Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Margarita L. Martinez-Fierro | -- | 2833 | 2022-12-19 16:30:08 | | | |
| 2 | Beatrix Zheng | Meta information modification | 2833 | 2022-12-20 06:43:52 | | | | |
| 3 | Samantha González | + 1 word(s) | 2834 | 2022-12-20 18:18:33 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Martinez-Fierro, M.L.; Delgado, S.G.; Garza-Veloz, I.; Trejo-Vazquez, F. Epidemiology and Pathogenesis of Inflammatory Bowel Disease. Encyclopedia. Available online: https://encyclopedia.pub/entry/38969 (accessed on 08 February 2026).
Martinez-Fierro ML, Delgado SG, Garza-Veloz I, Trejo-Vazquez F. Epidemiology and Pathogenesis of Inflammatory Bowel Disease. Encyclopedia. Available at: https://encyclopedia.pub/entry/38969. Accessed February 08, 2026.
Martinez-Fierro, Margarita L, Samantha González Delgado, Idalia Garza-Veloz, Fabiola Trejo-Vazquez. "Epidemiology and Pathogenesis of Inflammatory Bowel Disease" Encyclopedia, https://encyclopedia.pub/entry/38969 (accessed February 08, 2026).
Martinez-Fierro, M.L., Delgado, S.G., Garza-Veloz, I., & Trejo-Vazquez, F. (2022, December 19). Epidemiology and Pathogenesis of Inflammatory Bowel Disease. In Encyclopedia. https://encyclopedia.pub/entry/38969
Martinez-Fierro, Margarita L, et al. "Epidemiology and Pathogenesis of Inflammatory Bowel Disease." Encyclopedia. Web. 19 December, 2022.
Copy Citation
Ulcerative colitis (UC) and Crohn's disease (CD) have been recognized as occidentalized diseases, due to their higher rates of incidence and prevalence in occidental countries. CD mostly presents between 20 and 30 years, whereas UC mostly presents between 30 and 40 years, as well as from 60 to 70 years.
Crohn’s disease
ulcerative colitis
serotonin
bowel disease,
intestinal dysbiosis
1. Risk Factors and Clinical Manifestations
Ulcerative colitis (UC) and Crohn's disease (CD) have been considered as multi-factorial pathologies, including factors such as antibiotic use, viral and bacterial infections, processed and sugary foods, and alteration in the intestinal microbiota (intestinal dysbiosis) which have been related to an increased risk of inflammatory bowel disease (IBD) [1][2][3][4]. Notably, smoking has been described as having a controversial effect on IBD, as it acts as a risk factor in CD due to the production of free radicals, thus perpetuating inflammation; meanwhile, in UC, smoking has been identified as playing a protective role [1][5]. IBD is associated with factors such as abdominal delivery (CD, OR = 1.38, 95% CI: 1.12–1.70; UC, OR = 1.08, 95% CI: 0.87–1.33), antibiotics exposure (CD, OR = 1.74, 95% CI: 1.35–2.23; UC, OR = 1.08, 95% CI: 0.91–1.27), and sucrose ingestion (CD, RR = 1.09, 95% CI: 1.02–1.16; UC, RR = 1.10, 95% CI: 1.02–1.18), among others [1]. The dietary composition has been also considered as a risk factor, as it has the capacity to disrupt the normal gut microbiota, especially when foods such as sodas, chocolate, and artificial sweeteners are included [6]. Intestinal permeability has been found to be increased in mice fed a high-sugar diet [7]. Besides the influence of diet, increased serum LPS levels and decreased microbiota diversity can lead to reduced production of SCFAs [8].
CD is characterized by transmural damage. Patients may present with perianal pain, bleeding, incontinence, fistulization, abscesses, and hemorrhoidal illness [9]. CD can also be characterized by the presence of extraintestinal manifestations, the most common being enthesitis and axial or peripheral arthritis [10][11]. On the other hand, UC can produce chronic inflammation of the colonic mucosa, leading to manifestations such as proctitis, bloody stools, abdominal pain, fatigue, fecal incontinence, arthralgia, and erythema nodosum [12]. In both UC and CD, there is also involvement of the central nervous system (CNS), specifically leading to psychological or psychiatric manifestations [13]. It has been observed that between 15% and 25% of patients with IBD developed depression, while 30% presented with anxiety [14]. Furthermore, it may be accompanied by sleep difficulties and fatigue [15]. As part of the neurological involvement in patients with IBD, deficits in attention and executive function in adults have been observed [16].
Genetic Susceptibility and Inflammatory Bowel Disease
IBD, similarly to other inflammatory diseases such as autoimmune diseases, has been related to different genes; although IBD has not been recognized as a hereditary disease, several articles provide information about increased susceptibility, disease activity and treatment response related with these genes and their genetic variants (Table 1). As an example, in a trans-ancestry association study, in European, East Asian, Indian and Iranian populations, several risk loci for IBD were found. These loci included NOD2, ATG16L1, and IL-23R [17]. NOD2 gene variants such as p.Arg702Trp were able to provide an increased risk of IBD [18][19]. The rs2241880 gene variant of ATG16L1 has also been closely related with the maintenance of human intestinal cell homeostasis and autophagy processes in patients with IBD [20][21]. In an interesting review by Nour Younis et al., a wide range of genes related to IBD, together with their reported genetic variants, were considered [22]. Based on wide-genome studies, gene variants in genes such as ATG16L1 (rs2241880: OR = 0.74; 95% CI: 0.65–0.84; p < 0.001), PTPN2 (Protein tyrosine phosphatase non-receptor type 2) and of IL-23R (rs11209026 allele A; OR = 0.32; 95% CI: 0.17–0.60; p < 0.001) were related to increased susceptibility to IBD, and even to disease course and treatment outcomes [22].
