and their remnants.
omega-3 FA
fatty acids
cardiovascular disease
1. Dietary Sources and Guidelines
Polyunsaturated fatty acids (PU FAs), are generally classified into two broad groups: n-3 and n-6. PUFAs with double bonds starting at position six from the methyl end are considered Ω6 series, while those starting at position three are regarded as Ω3 series. Inflammatory mediators are produced by n-6 PUFAs, and n-3 PUFAs form neutral or anti-inflammatory signaling molecules. Arachidonic acid lies within the phospholipids that are present in cell membranes, and it is this Ω6 PUFA that plays an essential role in producing eicosanoids in the body. Although PUFAs are considered a family, their potency varies based on whether their origin is plant- or marine-based. The three major and more studied Ω3 PUFAs are alpha-linolenic acid (ALA), eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA).
ALA (ALA, 18:3n-3) is an 18-carbon atom FA with three double bonds, found primarily in plants, including flaxseed, soybean, canola oil, chia seeds, walnuts, and flax [1][2][3]. It is considered an essential FA as humans cannot synthesize it, and it is an important source of very long-chain PUFAs such as EPA and DHA (Figure 1). Apart from being able to be synthesized from plants, EPA and DHA are found in fish, more so in FOs coming from herring, mackerel, trout, salmon, and sardines. To assess the amount of EPA and DHA in the body, red blood cell (RBC) FA composition is used to reflect the cellular membranes throughout the body. A convenient method for relating long-chain Ω3 PUFA levels with coronary heart disease (CHD) risk was proposed by linking the sum of Ω3 PUFAs (EPA