Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Xiaohong Wang | -- | 6126 | 2022-12-09 01:25:59 | | | |
| 2 | Xiaohong Wang | -168 word(s) | 5958 | 2022-12-09 09:56:56 | | | | |
| 3 | Dean Liu | -4091 word(s) | 1867 | 2022-12-12 08:46:37 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Xu, Y.; Song, D.; Wang, X. 3D Bioprinting for Pancreas Engineering/Manufacturing. Encyclopedia. Available online: https://encyclopedia.pub/entry/38375 (accessed on 07 February 2026).
Xu Y, Song D, Wang X. 3D Bioprinting for Pancreas Engineering/Manufacturing. Encyclopedia. Available at: https://encyclopedia.pub/entry/38375. Accessed February 07, 2026.
Xu, Yukun, Dabin Song, Xiaohong Wang. "3D Bioprinting for Pancreas Engineering/Manufacturing" Encyclopedia, https://encyclopedia.pub/entry/38375 (accessed February 07, 2026).
Xu, Y., Song, D., & Wang, X. (2022, December 09). 3D Bioprinting for Pancreas Engineering/Manufacturing. In Encyclopedia. https://encyclopedia.pub/entry/38375
Xu, Yukun, et al. "3D Bioprinting for Pancreas Engineering/Manufacturing." Encyclopedia. Web. 09 December, 2022.
Copy Citation
Diabetes is the most common chronic disease in the world, and it brings a heavy burden to people’s health. Against this background, diabetic research, including islet functionalization has become a hot topic in medical institutions all over the world. Especially with the rapid development of microencapsulation and three-dimensional (3D) bioprinting technologies, organ engineering and manufacturing have become the main trends for disease modeling and drug screening. Especially the advanced 3D models of pancreatic islets have shown better physiological functions than monolayer cultures, suggesting their potential in elucidating the behaviors of cells under different growth environments.
3D bioprinting
organ engineering/manufacturing
vascularization
1. Natural Polymers for Pancreas 3D Printing
Natural polymers are macromolecular compounds that exist in nature, including proteins, polysaccharides, and their combinations, such as glycoproteins and proteoglycans [1][2]. Most of the natural polymers, such as gelatin, alginate, fibrinogen, and hyaluronic acid, are water-soluble, dissolving in inorganic solvents such as cell culture medium. The polymer solutions usually have good fluidities, excellent cytocompatibility, and can form water-rich hydrogels through the physical, chemical, and enzymatic cross-linking of the polymer molecules [3][4][5]. The water-rich hydrogels can not only embed living cells, growth factors, and other bioactive agents, transporting nutrients/oxygen to cells, but also discharge metabolic wastes produced by cells through the interpenetrating networks [6][7].
As the main component of 3D printable ‘bioinks’, natural polymers have been widely used in pancreas 3D printing. The 3D printed islets embedded in natural hydrogels can maintain excellent biological activities with glucose regulation functions [8]. The first pancreas 3D printing technology was reported by Prof. Wang early in 2009 using gelatin/alginate/fibrin hydrogels [9] in which, adipose stem cells (ASCs), embedded in gelatin/alginate/fibrinogen solutions, and islets were printed into large-scale living organs with similar biological and physiological functions of their natural counterparts. When the pancreatic islets were deposited at designated locations with the ASC-laden gelatin/alginate/fibrin hydrogel, the ASCs can be induced to differentiate into vascular endothelial cells (ECs) and adipocytes dividually (or separately). The differentiation and self-organization of ASCs can be totally controlled by the growth factor combinations and the incorporated islets. This is a huge milestone in complex organ engineering/manufacturing areas, which has shown great potential in the establishment of physiological models of MS. When different drugs are applied to this model, the physiological responses are consistent with the in vivo experiments, suggesting that this model has strong advantages in high-throughput drug screening, pathological model establishment, as well as contributing to a better understanding of the multiple sclerosis pathogenesis of cells and drug development strategies [10] (Figure 1).