Table 1. Genes related to Inflammatory Bowel Disease.
| Gene | Locus | Effect | Reference |
|---|---|---|---|
| NOD2 | 16q12.1 | IBD increased risk | [17][18][22][23] |
| ATG16L1 | 2q37.1 | Impaired intracellular bacteria clearance in IBD, intestinal autophagy | [20][22][24][25] |
| PTPN2 | 18p11.21 | IBD increased risk | [22][26][27] |
| IL-23R | 1p31.3 | IBD susceptibility, Crohn’s disease risk | [22][28][29] |
| IL-10 | 1q32.1 | IBD steroid dependency, early onset IBD | [22][30][31] |
| HNF4α | 20q13.12 | IBD susceptibility | [22][32][33] |
NOD2: Nucleotide-binding oligomerization domain-containing protein 2; ATG16L1: Autophagy-related 16-like 1 protein; PTPN2: Protein tyrosine phosphatase non-receptor type 2; IL-23R: Interleukin 23 receptor; IL-10: Interleukin 10; HNF4α: Hepatocyte Nuclear Factor 4 alpha.
2. Intestinal Barrier Disruption and Over-Activated Immune Response in Inflammatory Bowel Disease
The intestinal epithelial barrier, together with the intestinal microbiota, are considered an elemental functional unit in the physiology and pathophysiology of the GI. As part of the events that contribute to the pathogenesis of IBD, an alteration in the structure of the intestinal barrier can lead to an altered immune response and intestinal dysbiosis. This phenomenon has been clearly established in pathogen-free mice, where an alteration in the intestinal epithelial cells was observed, together with several abnormalities in the microvilli and a decrease in cellular renovation of the gut (Figure 1) [34]. TJs, such as zonula occludens 1 (ZO-1) and zonula occludens 2 (ZO-2), can be influenced and affected by the intestinal microbiota. TJs can be regulated by bacteria, such as Lactobacillus rhamnosus, Acidophilus plantarum, and Bifidobacterium infantis, through the activation of TLRs, causing increases in the expression of claudin 3, ZO-1, and claudin 4 [35]. In patients with a previous established diagnosis of IBD, an increased paracellular permeability has been observed in almost 40% of patients with CD [36].
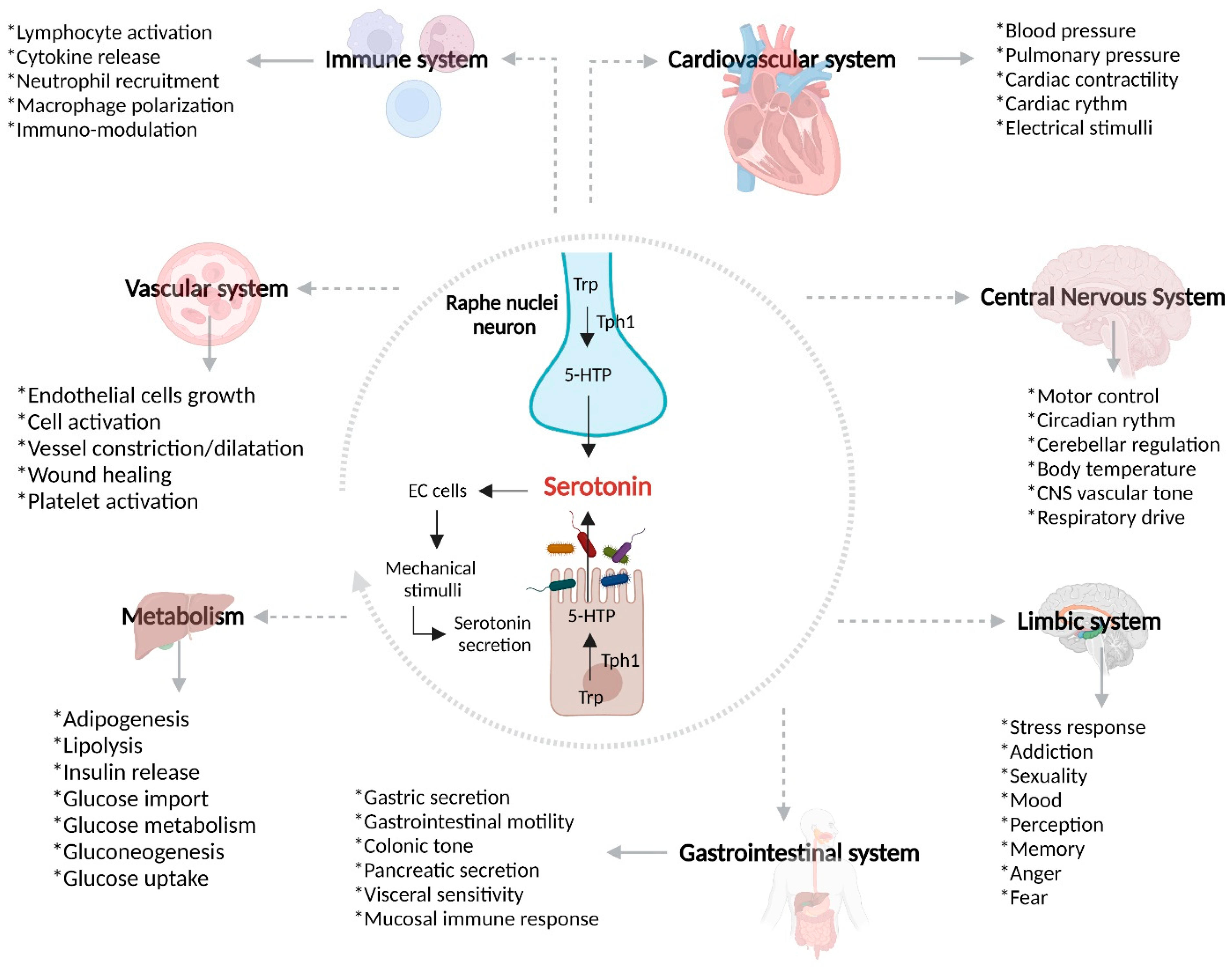
Figure 1. Intestinal dysbiosis and immune response in IBD. The over-growth of harmful bacteria and gut barrier defects cause an influx of bacterial components, leading to activation of the immune response (both innate and adaptive). In UC, Th2 differentiation prevails, while in CD, a Th1 and Th17 adaptive immune response prevails. The influx of bacterial components, adaptive immune response, and harmful bacterial over-growth lead to increased gut permeability, resulting in increased gut 5-HT causing an inflammatory process. DCs, dendritic cells; IFN-γ, interferon-γ; IL-17, interleukin 17; IL-13, interleukin 13; TNF-α, tumor-necrosis factor α.