and DHA) in RBCs to the Ω3 index (O3I) [4][5][6], finding an inverse correlation of the O3I with CHD. Accordingly, the American Heart Association (AHA) recommends eating 3 ounces of cooked fish (particularly fatty fish) two times per week [7]. Despite recommendations and the known benefits of Ω3FA, in the periods 2005–2006 and 2015–2016, the National Health and Nutrition Examination Survey reported a 6.8% decrease in the number of Americans older than twenty years old who consumed seafood more than once a week [8] (Figure 2).
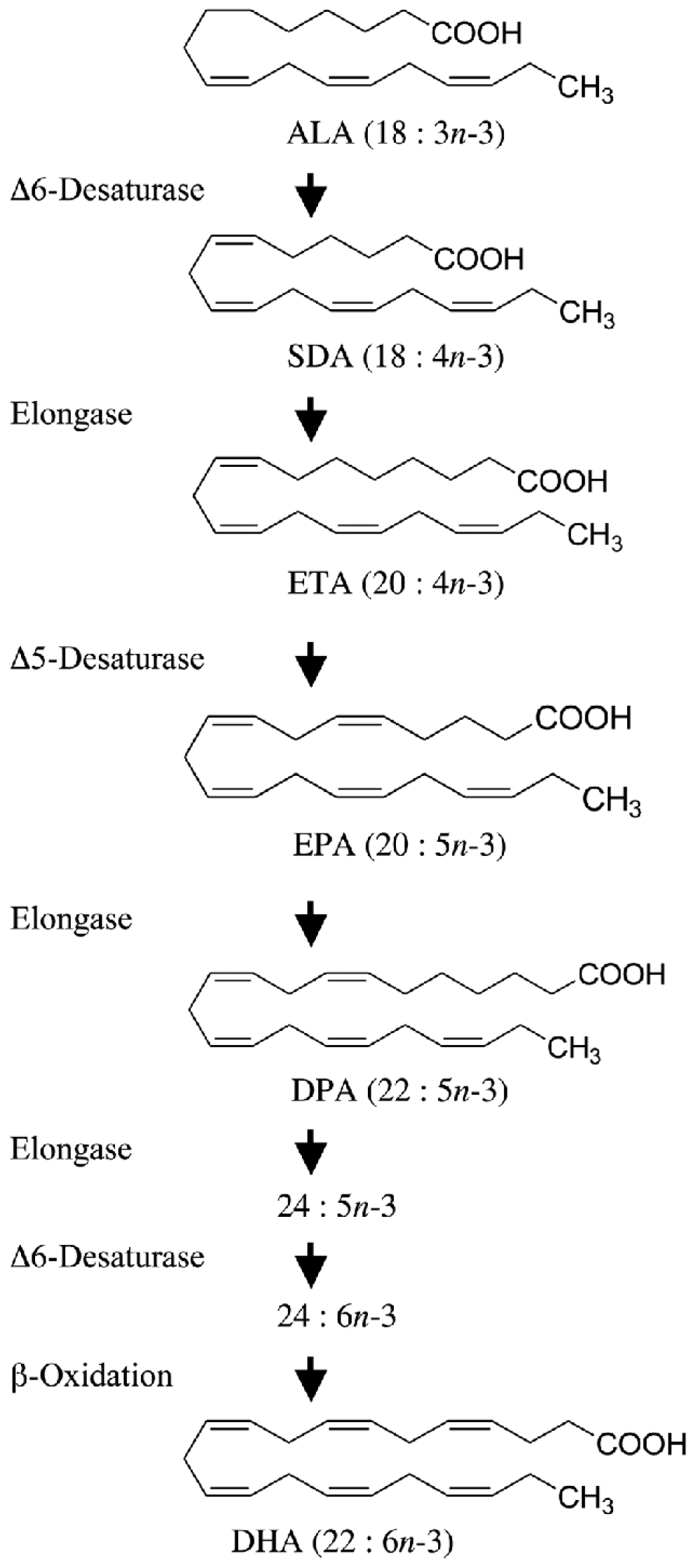
Figure 1. Alpha-linolenic acid (ALA) as a substrate of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA).
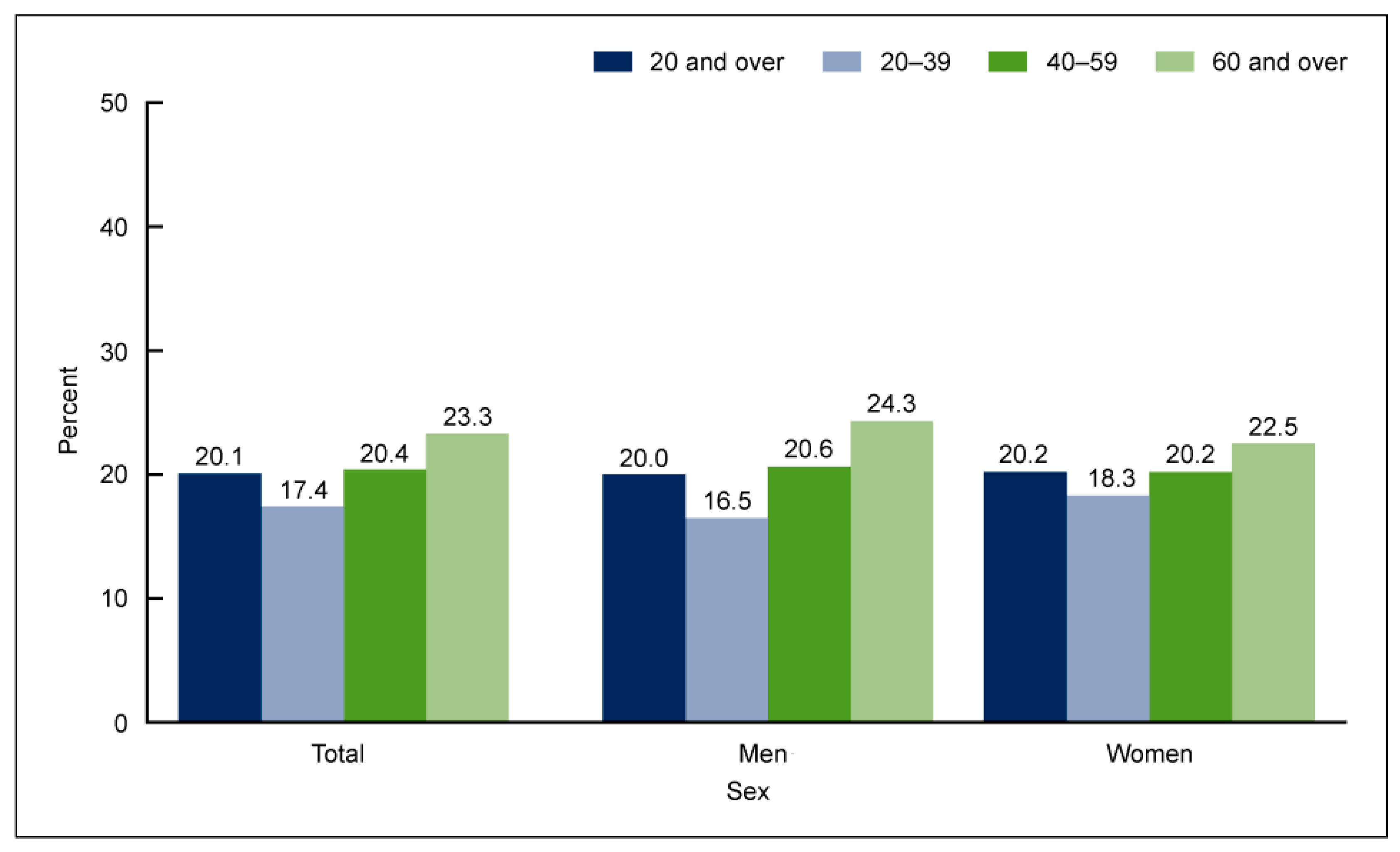
Figure 2. Percentage of adults aged 20 and over consuming seafood at least two times per week, by age and sex, in the United States in 2013–2016 [8].
The characteristics of patients with lower seafood consumption were examined by Love et al. [9], finding that individuals with lower incomes consumed less seafood (120.2 g/week) than individuals from high-income groups (141.8 g/week), with even lower consumption of seafood containing long-chain n-3 PUFAs (lower income: 21.3 g/week. vs. higher income 46.8 g/week) [9]. These results were later corroborated after adjusting for age and sex as potential confounders, again finding that people with lower income consumed 18% less seafood than the people with higher income (p = 0.03), with an added lower intake of nuts, seeds (p < 0.001), soy (p < 0.001), and all protein foods (p < 0.001). There were several reasons attributed to this difference; one of them was the price difference between fresh seafood with