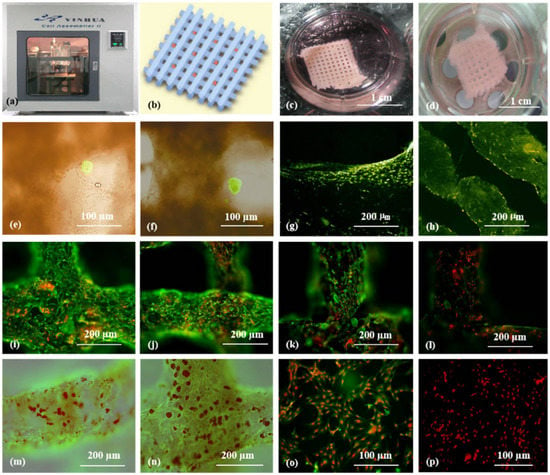
Figure 1. Cell-laden hydrogel constructs printed by Professor Wang: (a–d) grid gelatin/alginate/fibrin constructs containing ASCs and islets; (e,f) immunofluorescence staining of islet cells in the constructs; (g,h) immunofluorescence staining of ASCs differentiated into endothelial cells with EGF; (i–l) endothelial cell (green) immunostaining, nuclear (red) propidium iodide (PI) staining; (m,n) immunostained endothelial cells (green), and adipocytes (red) stained with oil red O; (o,p) immunostaining of two-dimensional (2D) cultured endothelial cells (green), differentiated from ASCs with pyridine iodide staining nucleus (red). Reprinted from Ref. [10].
Later, in 2019, Duin et al. encapsulated islets into an alginate/methylcellulose hydrogel and constructed macroporous hydrogel structures via a simple 3D bioprinting technique. It was shown that the islets within the hydrogel had good viability and morphology and could continuously produce insulin and glucagon throughout the observation stage in responding to glucose stimulation [11] (Figure 2).
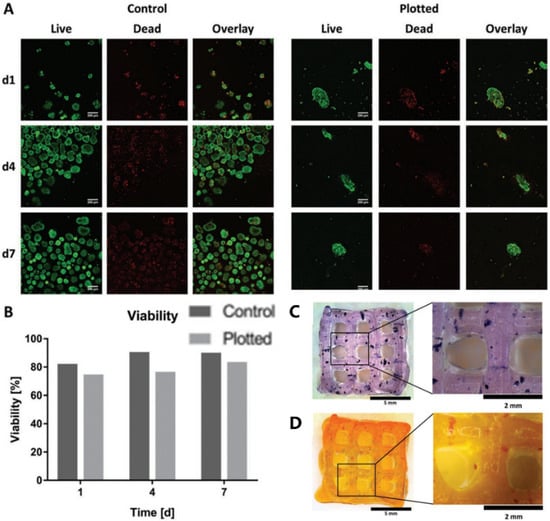
Figure 2. Islet viability assay and staining. (A) Live/dead staining of islets in Alg/MC gel (right) and free control islets (left). Live and dead cells are shown in green and red, respectively. (B) Semi-quantitative assessment of islet viability based on live/dead staining as shown in (A), n > 60 islets. (C) Islet-containing scaffolds were stained with thiazolyl blue tetrazolium bromide (MTT). (D) Islet-containing scaffolds were stained with dithizone (DTZ). Reprinted with permission from Ref. [11], Copyright 2019, John Wiley and Sons.
In 2021, Hu et al. developed a new ‘bioink’ based on natural alginate molecules. They added polymer Pluronic F127 to the alginate solution, which greatly improved the printability of the alginate-based hydrogel and the flexibility of the cross-linked structure. Meanwhile, hypomethylated pectin was added to reduce inflammation. The experimental results showed that the cellular constructs printed with pectin-alginate-pluronic ‘bioink’ could reduce tissue rejections by inhibiting TLR2/1 and ensure the survival of the insulin-producing β cells under inflammatory stress. It provides an improved strategy for the long-term survival of the transplanted islets in the treatment of type 1 diabetes [12].