Maintenance of the intestinal epithelial integrity depends on the mucus layer; nevertheless, Akkermansia muciniphila, Ruminococcus spp., Enterococcus, Bifidobacterium spp., and Bacteroides can degrade mucin and favor the colonization of harmful bacteria [37]. In patients with IBD, increases in Ruminococcus gnavus, Rumminococcus torques, Bacteroides fragilis, and Bacteroides vulgatus, containing mucolytic enzymes such as α-galactosidase, sulphatase, neuraminidase, and β-galactosidase, have been observed [38]. An alteration in the relation between Bacteroidetes, Firmicutes, and Actinobacteria, as well as a decrease in Proteobacteria and an increase in new bacterial groups, promotes an alteration to homeostasis in a process called intestinal dysbiosis. The equilibrium between host micro-organisms (also called symbiosis) can be affected by nutritive factors, fats, carbohydrates, and drug or antibiotic abuse, among others [2]. Bacteria such as E. Coli, Klebsiella spp., Proteus, Enterobacter, Shigella spp., Salmonella spp., and Serratia have been studied as pathogen micro-organisms which are capable of inducing inflammation and intestinal manifestations [34][35].
A decrease has been observed in the fecal concentrations of Bacteroides fragilis and B. vulgatus, both of which have protective potential, where their absence could lead to perpetuated inflammation and the development of IBD [36]. Reductions in the levels of Firmicutes and Proteobacteria were found to be the most reported and consistent changes in patients with IBD. Meanwhile, a metagenomic analysis reported an increase in enterobacteria, most commonly E. coli [35][37][39]. F. prausnitzii possesses anti-inflammatory properties; however, it has been shown to be decreased in patients with IBD (specifically, CD) [40][41]. Some probiotics are able to reinforce the intestinal barrier through the production of defensins and zonula occludens 2 proteins [42]. On the other hand, in patients with IBD, an increase in Malassezia restricta—a fungus generally found in the skin, which is able to promote the production of pro-inflammatory cytokines by immune cells—has been observed, specifically in those who were described as having a mutation in the CARD9 gene, which has been described in IBD [43].
IBD patients with over-expression of carcinoembryonic antigen cell adhesion molecule 6 (CEACAM 6) are more susceptible to EIEC infection, due to the ability of E. coli to bind to CEACAM6 [35]. EIEC has the potential to promote the production of TNF-α by macrophages and survive inside them, thanks to genes such as ATG16L1, immunity-related GTPase family protein (IRGM), and NOD2. When these genes suffer some mutation, these capacities disappear and the EIEC proteins (FimH) are able to bind to TLR4, generating, as a consequence, an inflammatory response [36]. Furthermore, a relation between serotonin, SERT, and TLR-2 has been observed: Ahmad Qasem et al. found that, after infecting Caco-2 cells with Mycobacteria paratuberculosis (MAP), there was an increase in the levels of pro-inflammatory cytokines and TLR2 and, consequently, due to the stimulation of this pro-inflammatory cascade, decreased SERT and IL-10 expression [44].
Due to the linkage between intestinal dysbiosis and GBA, several in vitro and in vivo models have demonstrated the important influence of certain pathogenic bacteria present in patients with IBD and/or the beneficial effect of specific bacteria over SERT and serotonin signaling, as detailed in Table 2.
Table 2. Relationships between bacteria and SERT function.
| Bacteria | Mechanism | Model | References |
|---|---|---|---|
| Enteropathogenic E. coli | Activation of protein tyrosine phosphatase, a process that leads to SERT inhibition | Caco-2 cells infected with E. coli | [45][46] |
| Listeria monocytogenes |
Reduced SERT expression related to a transcriptional change in TLR10 and TLR2 | Caco-2/TC7 cells infected with Listeria monocytogenes | [47] |
| Akkermansia muciniphila |
Interaction between activated TLR2 and SERT causes reduced SERT expression | Caco-2 cells infected with Akkermansia muciniphila | [48] |
| Lactobacillus acidophilus |
Up-regulation of SERT mRNA | Lactobacillus acidophilus and B. longum interaction with HT-29 and Caco-2 cells | [49] |
| Lactobacillus rhamnosus |
SERT Gene and protein up-regulation | Wistar rats implemented with probiotics and prebiotics | [50] |
| Campylobacter jejuni | EC hyperplasia and reduced SERT expression | C57BL/6 mice infected with T. Spiralis and C. jejuni | [51] |
| Salmonella typhimurium |
Inhibition of SERT by TLR4 activation | Mice and Caco-2 cells infected with S. typhimurium | [52][53] |
2.1. Immune Over-Activation in IBD
Innate lymphoid cells (ILCs) come from a common lymphoid progenitor [54] and are differentiated into Natural Killer cells (NK), innate lymphoid cells 1 (ILC1s), innate lymphoid cells 2 (ILC2s), and innate lymphoid cells 3 (ILC3s) [42]. When ILC1s receive stimuli through IL-12, IL-15, and IL-18, there is a release of interferon gamma (IFN-γ), which promotes the ability of macrophages and DCs to remove intracellular bacteria-presenting antigens through the expression of major histocompatibility complex (MHC) and adhesion molecules. In contrast to ILC1s, ILC2s have the ability to release IL-5, IL-9, and IL-13 under certain stimuli, while ILC3s are producers of IL-22 and IL-17 [39]. An alteration in ILC1s and ILC3s in IBD has been described, as IL-12 is able to induce the differentiation of ILC3s into ILC1S to produce IFN-γ; furthermore, ILC3s can pass into a process of maturation when interacting with the microbiota. Pathogen-free mice with decreased IL-22 level presented with alterations, which led to disruption of the intestinal symbiosis [55]. Furthermore, the interaction of IFN-γ with ILCs promotes the migration of neutrophils, lymphocytes, and macrophages, as well as the activation of endothelial cells, thus causing a disruption in the intestinal barrier by affecting TJs [37].