high n–3 PUFAs and those with low n–3 PUFAs. Fresh seafood with high n–3 PUFAs was 32% more expensive than fresh seafood with low n–3 PUFAs (p < 0.002). These findings could explain why prior authors have found an inverted independent link between socioeconomic status and the risk of ASCVD [10][11][12][13][14].
Compliance with diet should be a priority, as healthcare costs associated with increasing 20% adherence to diet are estimated to result in annual cost savings of approximately USD 31.5 billion. Cost savings related to CVD account for half of these savings [15].
2. Molecular Mechanisms
Cell membrane phospholipids contain FAs, which play a significant role in various functions, metabolic reactions, and signaling processes. Different levels of PUFAs in cell membranes are required to exert actions and to maintain proper functioning and tissue responsiveness to signaling. These levels depend on a sufficient intake of PUFAs from the diet.
Cellular cholesterol requirements are satisfied by either exogenous cholesterol inflow paths involving multiple lipoprotein receptors or endogenous cholesterol synthesis, which is regulated by the 3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMGR), a rate-limiting enzyme. The catabolism of cholesterol is exerted by the upregulation of the liver’s low-density lipoprotein (LDL) receptor (LDLR) gene and protein expression. A combination of the LDL and LDLR binds to the ligand and internalizes in lysosomes. The ligand uncouples from the receptor due to the lower pH of the lysosomes. In this process, the lipids in the receptor are degraded, and the receptor is then returned to the cell membrane for further lipid binding.