To overcome the fundamental problems for islet or pancreatic cell transplantation, such as lacking adequate blood vessels in the constructs and allogeneic immune attack after implantation, the development of custom-designed bioartificial pancreases is urgently needed. This problem is expected to be solved using multi-nozzle 3D bioprinting technologies [13]. With the multiple nozzles, the distribution of many different cell types, including multicellular islets, can be controlled simultaneously to mimic the natural pancreas with the desired physiological functions.
2. Synthetic Polymers for Pancreas 3D Printing
Synthetic polymers are artificially manufactured macromolecular compounds that cannot be obtained from nature. They are often obtained through a certain polymerization reaction, using small molecules called monomers, with known structures and relatively low molecular weights as raw materials [13]. Synthetic polymers are widely used in various fields such as electronics, automobiles, and transportation due to their excellent chemical and physical properties. Synthetic polymers, such as polylactic acid (PLA), polylactic-co-glycolic acid (PLGA), polyurethane (PU), and polycaprolactone (PCL) with good mechanical properties, in vivo histocompatibility, and structural stability, have been 3D printed widely as tissue engineering scaffolds for cell attachment and vascular/neural network building templates for organ implantation [10][14][15][16][17] (Figure 3).

Figure 3. Vascularized and neuralized liver tissue models constructed by Professor Wang: (a,b) combined four nozzle printer; (c) a CAD model of the vascularized and neuralized liver tissue; (d) the 3D printed constructs containing vascularized and neuralized liver tissues; (e) immunofluorescence staining of endothelial cells and Schwann cells around the branching channels of the constructs; (f) nerve fibers formed in the 3D constructs; (g) hepatocytes in the 3D constructs, some of the cells in proliferation stage with two nucleus; (h) the interface between the endothelial cells and Schwann cells; (i) nerve fibers formed in the constructs. Reprinted from Ref. [10].
Compared with natural polymers, most synthetic polymers have super mechanical properties, and 3D printed structures can be maintained in vivo for a long time [18]. For example, Song et al. printed a PLA structure by tuning the parameters of a low-cost 3D printer that could be accommodated by clusters of SC-β cells in a degradable fibrin gel. A finite element model of cellular oxygen diffusion consumption was used to determine the diameter of cell clusters to avoid severe hypoxia before vascularization. After the constructs were transplanted into mice, insulin was secreted in response to glucose injection, and the transplanted constructs maintained their structural integrity for 12 weeks. Unlike the pure cell encapsulation techniques, this approach could serve as a platform for advanced diabetes therapies using 3D printed cell replacements [19].
In another study, Farina presented a novel 3D printing and functionalized encapsulation system for the subcutaneous transplantation of pancreatic islets or islet-like cells. When the surface of the 3D printed PLA structure underwent some treatments, the hydrophilicity of the synthetic polymers was increased, which could facilitate cell attachment and proliferation. The implantation of a growth factor-rich platelet gel in a surface-treated encapsulation system could help to create a vascularized environment prior to loading human islets. Islets encased in this device could be protected from acute hypoxia and retain their function [20].
Similarly, Marchioli developed a PCL scaffold that could actively promote vascularization in extrahepatic islet transplantation. The PCL scaffold with a heparinized surface could electrostatically bind vascular endothelial growth factor (VEGF) to the alginate-encapsulated islets. Compared with the untreated PCL scaffold, heparin immobilization could increase the retention of the VEGF in the scaffold up to 3.6-fold. In a chicken chorioallantoic membrane model, the VEGF immobilized on the surface of the PCL scaffold could promote angiogenesis. After 7 days of implantation, the alginate-encapsulated islets exhibited functional responses to the glucose stimulation similar to the free-floating islets. The model has the potential to support rapid vascularization and islet endocrine function [21] (Figure 4).