Besides the importance of ILCs, cells such as macrophages are able to act as a bridge between the innate and adaptive immune response. Macrophages are susceptible to a process called polarization, which allows for the differentiation of macrophages into M1 and M2 sub-types, depending on the received stimuli. M1 macrophages are able to trigger an inflammatory response through the production of pro-inflammatory biomarkers such as IL6, IL-12, and TNF-α, while M2 macrophages possess anti-inflammatory properties [38]. In patients with IBD, an increase in the levels of IL-33 and hyperplasia of caliciform cells has been observed, accompanied by macrophage M2 polarization [56]. In particular, in macrophages of patients with IBD, the intracellular replication of bacteria including E. coli, Micobacterium, Salmonella, Shigella, Coxiella, Brucella, Legionella, and Listeria has been reported [57].
Together with macrophages, neutrophils are the most abundant innate immune cell (approximately 70%). In IBD, these cells are responsible for the increased production of ROS, which causes damage to the intestinal epithelial barrier and can activate an inflammatory cascade [24]. Neutrophils are capable of forming a special defense mechanism—Neutrophil Extracellular Traps (NETs)—which are responsible for catching the pathogen component in a microbicidal environment, guaranteeing regulation of the immune response and a highly efficient defense mechanism [58]. Neutrophils migrate to the area of inflammation through interaction with components such as selectins and intracellular/vascular adhesion molecules (e.g., ICAM-1 and VCAM-1) [59]. One of the main findings in histopathological samples of patients with IBD was neutrophil infiltration with the presence of citrullinated histone H3 (citH3) and some other specific NETs products; this formation of NETs in patients with IBD can be considered a result of release and stimulation by TNF-α [60].
Together with macrophages and neutrophils, DCs play an important role in maintaining immune tolerance, considering the effects of nutrients and commensal bacteria [61]. In mucosal samples of patients with IBD, a decrease in sub-populations of CD103+ has been observed, as well as an increase in the expression of TLRs, thus generating an increase in immune responses and leading to a loss of immune tolerance [62]. In CD, a imbalance has been documented in the DCs, which may contribute to an excessive T cell response; in samples from patients with CD, a high expression of TLRs (TLR4) was also observed, as well as an increase in CD11c+, which produces IL-12 and IL-16 [63].
Once the innate immune response is over, the adaptive immunity is activated. This type of immunity is characterized by the generation of memory, with the capacity to confer long-term immunity, mediated by T and B lymphocytes [64].
Th1 Response and Crohn’s Disease
An interplay between the microbiota, immune system, and IBD has been described in terms of decreases in Bacteroides and Firmicutes, along with increases in Clostridium, Gammaproteobacteria, Actinobacteria, enteroinvasive Escherichia coli (EIEC), and ILC1s, with high expression of IL-17A, IL-22, and IL-23 receptor (IL-23R) [65].
CD4 T helper lymphocytes differentiate to Th1, Th2, Treg, Th17, TFH, or Th9 under specific stimuli; notably, a Th1 and Th17 response has been observed as part of the pathophysiology of CD [65]. As previously mentioned, T lymphocytes can differentiate under various chemical stimuli into Th1 lymphocytes, which are producers of IFN-γ, IL-12, IL-17, and IFN-γ [66]. The IL-17/IL-23 axis is a key actor in CD: when IL-23 binds to its receptor, IL-23R, which is expressed in cells including DCs, macrophages, neutrophils, NK cells and ILCs [67], the activation of a kinase (jak2) and a tyrosin kinase (tyk2) causes phosphorylation of the receptor and the transcription 3 activator (STAT3) [68]. Single nucleotide variants (SNVs) reported in the IL-23R gene in chromosome 1p31 (rs10889677) lead to increased IL-23R level, favoring chronic inflammation in CD [67]. Furthermore, there exists a relationship between IL-23 and Th17 activation, with subsequent accumulation of IL-17 producer cells in patients with CD [68].
Th2 Response and Ulcerative Colitis
T lymphocytes differentiate to Th2 lymphocytes after stimulation by IL-4, IL-33, and a transcription factor (GATA3), leading to the final production of IL-4 and IL-13 [69]. Another effect that has been related to IL-13 is the damage that it can impose upon the intestinal epithelial barrier, as IL-13 is able to increase apoptosis in epithelial cells as well as induce disruptions in cellular unions such as claudins-2 [70]. It has been suggested that IL-13 is capable of activating a pro-apoptotic molecule—caspase 3 [71].
3. Serotonin and Gut–Brain Axis Dysfunction in IBD
Due to the link between intestinal dysbiosis and GBA, it has been considered that there is an important influence of certain pathogenic bacteria present in patients with IBD and/or a beneficial effect of specific bacteria over SERT and serotonin signaling, as detailed in Table 2.
The GBA coordinates the release of the adrenocorticotropic hormone under stress, which can cause increases in intestinal permeability and glucocorticoid secretion [72]. The increased intestinal permeability associated with a high level of stress favors communication between the microbiota and nervous system [73][74]. Being a complex network, it has an influence over the neuroplasticity of the ENS during inflammation, leading to structural changes, including degradation and loss of enteric ganglion cells, causing an alteration in the normal neurotransmission and, therefore, gastrointestinal mechanosensitive alterations [75]. In view of the pro-inflammatory profile described by IL-1β, IL-6, and TNF-α, among others, leading to inhibition of the vagus nerve and activation of HPA axis (Figure 2) [76], S Haub et al. found that gut inflammation and a lack of IL-10 and SERT lead to abnormal serotonin signaling [77]. Besides the influence of serotonin and SERT over the maintenance of homeostasis in the GI tract, SERT acts as a determinant of the maintenance of bone mass in patients with IBD. Interestingly, osteoporosis is frequent in patients with IBD [78]. B. Lavoie et al. have demonstrated that, in mice, a lack of SERT due to induced colitis led to an incredible loss of trabecular bone mass. It has been found that the serotonin secreted by EC cells acts as a negative regulator of bone density through the inhibition of osteoblasts, which is a cell type responsible for producing and remodeling bone mass [79].