Through a negative feedback mechanism tightly controlled by two proteins, the LDLR gene transcription is regulated by cholesterol availability, the sterol regulatory element-binding protein (SREBP), which requires cleavage to become active, and the SREBP cleavage-activating protein (SCAP), which cleaves it for activation. The SCAP proteins transport SREBPs to the Golgi apparatus when cholesterol levels in cells are low. Once in the Golgi apparatus, they are cleaved in order to activate enzymes involved in the synthesis of lipids. It has been shown that the sterol sensor protein SCAP undergoes conformational changes in response to high cholesterol levels in the cell, allowing it to adhere to the endoplasmic reticulum protein Insig-1, forming a ternary complex known as SREBP/SCAP/Insig-1, trapping it in the endoplasmic reticulum, stopping cholesterol synthesis and uptake (via the LDLR) and maintaining cell cholesterol homeostasis. As demonstrated through posttranslational downregulation of the LDLR, PCSK-9 plays a significant role in cholesterol metabolism by binding specifically to the LDLR on the cell membrane in order to form a PCSK9/LDLR complex, which then prevents the recycling of the LDLR by redirecting it to the lysosome, where it will be degraded. The disruption of this pathway can result in the accumulation of cholesterol and the formation of foam cells in organs.
ALA promotes cholesterol conversion into bile acids by the cholesterol 7α-hydroxylase (CYP7). As a result of removing hepatic cholesterol from the circulation by the synthesis of bile acids, ALA promotes SREBP activation (via SCAP activity), upregulating LDLR expression and favoring the clearance of LDL cholesterol from the body. Ω3FAs such as EPA and DHA reduce triglycerides (TGs) levels by direct inhibiting liver diacylglycerol acetyl-transferase, which catalyzes the formation of TG from diacylglycerol, essential for TG intestinal absorption and fatty acyl-CoA, and the inhibition of the phosphatidic acid phosphohydrolase required for triacylglycerol (TAG) synthesis from glycerol 3-phosphate [16]. Other molecular effects are exerted by inhibiting the acyl-CoA:1,2-diacylglycerol acyltransferase, increasing mitochondrial and peroxisomal-beta-oxidation in the liver, decreasing lipogenesis, and increasing plasma lipoprotein lipase activity. EPA’s ability to stabilize cell membranes, along with its ability to lower cholesterol, may contribute to the 30% and 40% reductions seen in deaths from CVD and sudden cardiac death (SCD) and the 56% reduction in cardiac arrest [17].
Ω3FAs lower TG-rich lipoproteins and increase anti-aggregatory and vasodilatory prostanoids such as prostacyclin, combating thrombosis and vasospasm. It can incorporate into the mitochondria and plasma membranes, stabilizing them and preventing them from oxidation, which is believed to have a role in preventing arrhythmias. Additionally, Ω3FAs’ are precursors to the synthesis of specialized mediators capable of combating inflammation and have been demonstrated to decrease proinflammatory cytokines such as interleukine-6 and tumor necrosis factor-α and inhibit the activation of the ikappaB kinase and nuclear factor-κB, as well as several other transcription factors that inhibit reactive oxygen species [18][19]. These anti-inflammatory properties are believed to interfere less with self-defense than direct anti-inflammatory treatments. A combination of these mechanisms is believed to contribute to the CVD protection associated with Ω3FA consumption and the added benefit on multiple other systems and pathologies [20].
3. Controversies Surrounding Ω3
It has been debated since the Inuits study whether Ω3FA consumption is solely responsible for the CV benefits observed in this population, whether fish intake is beneficial on its own, or whether an overall healthier diet resulting from a higher fish intake is beneficial in lowering CVD risk. The Diet and Reinfarction Trial (DART) [21] was the first RCT to show a reduction in mortality during the two years after myocardial infarction (MI) among men who were advised to eat about 300 g of FO per week or who took an equivalent amount of n-3 fatty acids in the form of FO supplements. Later, these findings were confirmed by the GISSI– Prevenzione trial [22], the Lyon Diet Heart Study [23], and various cohort studies.
