Diabetes is the most common chronic disease in the world, and it brings a heavy burden to people’s health. Against this background, diabetic research, including islet functionalization has become a hot topic in medical institutions all over the world. Especially with the rapid development of microencapsulation and three-dimensional (3D) bioprinting technologies, organ engineering and manufacturing have become the main trends for disease modeling and drug screening. Especially the advanced 3D models of pancreatic islets have shown better physiological functions than monolayer cultures, suggesting their potential in elucidating the behaviors of cells under different growth environments.
- 3D bioprinting
- organ engineering/manufacturing
- vascularization
1. Natural Polymers for Pancreas 3D Printing
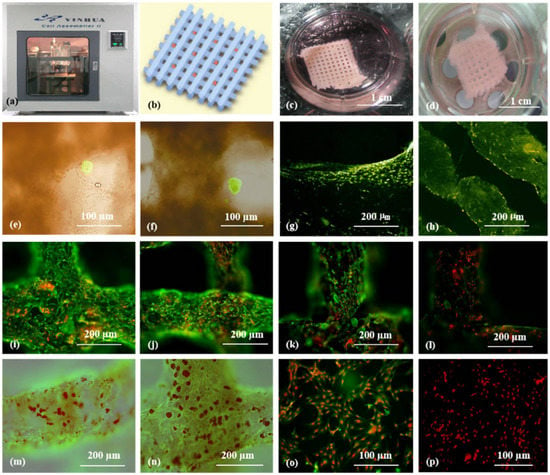
2. Synthetic Polymers for Pancreas 3D Printing

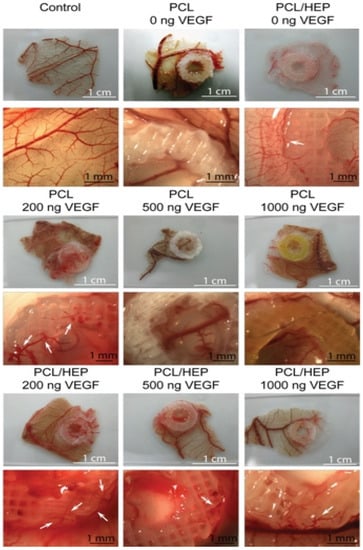
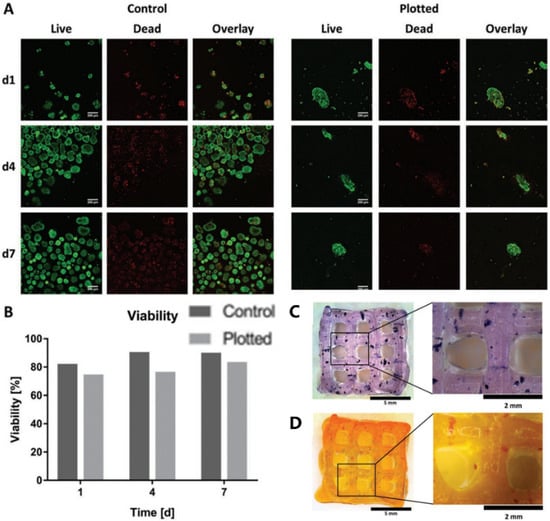
3. ECM and dECM for Pancreas 3D Printing
- Barnett, R. Type 1 diabetes. Lancet. 2018, 20, 391(10117):195, https://doi.org/ 10.1016/S0140-6736(18)30024-2.
- Gloyn, A.L.; Drucker, D.J. Precision medicine in the management of type 2 diabetes. Lancet Diabetes Endocrinol. 2018, 11, 891-900, https://doi.org/ 10.1016/S2213-8587(18)30052-4.
- Bjerg, L.; Gudbjörnsdottir, S.; Franzén, S.; Carstensen, B.; Witte, D.R.; Jørgensen, M.E.; Svensson, A.M. Duration of diabetes-related complications and mortality in type 1 diabetes: a national cohort study. Int. J. 202, 4, 1250-1259, https://doi.org/ 10.1093/ije/dyaa290.
- Ahearn, A.J.; Parekh, J.R.; Posselt, AM. Islet transplantation for Type 1 diabetes: where are we now? Expert Rev Clin Immunol. 2015, 1, 59-68, https://doi.org/ 10.1586/1744666X.2015.978291.
- Menger, M.M.; Nalbach, L.; Roma, L.P.; Körbel, C.; Wrublewsky, S.; Glanemann, M.; Laschke, M.W.; Menger, M.D.; Ampofo, E. Erythropoietin accelerates the revascularization of transplanted pancreatic islets. Br J Pharmacol. 2020, 7, 1651-1665, https://doi.org/ 10.1111/bph.14925.