Figure 2. Overview of SERT, gut microbiota, and IBD pathogenesis. Serotonin is a monoamine exerting a wide range of biological effects through the intestinal microbiota, serotonin receptors, and SERT. After the biosynthesis of serotonin in enterochromaffin cells, there is the possibility that, in the context of gut inflammation, SERT may be affected by different pro-inflammatory mediators and bacteria, leading to dysregulation of mRNA, SERT proteins, and serotonin bioavailability. Trp, tryptophan; 5-HTP, 5-hydroxytryptophan; NOD2, Nucleotide oligomerization domain 2; TLR-2, Toll-like receptor 2; EC, enterochromaffin cells.
References
- Piovani, D.; Danese, S.; Peyrin-Biroulet, L.; Nikolopoulos, G.K.; Lytras, T.; Bonovas, S. Environmental Risk Factors for Inflammatory Bowel Diseases: An Umbrella Review of Meta-analyses. Gastroenterology 2019, 157, 647–659.e644.
- Kelsen, J.R.; Russo, P.; Sullivan, K.E. Early-Onset Inflammatory Bowel Disease. Immunol. Allergy Clin. N. Am. 2019, 39, 63–79.
- Zhang, Y.Z.; Li, Y.Y. Inflammatory bowel disease: Pathogenesis. World J. Gastroenterol. 2014, 20, 91–99.
- Reddavide, R.; Rotolo, O.; Caruso, M.G.; Stasi, E.; Notarnicola, M.; Miraglia, C.; Nouvenne, A.; Meschi, T.; De’ Angelis, G.L.; Di Mario, F.; et al. The role of diet in the prevention and treatment of Inflammatory Bowel Diseases. Acta Bio-Med. Atenei Parm. 2018, 89, 60–75.
- Piovani, D.; Pansieri, C.; Kotha, S.R.R.; Piazza, A.C.; Comberg, C.L.; Peyrin-Biroulet, L.; Danese, S.; Bonovas, S. Ethnic Differences in the Smoking-related Risk of Inflammatory Bowel Disease: A Systematic Review and Meta-analysis. J. Crohn’s Colitis 2021, 15, 1658–1678.
- Owczarek, D.; Rodacki, T.; Domagala-Rodacka, R.; Cibor, D.; Mach, T. Diet and nutritional factors in inflammatory bowel diseases. World J. Gastroenterol. 2016, 22, 895–905.
- Laffin, M.; Fedorak, R.; Zalasky, A.; Park, H.; Gill, A.; Agrawal, A.; Keshteli, A.; Hotte, N.; Madsen, K.L. A high-sugar diet rapidly enhances susceptibility to colitis via depletion of luminal short-chain fatty acids in mice. Sci. Rep. 2019, 9, 12294.
- Wark, G.; Samocha-Bonet, D.; Ghaly, S.; Danta, M. The Role of Diet in the Pathogenesis and Management of Inflammatory Bowel Disease: A Review. Nutrients 2020, 13, 135.
- Lightner, A.L. Perianal Crohn’s Disease. Dis. Colon Rectum 2020, 63, 1023–1026.
- Gheita, T.A.; El, G., II; El-Fishawy, H.S.; Aboul-Ezz, M.A.; Kenawy, S.A. Involvement of IL-23 in enteropathic arthritis patients with inflammatory bowel disease: Preliminary results. Clin. Rheumatol. 2014, 33, 713–717.
- Peyrin-Biroulet, L.; Loftus, E.V., Jr.; Colombel, J.F.; Sandborn, W.J. Long-term complications, extraintestinal manifestations, and mortality in adult Crohn’s disease in population-based cohorts. Inflamm. Bowel Dis. 2011, 17, 471–478.
- Conrad, K.; Roggenbuck, D.; Laass, M.W. Diagnosis and classification of ulcerative colitis. Autoimmun. Rev. 2014, 13, 463–466.
- Kuznicki, P.; Kempinski, R.; Neubauer, K. The emerging role of mood disorders in inflammatory bowel diseases. Adv. Clin. Exp. Med. Off. Organ Wroc. Med. Univ. 2020, 29, 1505–1510.
- Hatamnejad, M.R.; Baradaran Ghavami, S.; Shirvani, M.; Asghari Ahmadabad, M.; Shahrokh, S.; Farmani, M.; Sherkat, G.; Asadzadeh Aghdaei, H.; Zali, M.R. Selective serotonin reuptake inhibitors and inflammatory bowel disease; Beneficial or malpractice. Front. Immunol. 2022, 13, 980189.
- Moulton, C.D.; Pavlidis, P.; Norton, C.; Norton, S.; Pariante, C.; Hayee, B.; Powell, N. Depressive symptoms in inflammatory bowel disease: An extraintestinal manifestation of inflammation? Clin. Exp. Immunol. 2019, 197, 308–318.
- Hopkins, C.W.P.; Powell, N.; Norton, C.; Dumbrill, J.L.; Hayee, B.; Moulton, C.D. Cognitive Impairment in Adult Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. J. Acad. Consult.-Liaison Psychiatry 2021, 62, 387–403.
- Liu, J.Z.; van Sommeren, S.; Huang, H.; Ng, S.C.; Alberts, R.; Takahashi, A.; Ripke, S.; Lee, J.C.; Jostins, L.; Shah, T.; et al. Association analyses identify 38 susceptibility loci for inflammatory bowel disease and highlight shared genetic risk across populations. Nat. Genet. 2015, 47, 979–986.