The OMEGA trial tested the effects of adding Ω3-acid ethyl esters-90 (1 g/d for one year) to current guidelines. The primary endpoint was SCD in survivors of acute MI. Secondary endpoints were non-fatal clinical CVD events and total mortality. The patients were followed up for 365 days; herein, investigators found no difference between omega and control groups in the rates of SCD (1.5% and 1.5%; p = 0.84), total mortality (4.6% and 3.7%; p = 0.18), major adverse cerebrovascular and CVD events (10.4% and 8.8%; p = 0.1), and revascularization in survivors (27.6% and 29.1%; p = 0.34); however, a significant limitation of this trial was a lack of statistical power and a reduced rate of SCD, total mortality, and major adverse CVD events (MACE) after one year of follow-up. In 2019, the strength of Ω3FAs was again demonstrated in the RCT REDUCE-IT trial, demonstrating the added benefit of consuming 4 g/day of icosapent ethyl (IPE), divided into twice-daily doses (2 g two times per day), to reduce ischemic events and CVD death [24].
While individual trial results are inconsistent, the results of pooled RCTs suggest a cardioprotective effect of Ω3FAs. The possible explanation for this heterogeneity includes differences in dosages between groups, the differences between study follow-ups, sample sizes, and lower event rates [25]. Further controversies regarding the effect of DHA on preventing CVD were drawn after some studies found rising LDL-C among patients given DHA. This controversy was later clarified in clinical trials, where using adequate doses of 4 g/day failed to demonstrate any increase in LDL cholesterol [26][27][28][29][30].
Ω3FAs have also been discussed for their arrhythmogenic effects. Initial studies in animal labs demonstrated that supplementation with DHA, but not EPA, reduced arrhythmogenic structural changes to the atria resulting from simultaneous atrial and ventricular pacing [31]. However, four studies providing a combination of EPA–DHA to assess the risk of AF suggested, but did not prove, that the risk of AF with Ω3FA intake may be dose-related. Doses of 1.8 g/d had an increase in risk (hazard ratio (HR), 1.84) of atrial fibrillation (however, not achieving statistical significance), and doses of 4.0 g/d almost doubled the risk of this arrhythmia [32][33][34][35]. These findings were later contradicted by The MESA study (Multiethnic Study of Atherosclerosis), which examined the relationship between Ω3FAs (expressed as a percentage mass of total fatty acids) and the risk of major bleeding events and AF [36]. Here, it included a population free of CVD, finding that higher DHA levels were associated with fewer incidents of AF (HR, 0.80; CI, 0.65–0.98; p = 0.03) and that higher EPA and DHA were associated with significantly fewer hospitalizations for bleeding events (EPA (HR, 0.75 CI, 0.60–0.94, p = 0.01; EPA + DHA (HR, 0.84; CI, 0.73–0.98; p = 0.03)) [32][37].
4. Index
A diet low in Ω3FAs is associated with increased mortality and CHD risk [24][32][38][39], although, as concentrations of Ω3 vary among varieties of food and among types of fish and vegetables, measuring the amount of Ω3 consumed by a person is not a reliable indicator of the level of Ω3 in the organism. In addition, various factors can affect the blood levels of Ω3FAs after eating an Ω3-rich meal, such as the variability of the uptake of ingested EPA and DHA and the difference in bioavailability between individuals (EPA and DHA in pregnant or obese women compared to EPA and DHA in lean women). Once absorbed, Ω3FAs become part of the cell membranes and have the ability to affect several of their properties, including modulating the activity of membrane-bound enzymes and cell signaling pathways [40][41]. Therefore, to better assess the amount of Ω3FAs in the human body, researchers have proposed using the O3I. This term was first introduced in 2004 by William S Harris and Clemens Von Schacky [42], who described the O3I as the percentage of the total red blood cell membrane formed by EPA and DHA. Its levels were demonstrated to have a direct correlation with the risk of death from CHD, where an O3I value of 8% decreased the risk of CHD mortality and a percentage less than 4% increased this risk [42].
References
- U.S. Department of Agriculture, Agricultural Research Service. Food Data Central. 2019. Available online: Fdc.nal.usda.gov (accessed on 17 November 2022).
- Lavie, C.J.; Milani, R.V.; Mehra, M.R.; Ventura, H.O. Omega-3 polyunsaturated fatty acids and cardiovascular diseases. J. Am. Coll. Cardiol. 2009, 54, 585–594.