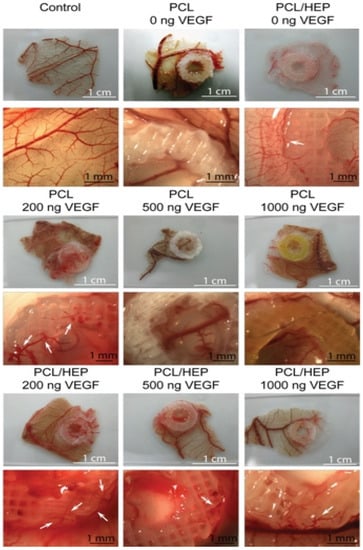
Figure 4. CAM assays were performed on PCL and heparin-coated 3D-printed PCL scaffolds with three different concentrations of VEGF. The 200 ng loading of VEGF induced stent formation with normal morphological vessels. Reprinted with permission from Ref. [21], Copyright 2016, Elsevier.
3. ECM and dECM for Pancreas 3D Printing
As stated above, ECM is a macromolecular substance secreted by cells with a complex network, supporting, connecting, and regulating cell behaviors with the occurrence of tissue and organ formation [22][23]. An acellular matrix is a process of the decellularization of allogeneic tissue to remove antigenic components that can cause immune rejection, while completely retaining the 3D structure of the ECM with some growth factors, such as the fibroblast growth factor, VEGF, that play a significant role in stem cell differentiation [24][25]. Some of the ECMs have relatively good mechanical properties compared with the single natural polymeric hydrogels. Some of the ECMs demonstrate good histocompatibility and low immune rejection when they are implanted in the body [26][27].
Similarly, an acellular extracellular matrix or dECM is a biological material derived from living organisms, and its 3D printed pancreas model is closer to the living environment of real islets, which is more conducive to the maintenance of islet function and the release of insulin [28]. In 2019, Kim et al. printed pancreatic-derived ECM (pdECM) for the creation of a native microenvironment for transplantable 3D pancreatic tissues. The results showed that the insulin secretion of human pluripotent stem cells and the maturity of insulin-producing cells were highly enhanced when they were cultured in the pdECM ‘bioinks’ and that the co-culture with human umbilical vein-derived endothelial cells could reduce the central islet necrosis under 3D culture conditions. The possibility of fabricating 3D islet structures with therapeutic graft dimensions was validated by the fusion with 3D bioprinting technology [28]. Hwang et al. developed a hybrid packaging system using 3D bioprinting technology, which consists of macroporous polymer capsules and nanoporous dECM hydrogels with islet-like aggregates. The exterior of the construct is designed as a go-through porous structure, β-cells can be encapsulated inside, and can maintain their activities with insulin secretion functions. The islet-like aggregates are formed through 3D bioprinting technology to improve cell vitalities and functions. The experimental results show that the hybrid packaging system has good biocompatibility, and the cells in the construct can connect through the go-through pores. These approaches are expected to solve the donor shortage problems to some degree and realize the clinical application of 3D printed pancreatic organs [29]. Wang et al. fabricated a novel ’bioink’ by combining pancreatic extracellular matrix (pECM) and hyaluronic acid methacrylate (HAMA) and used 3D printing technology to construct islet organoids. The islet cells maintained the biological functions in the structure through the Rac1/ROCK/MLCK signal pathway with improved bioactivities. When the pancreas structure was implanted into the diabetic model mice, the insulin level in the mice was significantly increased, and the blood glucose level in the mice remained at the normal level for 90 days. Compared with HAMA hydrogel, the HAMA/pECM hydrogel is more conducive to angiogenesis, and the blood vessel density is significantly increased, which brings hope for the construction of vascularized 3D pancreatic organs [30].
References
- Li, S.; Yan, Y.; Xiong, Z.; Weng, C.; Zhang, R.; Wang, X. Gradient hydrogel construct based on an improved cell assembling system. J. Bioact. Compat. Polym. 2009, 24, 84–99.
- Mahendiran, B.; Muthusamy, S.; Sampath, S.; Jaisankar, S.N.; Popat, K.C.; Selvakumar, R.; Krishnakumar, G.S. Recent trends in natural polysaccharide based bioinks for multiscale 3D printing in tissue regeneration: A review. Int. J. Biol. Macromol. 2021, 183, 564–588.