- Boukercha, A.; Mesbah-Amroun, H.; Bouzidi, A.; Saoula, H.; Nakkemouche, M.; Roy, M.; Hugot, J.P.; Touil-Boukoffa, C. NOD2/CARD15 gene mutations in North Algerian patients with inflammatory bowel disease. World J. Gastroenterol. 2015, 21, 7786–7794.
- Hugot, J.-P. Association of NOD-2 leucine-rich repeat variants with susceptibility to Crohn’s disease. Nature 2001, 411, 599–603.
- Glas, J.; Konrad, A.; Schmechel, S.; Dambacher, J.; Seiderer, J.; Schroff, F.; Wetzke, M.; Roeske, D.; Torok, H.P.; Tonenchi, L.; et al. The ATG16L1 gene variants rs2241879 and rs2241880 (T300A) are strongly associated with susceptibility to Crohn’s disease in the German population. Am. J. Gastroenterol. 2008, 103, 682–691.
- Cadwell, K.; Liu, J.Y.; Brown, S.L.; Miyoshi, H.; Loh, J.; Lennerz, J.K.; Kishi, C.; Kc, W.; Carrero, J.A.; Hunt, S.; et al. A key role for autophagy and the autophagy gene Atg16l1 in mouse and human intestinal Paneth cells. Nature 2008, 456, 259–263.
- Younis, N.; Zarif, R.; Mahfouz, R. Inflammatory bowel disease: Between genetics and microbiota. Mol. Biol. Rep. 2020, 47, 3053–3063.
- Mirkov, M.U.; Verstockt, B.; Cleynen, I. Genetics of inflammatory bowel disease: Beyond NOD2. Lancet. Gastroenterol. Hepatol. 2017, 2, 224–234.
- Larabi, A.; Barnich, N.; Nguyen, H.T.T. New insights into the interplay between autophagy, gut microbiota and inflammatory responses in IBD. Autophagy 2020, 16, 38–51.
- Wang, S.L.; Shao, B.Z.; Zhao, S.B.; Chang, X.; Wang, P.; Miao, C.Y.; Li, Z.S.; Bai, Y. Intestinal autophagy links psychosocial stress with gut microbiota to promote inflammatory bowel disease. Cell Death Dis. 2019, 10, 391.
- Festen, E.A.; Goyette, P.; Green, T.; Boucher, G.; Beauchamp, C.; Trynka, G.; Dubois, P.C.; Lagace, C.; Stokkers, P.C.; Hommes, D.W.; et al. A meta-analysis of genome-wide association scans identifies IL18RAP, PTPN2, TAGAP, and PUS10 as shared risk loci for Crohn’s disease and celiac disease. PLoS Genet. 2011, 7, e1001283.
- Yu, W.; Hegarty, J.P.; Berg, A.; Kelly, A.A.; Wang, Y.; Poritz, L.S.; Franke, A.; Schreiber, S.; Koltun, W.A.; Lin, Z. PTPN2 is associated with Crohn’s disease and its expression is regulated by NKX2-3. Dis. Mrk. 2012, 32, 83–91.
- Lacher, M.; Schroepf, S.; Helmbrecht, J.; Von Schweinitz, D.; Ballauff, A.; Koch, I.; Lohse, P.; Osterrieder, S.; Kappler, R.; Koletzko, S. Association of the interleukin-23 receptor gene variant rs11209026 with Crohn’s disease in German children. Acta Paediatr. 2010, 99, 727–733.
- Peyrin-Biroulet, L.; Parmentier-Decrucq, E.; Branche, J.; Desreumaux, P. IL-23R, a novel susceptibility gene for inflammatory bowel disease. Med. Sci. M/S 2007, 23, 250–252.
- Castro-Santos, P.; Suarez, A.; Lopez-Rivas, L.; Mozo, L.; Gutierrez, C. TNFalpha and IL-10 gene polymorphisms in inflammatory bowel disease. Association of -1082 AA low producer IL-10 genotype with steroid dependency. Am. J. Gastroenterol. 2006, 101, 1039–1047.
- Uhlig, H.H.; Schwerd, T.; Koletzko, S.; Shah, N.; Kammermeier, J.; Elkadri, A.; Ouahed, J.; Wilson, D.C.; Travis, S.P.; Turner, D.; et al. The diagnostic approach to monogenic very early onset inflammatory bowel disease. Gastroenterology 2014, 147, 990–1007.e1003.
- NW, H. Hepatocyte nuclear factor- 4alpha regulates expression of the serotonin transporter in intestinal epithelial cells. Physiology 2020, 6, 1294–1304.
- Meddens, C.A.; Harakalova, M.; van den Dungen, N.A.; Foroughi Asl, H.; Hijma, H.J.; Cuppen, E.P.; Bjorkegren, J.L.; Asselbergs, F.W.; Nieuwenhuis, E.E.; Mokry, M. Systematic analysis of chromatin interactions at disease associated loci links novel candidate genes to inflammatory bowel disease. Genome Biol. 2016, 17, 247.
- Takiishi, T.; Fenero, C.I.M.; Camara, N.O.S. Intestinal barrier and gut microbiota: Shaping our immune responses throughout life. Tissue Barriers 2017, 5, e1373208.
- Rose, E.C.; Odle, J.; Blikslager, A.T.; Ziegler, A.L. Probiotics, Prebiotics and Epithelial Tight Junctions: A Promising Approach to Modulate Intestinal Barrier Function. Int. J. Mol. Sci. 2021, 22, 6729.
- Martini, E.; Krug, S.M.; Siegmund, B.; Neurath, M.F.; Becker, C. Mend Your Fences: The Epithelial Barrier and its Relationship With Mucosal Immunity in Inflammatory Bowel Disease. Cell. Mol. Gastroenterol. Hepatol. 2017, 4, 33–46.