- O’Keefe, E.L.; Harris, W.S.; DiNicolantonio, J.J.; Elagizi, A.; Milani, R.V.; Lavie, C.J.; O’Keefe, J.H. Sea Change for Marine Omega-3s: Randomized Trials Show Fish Oil Reduces Cardiovascular Events. Mayo Clin. Proc. 2019, 94, 2524–2533.
- Fielding, B.A. Omega-3 index as a prognosis tool in cardiovascular disease. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 360–365.
- Thies, F.; Garry, J.M.C.; Yaqoob, P.; Rerkasem, K.; Williams, J.; Shearman, C.P.; Gallagher, P.J.; Calder, P.C.; Grimble, R.F. Association of n-3 polyunsaturated fatty acids with stability of atherosclerotic plaques: A randomised controlled trial. Lancet 2003, 361, 477–485.
- Erkkila, A.T.; Lehto, S.; Pyorala, K.; Uusitupa, M.I. n-3 Fatty acids and 5-y risks of death and cardiovascular disease events in patients with coronary artery disease. Am. J. Clin. Nutr. 2003, 78, 65–71.
- Rimm, E.B.; Appel, L.J.; Chiuve, S.E.; Djousse, L.; Engler, M.B.; Kris-Etherton, P.M.; Mozaffarian, D.; Siscovick, D.S.; Lichtenstein, A.H.; American Heart Association Nutrition Committee of the Council on Lifestyle and Cardiometabolic Health; et al. Seafood Long-Chain n-3 Polyunsaturated Fatty Acids and Cardiovascular Disease: A Science Advisory from the American Heart Association. Circulation 2018, 138, e35–e47.
- Terry, A.L.; Herrick, K.A.; Afful, J.; Ahluwalia, N. Seafood Consumption in the United States, 2013–2016. NCHS Data Brief 2018, 1–8.
- Love, D.C.; Thorne-Lyman, A.L.; Conrad, Z.; Gephart, J.A.; Asche, F.; Godo-Solo, D.; McDowell, A.; Nussbaumer, E.M.; Bloem, M.W. Affordability influences nutritional quality of seafood consumption among income and race/ethnicity groups in the United States. Am. J. Clin. Nutr. 2022, 116, 415–425.
- Marmot, M.G.; Stansfeld, S.; Patel, C.; North, F.; Head, J.; White, I.; Brunner, E.; Feeney, A. Health inequalities among British civil servants: The Whitehall II study. Lancet 1991, 337, 1387–1393.
- Mackenbach, J.P.; Cavelaars, A.E.; Kunst, A.E.; Groenhof, F. Socioeconomic inequalities in cardiovascular disease mortality; an international study. Eur. Heart J. 2000, 21, 1141–1151.
- Manrique-Garcia, E.; Sidorchuk, A.; Hallqvist, J.; Moradi, T. Socioeconomic position and incidence of acute myocardial infarction: A meta-analysis. J. Epidemiol. Community Health 2011, 65, 301–309.
- Khaing, W.; Vallibhakara, S.A.; Attia, J.; McEvoy, M.; Thakkinstian, A. Effects of education and income on cardiovascular outcomes: A systematic review and meta-analysis. Eur. J. Prev. Cardiol. 2017, 24, 1032–1042.
- Addo, J.; Ayerbe, L.; Mohan, K.M.; Crichton, S.; Sheldenkar, A.; Chen, R.; Wolfe, C.D.; McKevitt, C. Socioeconomic status and stroke: An updated review. Stroke 2012, 43, 1186–1191.
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Alonso, A.; Beaton, A.Z.; Bittencourt, M.S.; Boehme, A.K.; Buxton, A.E.; Carson, A.P.; Commodore-Mensah, Y.; et al. Heart Disease and Stroke Statistics—2022 Update: A Report from the American Heart Association. Circulation 2022, 145, e153–e639.
- Harris, W.S.; Bulchandani, D. Why do omega-3 fatty acids lower serum triglycerides? Curr. Opin. Lipidol. 2006, 17, 387–393.