- Hong, H.; Seo, Y.B.; Kim, D.Y.; Lee, J.S.; Lee, Y.J.; Lee, H.; Ajiteru, O.; Sultan, M.T.; Lee, O.J.; Kim, S.H.; et al. Digital light processing 3D printed silk fibroin hydrogel for cartilage tissue engineering. Biomaterials 2020, 232, 119679.
- Wang, X. Spatial effects of stem cell engagement in 3D printing constructs. J. Stem Cells Res. Rev. Rep. 2014, 1, 5–9.
- Wang, X. 3D printing of tissue/organ analogues for regenerative medicine. In Handbook of Intelligent Scaffolds for Regenerative Medicine, 2nd ed.; Pan Stanford Publishing: Palo Alto, CA, USA, 2016; pp. 557–570.
- Erkoc, P.; Uvak, I.; Nazeer, M.A.; Batool, S.R.; Odeh, Y.N.; Akdogan, O.; Kizilel, S. 3D Printing of cytocompatible gelatin-cellulose-alginate blend hydrogels. Macromol. Biosci. 2020, 10, e2000106.
- Jose, G.; Shalumon, K.T.; Chen, J.P. Natural polymers based hydrogels for cell culture applications. Curr. Med. Chem. 2020, 16, 2734–2776.
- Luetchford, K.A.; Chaudhuri, J.B.; De Bank, P.A. Silk fibroin/gelatin microcarriers as scaffolds for bone tissue engineering. Mater. Sci. Eng. C 2020, 106, 110116.
- Xu, M.; Yan, Y.; Liu, H.; Yao, Y.; Wang, X. Control adipose-derived stromal cells differentiation into adipose and endothelial cells in a 3D structure established by cell-assembly technique. J. Bioact. Compat. Polym. 2009, 24, 31–47.
- Wang, X. Advanced polymers for three-dimensional (3D) organ bioprinting. Micromachines 2019, 12, 814.
- Duin, S.; Schütz, K.; Ahlfeld, T.; Lehmann, S.; Lode, A.; Ludwig, B.; Gelinsky, M. 3D bioprinting of functional islets of langerhans in an alginate/methylcellulose hydrogel blend. Adv. Healthc. Mater. 2019, 7, e1801631.
- Hu, S.; Martinez-Garcia, F.D.; Moeun, B.N.; Burgess, J.K.; Harmsen, M.C.; Hoesli, C.; de Vos, P. An immune regulatory 3D-printed alginate-pectin construct for immunoisolation of insulin producing β-cells. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 123, 112009.
- Huang, Y.; He, K.; Wang, X. Rapid Prototyping of a hybrid hierarchical polyurethane-cell/hydrogel construct for regenerative medicine. Mater. Sci. Eng. C 2013, 33, 3220–3229.
- Xu, W.; Wang, X.; Yan, Y.; Zhang, R. Rapid prototyping of polyurethane for the creation of vascular systems. J. Bioact. Compat. Polym. 2008, 23, 103–114.
- Ashwin, B.; Abinaya, B.; Prasith, T.P.; Chandran, S.V.; Yadav, L.R.; Vairamani, M.; Patil, S.; Selvamurugan, N. 3D-poly (lactic acid) scaffolds coated with gelatin and mucic acid for bone tissue engineering. Int. J. Biol. Macromol. 2020, 162, 523–532.
- Babilotte, J.; Martin, B.; Guduric, V.; Bareille, R.; Agniel, R.; Roques, S.; Héroguez, V.; Dussauze, M.; Gaudon, M.; Le Nihouannen, D.; et al. Development and characterization of a PLGA-HA composite material to fabricate 3D-printed scaffolds for bone tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 118, 111334.
- Hung, K.C.; Tseng, C.S.; Dai, L.G.; Hsu, S.H. Water-based polyurethane 3D printed scaffolds with controlled release function for customized cartilage tissue engineering. Biomaterials 2016, 83, 156–168.