- Peters, C.P.; Mjosberg, J.M.; Bernink, J.H.; Spits, H. Innate lymphoid cells in inflammatory bowel diseases. Immunol. Lett. 2016, 172, 124–131.
- Yunna, C.; Mengru, H.; Lei, W.; Weidong, C. Macrophage M1/M2 polarization. Eur. J. Pharm. 2020, 877, 173090.
- Panda, S.K.; Colonna, M. Innate Lymphoid Cells in Mucosal Immunity. Front. Immunol. 2019, 10, 861.
- Zhou, L.; Zhang, M.; Wang, Y.; Dorfman, R.G.; Liu, H.; Yu, T.; Chen, X.; Tang, D.; Xu, L.; Yin, Y.; et al. Faecalibacterium prausnitzii Produces Butyrate to Maintain Th17/Treg Balance and to Ameliorate Colorectal Colitis by Inhibiting Histone Deacetylase 1. Inflamm. Bowel Dis. 2018, 24, 1926–1940.
- Quevrain, E.; Maubert, M.A.; Michon, C.; Chain, F.; Marquant, R.; Tailhades, J.; Miquel, S.; Carlier, L.; Bermudez-Humaran, L.G.; Pigneur, B.; et al. Identification of an anti-inflammatory protein from Faecalibacterium prausnitzii, a commensal bacterium deficient in Crohn’s disease. Gut 2016, 65, 415–425.
- Li, J.; Glover, S.C. Innate Lymphoid Cells in Inflammatory Bowel Disease. Arch. Immunol. Ther. Exp. 2018, 66, 415–421.
- Ahlawat, S.; Kumar, P.; Mohan, H.; Goyal, S.; Sharma, K.K. Inflammatory bowel disease: Tri-directional relationship between microbiota, immune system and intestinal epithelium. Crit. Rev. Microbiol. 2021, 47, 254–273.
- Qasem, A.; Naser, A.E.; Naser, S.A. Enteropathogenic infections modulate intestinal serotonin transporter (SERT) function by activating Toll-like receptor 2 (TLR-2) in Crohn’s disease. Sci. Rep. 2021, 11, 22624.
- Singhal, M.; Manzella, C.; Soni, V.; Alrefai, W.A.; Saksena, S.; Hecht, G.A.; Dudeja, P.K.; Gill, R.K. Role of SHP2 protein tyrosine phosphatase in SERT inhibition by enteropathogenic E. coli (EPEC). Am. J. Physiol. Gastrointest. Liver Physiol. 2017, 312, G443–G449.
- Esmaili, A.; Nazir, S.F.; Borthakur, A.; Yu, D.; Turner, J.R.; Saksena, S.; Singla, A.; Hecht, G.A.; Alrefai, W.A.; Gill, R.K. Enteropathogenic Escherichia coli infection inhibits intestinal serotonin transporter function and expression. Gastroenterology 2009, 137, 2074–2083.
- Latorre, E.; Pradilla, A.; Chueca, B.; Pagan, R.; Layunta, E.; Alcalde, A.I.; Mesonero, J.E. Listeria monocytogenes Inhibits Serotonin Transporter in Human Intestinal Caco-2 Cells. Microb. Ecol. 2016, 72, 730–739.
- Wang, J.; Xu, W.; Wang, R.; Cheng, R.; Tang, Z.; Zhang, M. The outer membrane protein Amuc_1100 of Akkermansia muciniphila promotes intestinal 5-HT biosynthesis and extracellular availability through TLR2 signalling. Food Funct. 2021, 12, 3597–3610.
- Cao, Y.N.; Feng, L.J.; Wang, B.M.; Jiang, K.; Li, S.; Xu, X.; Wang, W.Q.; Zhao, J.W.; Wang, Y.M. Lactobacillus acidophilus and Bifidobacterium longum supernatants upregulate the serotonin transporter expression in intestinal epithelial cells. Saudi J. Gastroenterol. Off. J. Saudi Gastroenterol. Assoc. 2018, 24, 59–66.
- Li, H.; Wang, P.; Huang, L.; Li, P.; Zhang, D. Effects of regulating gut microbiota on the serotonin metabolism in the chronic unpredictable mild stress rat model. Neurogastroenterol. Motil. Off. J. Eur. Gastrointest. Motil. Soc. 2019, 31, e13677.
- Wheatcroft, J.; Wakelin, D.; Smith, A.; Mahoney, C.R.; Mawe, G.; Spiller, R. Enterochromaffin cell hyperplasia and decreased serotonin transporter in a mouse model of postinfectious bowel dysfunction. Neurogastroenterol. Motil. Off. J. Eur. Gastrointest. Motil. Soc. 2005, 17, 863–870.
- Kim, S.W.; Kim, S.; Son, M.; Cheon, J.H.; Park, Y.S. Melatonin controls microbiota in colitis by goblet cell differentiation and antimicrobial peptide production through Toll-like receptor 4 signalling. Sci. Rep. 2020, 10, 2232.
- Mendoza, C. Lypopolysaccharide inducces alteration of serotonin transporter in human intestinal epithelial cells. Innate Immun. 2009, 15, 243–250.
- Saez, A.; Gomez-Bris, R.; Herrero-Fernandez, B.; Mingorance, C.; Rius, C.; Gonzalez-Granado, J.M. Innate Lymphoid Cells in Intestinal Homeostasis and Inflammatory Bowel Disease. Int. J. Mol. Sci. 2021, 22, 7618.
- Luo, W.; Tian, L.; Tan, B.; Shen, Z.; Xiao, M.; Wu, S.; Meng, X.; Wu, X.; Wang, X. Update: Innate Lymphoid Cells in Inflammatory Bowel Disease. Dig. Dis. Sci. 2022, 67, 56–66.
- Seo, D.H.; Che, X.; Kwak, M.S.; Kim, S.; Kim, J.H.; Ma, H.W.; Kim, D.H.; Kim, T.I.; Kim, W.H.; Kim, S.W.; et al. Interleukin-33 regulates intestinal inflammation by modulating macrophages in inflammatory bowel disease. Sci. Rep. 2017, 7, 851.