- Gaba, P.; Bhatt, D.L.; Steg, P.G.; Miller, M.; Brinton, E.A.; Jacobson, T.A.; Ketchum, S.B.; Juliano, R.A.; Jiao, L.; Doyle, R.T., Jr.; et al. Prevention of Cardiovascular Events and Mortality with Icosapent Ethyl in Patients with Prior Myocardial Infarction. J. Am. Coll. Cardiol. 2022, 79, 1660–1671.
- De Caterina, R.; Massaro, M. Omega-3 fatty acids and the regulation of expression of endothelial pro-atherogenic and pro-inflammatory genes. J. Membr. Biol. 2005, 206, 103–116.
- Welty, F.K.; Alfaddagh, A.; Elajami, T.K. Targeting inflammation in metabolic syndrome. Transl. Res. 2016, 167, 257–280.
- Mason, R.P.; Libby, P.; Bhatt, D.L. Emerging Mechanisms of Cardiovascular Protection for the Omega-3 Fatty Acid Eicosapentaenoic Acid. Arter. Thromb. Vasc. Biol. 2020, 40, 1135–1147.
- Burr, M.L.; Gilbert, J.F.; Holliday, R.M.; Elwood, P.C.; Fehily, A.M.; Rogers, S.; Sweetnam, P.M.; Deadman, N.M. Effects of Changes in Fat, Fish, and Fibre Intakes on Death and Myocardial Reinfarction: Diet and Reinfarction Trial (Dart). Lancet 1989, 334, 757–761.
- Jialal, I.; Devaraj, S.; Huet, B.A.; Traber, M. GISSI-Prevenzione trial. Lancet 1999, 354, 1554.
- Kris-Etherton, P.; Eckel, R.H.; Howard, B.V.; St Jeor, S.; Bazzarre, T.L.; Nutrition Committee Population Science Committee; Clinical Science Committee of the American Heart Association. AHA Science Advisory: Lyon Diet Heart Study. Benefits of a Mediterranean-style, National Cholesterol Education Program/American Heart Association Step I Dietary Pattern on Cardiovascular Disease. Circulation 2001, 103, 1823–1825.
- Bhatt, D.L.; Miller, M.; Brinton, E.A.; Jacobson, T.A.; Steg, P.G.; Ketchum, S.B.; Doyle, R.T., Jr.; Juliano, R.A.; Jiao, L.; Granowitz, C.; et al. REDUCE-IT USA: Results From the 3146 Patients Randomized in the United States. Circulation 2020, 141, 367–375.
- Quispe, R.; Alfaddagh, A.; Kazzi, B.; Zghyer, F.; Marvel, F.A.; Blumenthal, R.S.; Sharma, G.; Martin, S.S. Controversies in the Use of Omega-3 Fatty Acids to Prevent Atherosclerosis. Curr. Atheroscler. Rep. 2022, 24, 571–581.
- Jacobson, T.A.; Glickstein, S.B.; Rowe, J.D.; Soni, P.N. Effects of eicosapentaenoic acid and docosahexaenoic acid on low-density lipoprotein cholesterol and other lipids: A review. J. Clin. Lipidol. 2012, 6, 5–18.
- Weintraub, H.S. Overview of prescription omega-3 fatty acid products for hypertriglyceridemia. Postgrad. Med. 2014, 126, 7–18.
- Singh, S.; Arora, R.R.; Singh, M.; Khosla, S. Eicosapentaenoic Acid Versus Docosahexaenoic Acid as Options for Vascular Risk Prevention: A Fish Story. Am. J. Ther. 2016, 23, e905–e910.
- Wei, M.Y.; Jacobson, T.A. Effects of eicosapentaenoic acid versus docosahexaenoic acid on serum lipids: A systematic review and meta-analysis. Curr. Atheroscler. Rep. 2011, 13, 474–483.
- Fialkow, J. Omega-3 Fatty Acid Formulations in Cardiovascular Disease: Dietary Supplements are Not Substitutes for Prescription Products. Am. J. Cardiovasc. Drugs 2016, 16, 229–239.