- Liu, F.; Chen, Q.; Liu, C.; Ao, Q.; Tian, X.; Fan, J.; Tong, H.; Wang, X. Natural polymers for organ 3D bioprinting. Polymers 2018, 11, 1278.
- Lei, M.; Wang, X. Biodegradable polymers and stem cells for bioprinting. Molecules 2016, 21, 539.
- Farina, M.; Ballerini, A.; Fraga, D.W.; Nicolov, E.; Hogan, M.; Demarchi, D.; Scaglione, F.; Sabek, O.M.; Horner, P.; Thekkedath, U.; et al. 3D printed vascularized device for subcutaneous transplantation of human islets. Biotechnol. J. 2017, 9, 1700169.
- Marchioli, G.; Luca, A.D.; de Koning, E.; Engelse, M.; Van Blitterswijk, C.A.; Karperien, M.; Van Apeldoorn, A.A.; Moroni, L. Hybrid polycaprolactone/alginate scaffolds functionalized with VEGF to promote de Novo vessel formation for the transplantation of islets of Langerhans. Adv. Healthc. Mater. 2016, 13, 1606–1616.
- Foyt, D.A.; Norman, M.D.A.; Yu, T.T.L.; Gentleman, E. Exploiting Advanced Hydrogel Technologies to Address Key Challenges in Regenerative Medicine. Adv. Healthc. Mater. 2018, 8, e1700939.
- Ge, F.; Lu, Y.; Li, Q.; Zhang, X. Decellularized extracellular matrices for tissue engineering and regeneration. Adv. Exp. Med. Biol. 2020, 1250, 15–31.
- Zhao, X.; Liu, L.; Wang, J.; Xu, Y.F.; Zhang, W.M.; Khang, G.; Wang, X. In vitro vascularization of a combined system based on a 3D bioprinting technique. J. Tissue Eng. Regen. Med. 2016, 10, 833–842.
- Yao, Q.; Zheng, Y.W.; Lan, Q.H.; Kou, L.; Xu, H.L.; Zhao, Y.Z. Recent development and biomedical applications of decellularized extracellular matrix biomaterials. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 104, 109942.
- Kim, B.S.; Kwon, Y.W.; Kong, J.S.; Park, G.T.; Gao, G.; Han, W.; Kim, M.B.; Lee, H.; Kim, J.H.; Cho, D.W. 3D cell printing of in vitro stabilized skin model and in vivo pre-vascularized skin patch using tissue-specific extracellular matrix bioink: A step towards advanced skin tissue engineering. Biomaterials 2018, 168, 38–53.
- Taylor, D.A.; Sampaio, L.C.; Ferdous, Z.; Gobin, A.S.; Taite, L.J. Decellularized matrices in regenerative medicine. Acta Biomater. 2018, 74, 74–89.
- Kim, J.; Kim, M.; Hwang, D.G.; Shim, I.K.; Kim, S.C.; Jang, J. Pancreatic tissue-derived extracellular matrix bioink for printing 3D cell-laden pancreatic tissue constructs. J. Vis. Exp. 2019, 154, e60434.
- Wang, X.; Tuomi, J.; Mäkitie, A.A.; Poloheimo, K.-S.; Partanen, J.; Yliperttula, M. The integrations of biomaterials and rapid prototyping techniques for intelligent manufacturing of complex organs. In Advances in Biomaterials Science and Applications in Biomedicine; Lazinica, R., Ed.; InTech: Rijeka, Croatia, 2013; pp. 437–463.
- Wang, D.; Guo, Y.; Zhu, J.; Liu, F.; Xue, Y.; Huang, Y.; Zhu, B.; Wu, D.; Pan, H.; Gong, T.; et al. Hyaluronic acid methacrylate/pancreatic extracellular matrix as a potential 3D printing bioink for constructing islet organoids. Acta Biomater. 2022, 22, 00375.
More
Information
Subjects:
Engineering, Biomedical
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.4K
Entry Collection:
Biopharmaceuticals Technology
Revisions:
3 times
(View History)
Update Date:
12 Dec 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