- Tawfik, A. Escherichia Coli-host macrophage interactions in the pathogenesis of inflammatory bowel disease. World J. Gastroenterol. 2014, 20, 8751.
- Drury, B.; Hardisty, G.; Gray, R.D.; Ho, G.T. Neutrophil Extracellular Traps in Inflammatory Bowel Disease: Pathogenic Mechanisms and Clinical Translation. Cell. Mol. Gastroenterol. Hepatol. 2021, 12, 321–333.
- Zhou, G.X. Potential roles of neutrophils in regulating intestinal inflammation of inflammatory bowel disease. J. Dig. Dis. 2017, 18, 495–503.
- Li, T.; Wang, C.; Liu, Y.; Li, B.; Zhang, W.; Wang, L.; Yu, M.; Zhao, X.; Du, J.; Zhang, J.; et al. Neutrophil Extracellular Traps Induce Intestinal Damage and Thrombotic Tendency in Inflammatory Bowel Disease. J. Crohn’s Colitis 2020, 14, 240–253.
- Waisman, A.; Lukas, D.; Clausen, B.E.; Yogev, N. Dendritic cells as gatekeepers of tolerance. Semin. Immunopathol. 2017, 39, 153–163.
- Bernardo, D.; Chaparro, M.; Gisbert, J.P. Human Intestinal Dendritic Cells in Inflammatory Bowel Diseases. Mol. Nutr. Food Res. 2018, 62, e1700931.
- Silva, M.A. Intestinal dendritic cells and epithelial barrier dysfunction in Crohn’s disease. Inflamm. Bowel Dis. 2009, 15, 436–453.
- Netea, M.G.; Joosten, L.A.; Latz, E.; Mills, K.H.; Natoli, G.; Stunnenberg, H.G.; O’Neill, L.A.; Xavier, R.J. Trained immunity: A program of innate immune memory in health and disease. Science 2016, 352, aaf1098.
- Ballester Ferre, M.P.; Bosca-Watts, M.M.; Minguez Perez, M. Crohn’s disease. Med. Clin. 2018, 151, 26–33.
- Okazawa, A. Th1-mediated intestinal inflammation in Crohn’s disease may be induced by activation of lamina propria lymphocytes through synergistic stimulation of interleukin-12 and interleukin-18 without T cel receptor engagement. Am. J. Gastroenterol. 2002, 97, 3108–3117.
- Sedda, S.; Bevivino, G.; Monteleone, G. Targeting IL-23 in Crohn’s disease. Expert Rev. Clin. Immunol. 2018, 14, 907–913.
- Schmitt, H.; Neurath, M.F.; Atreya, R. Role of the IL23/IL17 Pathway in Crohn’s Disease. Front. Immunol. 2021, 12, 622934.
- Ogino, H.; Fukaura, K.; Iboshi, Y.; Nagamatsu, Y.; Okuno, H.; Nishioka, K.; Nishihara, Y.; Tanaka, Y.; Chinen, T.; Ihara, E.; et al. Role of the IL-23-T-bet/GATA3 Axis for the Pathogenesis of Ulcerative Colitis. Inflammation 2021, 44, 592–603.
- Heller, F.; Florian, P.; Bojarski, C.; Richter, J.; Christ, M.; Hillenbrand, B.; Mankertz, J.; Gitter, A.H.; Burgel, N.; Fromm, M.; et al. Interleukin-13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterology 2005, 129, 550–564.
- Mannon, P.; Reinisch, W. Interleukin 13 and its role in gut defence and inflammation. Gut 2012, 61, 1765–1773.
- Farzi, A.; Frohlich, E.E.; Holzer, P. Gut Microbiota and the Neuroendocrine System. Neurother. J. Am. Soc. Exp. NeuroTher. 2018, 15, 5–22.
- Filaretova, L. The realization of the brain-gut interactions with corticotropin-releasing factor and glucocorticoids. Curr. Neuropharmacol. 2016, 14, 876–881.
- de Weerth, C. Do bacteria shape our development? Crosstalk between intestinal microbiota and HPA axis. Neurosci. Biobehav. Rev. 2017, 83, 458–471.
- Banfi, D.; Moro, E.; Bosi, A.; Bistoletti, M.; Cerantola, S.; Crema, F.; Maggi, F.; Giron, M.C.; Giaroni, C.; Baj, A. Impact of Microbial Metabolites on Microbiota-Gut-Brain Axis in Inflammatory Bowel Disease. Int. J. Mol. Sci. 2021, 22, 1623.
- Bonaz, B.L.; Bernstein, C.N. Brain-gut interactions in inflammatory bowel disease. Gastroenterology 2013, 144, 36–49.
- Haub, S.; Ritze, Y.; Bergheim, I.; Pabst, O.; Gershon, M.D.; Bischoff, S.C. Enhancement of intestinal inflammation in mice lacking interleukin 10 by deletion of the serotonin reuptake transporter. Neurogastroenterol. Motil. Off. J. Eur. Gastrointest. Motil. Soc. 2010, 22, 826–834.e229.
- Oh, H.J.; Ryu, K.H.; Park, B.J.; Yoon, B.H. Osteoporosis and Osteoporotic Fractures in Gastrointestinal Disease. J. Bone Metab. 2018, 25, 213–217.
- Lavoie, B.; Roberts, J.A.; Haag, M.M.; Spohn, S.N.; Margolis, K.G.; Sharkey, K.A.; Lian, J.B.; Mawe, G.M. Gut-derived serotonin contributes to bone deficits in colitis. Pharmacol. Res. 2019, 140, 75–84.
More
Information
Subjects:
Gastroenterology & Hepatology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.0K
Entry Collection:
Gastrointestinal Disease
Revisions:
3 times
(View History)
Update Date:
20 Dec 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