- Ramadeen, A.; Connelly, K.A.; Leong-Poi, H.; Hu, X.; Fujii, H.; Laurent, G.; Domenichiello, A.F.; Bazinet, R.P.; Dorian, P. Docosahexaenoic acid, but not eicosapentaenoic acid, supplementation reduces vulnerability to atrial fibrillation. Circ. Arrhythmia Electrophysiol. 2012, 5, 978–983.
- Bhatt, D.L.; Steg, P.G.; Miller, M.; Brinton, E.A.; Jacobson, T.A.; Ketchum, S.B.; Doyle, R.T., Jr.; Juliano, R.A.; Jiao, L.; Granowitz, C.; et al. Cardiovascular Risk Reduction with Icosapent Ethyl for Hypertriglyceridemia. N. Engl. J. Med. 2019, 380, 11–22.
- Albert, C.M.; Cook, N.R.; Pester, J.; Moorthy, M.V.; Ridge, C.; Danik, J.S.; Gencer, B.; Siddiqi, H.K.; Ng, C.; Gibson, H.; et al. Effect of Marine Omega-3 Fatty Acid and Vitamin D Supplementation on Incident Atrial Fibrillation: A Randomized Clinical Trial. JAMA 2021, 325, 1061–1073.
- Nicholls, S.J.; Lincoff, A.M.; Garcia, M.; Bash, D.; Ballantyne, C.M.; Barter, P.J.; Davidson, M.H.; Kastelein, J.J.P.; Koenig, W.; McGuire, D.K.; et al. Effect of High-Dose Omega-3 Fatty Acids vs Corn Oil on Major Adverse Cardiovascular Events in Patients at High Cardiovascular Risk: The STRENGTH Randomized Clinical Trial. JAMA 2020, 324, 2268–2280.
- Kalstad, A.A.; Myhre, P.L.; Laake, K.; Tveit, S.H.; Schmidt, E.B.; Smith, P.; Nilsen, D.W.T.; Tveit, A.; Fagerland, M.W.; Solheim, S.; et al. Effects of n-3 Fatty Acid Supplements in Elderly Patients after Myocardial Infarction: A Randomized, Controlled Trial. Circulation 2021, 143, 528–539.
- Kapoor, K.; Alfaddagh, A.; Al Rifai, M.; Bhatt, D.L.; Budoff, M.J.; Nasir, K.; Miller, M.; Welty, F.K.; McEvoy, J.W.; Dardari, Z.; et al. Association Between Omega-3 Fatty Acid Levels and Risk for Incident Major Bleeding Events and Atrial Fibrillation: MESA. J. Am. Heart Assoc. 2021, 10, e021431.
- Yokoyama, M.; Origasa, H.; Matsuzaki, M.; Matsuzawa, Y.; Saito, Y.; Ishikawa, Y.; Oikawa, S.; Sasaki, J.; Hishida, H.; Itakura, H.; et al. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): A randomised open-label, blinded endpoint analysis. Lancet 2007, 369, 1090–1098.
- Hu, Y.; Hu, F.B.; Manson, J.E. Marine Omega-3 Supplementation and Cardiovascular Disease: An Updated Meta-Analysis of 13 Randomized Controlled Trials Involving 127,477 Participants. J. Am. Heart Assoc. 2019, 8, e013543.
- Bernasconi, A.A.; Lavie, C.J.; Milani, R.V.; Laukkanen, J.A. Omega-3 Benefits Remain Strong Post-STRENGTH. Mayo Clin. Proc. 2021, 96, 1371–1372.
- Stillwell, W.; Wassall, S.R. Docosahexaenoic acid: Membrane properties of a unique fatty acid. Chem. Phys. Lipids 2003, 126, 1–27.
- Calder, P.C. n-3 fatty acids, inflammation and immunity: New mechanisms to explain old actions. Proc. Nutr. Soc. 2013, 72, 326–336.
- Harris, W.S.; Von Schacky, C. The Omega-3 Index: A new risk factor for death from coronary heart disease? Prev. Med. 2004, 39, 212–220.
More
Information
Subjects:
Cardiac & Cardiovascular Systems
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
975
Entry Collection:
Hypertension and Cardiovascular Diseases
Revisions:
2 times
(View History)
Update Date:
20 Dec 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