- Troppmann, C. Complications after pancreas transplantation. Curr Opin Organ Transplant. 2010, 1, 112-8, https://doi.org/ 10.1097/MOT.0b013e3283355349.
- Gibly, R.F.; Graham J.G.; Luo, X.; Lowe, W.L. Jr; Hering, B.J.; Shea, L.D. Advancing islet transplantation: from engraftment to the immune response. 2011, 10, 2494-505, https://doi.org/ 10.1007/s00125-011-2243-0.
- Hyder, A.; Laue, C.; Schrezenmeir, J. Effect of the immunosuppressive regime of Edmonton protocol on the long-term in vitro insulin secretion from islets of two different species and age categories. Toxicol In Vitro. 2005, 4, 541-6, https://doi.org/ 10.1016/j.tiv.2005.01.005.
- Sakai, S.; Inagaki, H.; Inamoto, K.; Taya, M. Wrapping tissues with a pre-established cage-like layer composed of living cells. 2012, 28, 6721-7, https://doi.org/ 10.1016/j.biomaterials.2012.06.027.
- Desai, T.; Shea, L.D. Advances in islet encapsulation technologies. Nat Rev Drug Discov. 2017, 5, 338-350, https://doi.org/ 10.1038/nrd.2016.232.
- Strand, B.L.; Coron, A.E.; Skjak-Braek, G. Current and future perspectives on alginate encapsulated pancreatic i Stem Cells Transl Med. 2017, 4, 1053-1058, https://doi.org/ 10.1002/sctm.16-0116.
- Song, D.; Xu, Y.; Liu, S.; Wen, L.; Wang, X. Progress of 3D bioprinting in organ m Polymers (Basel). 2021, 18, 3178, https://doi.org/ 10.3390/polym13183178.
- Zhou, Q.; Melton DA. Pancreas regeneration. 2018, 7705, 351-358, https://doi.org/ 10.1038/s41586-018-0088-0.
- van der, Meulen T.; Mawla, A.M.; DiGruccio, M.R.; Adams, M.W.; Nies, V.; Dólleman, S.; Liu, S.; Ackermann, A.M.; Cáceres, E.; Hunter, A.E.; Kaestner, K.H.; Donaldson, C.J.; Huising, M.O. Virgin beta cells persist throughout life at a neogenic niche within pancreatic i Cell Metab. 2017, 4, 911-926.e6, https://doi.org/ 10.1016/j.cmet.2017.03.017.
- Lemaire, K.; Thorrez, L.; Schuit, F. Disallowed and allowed gene expression: two faces of mature islet beta c Annu Rev Nutr. 2016, 36, 45-71, https://doi.org/ 10.1146/annurev-nutr-071715-050808.
- Rutter, G.A.; Pullen, T.J.; Hodson, D.J.; Martinez-Sanchez, A. Pancreatic β-cell identity, glucose sensing and the control of insulin secretion. Biochem J. 2015, 2, 203-18, https://doi.org/ 10.1042/BJ20141384.
- Xu, Y.; Li, D.; Wang, X. The construction of vascularized pancreas based on 3D printing techniques. In: Organ Manufacturing; Wang, X., Ed.; Nova Science Publishers Inc.: Hauppauge, NY, USA, 2015, pp, 245–268.
- Espona-Noguera, A.; Ciriza, J.; Cañibano-Hernández, A.; Villa, R.; Saenz Del Burgo, L.; Alvarez, M.; Pedraz, J.L. 3D printed polyamide macroencapsulation devices combined with alginate hydrogels for insulin-producing cell-based therapies. Int J Pharm. 2019, 566, 604-614, https://doi.org/ 10.1016/j.ijpharm.2019.06.009.
- Borg, D.J.; Bonifacio, E. The use of biomaterials in islet transplantation. Curr Diab Rep. 2011, 5, 434-44, https://doi.org/ 10.1007/s11892-011-0210-2.
- Kothale, D.; Verma, U.; Dewangan, N.; Jana, P.; Jain, A.; Jain, D. Alginate as promising natural polymer for pharmaceutical, food, and biomedical a Curr Drug Deliv. 2020, 9, 755-775, https://doi.org/ 10.2174/1567201817666200810110226.
- Hogan, M.F.; Hull, R.L. The islet endothelial cell: a novel contributor to beta cell secretory dysfunction in diabetes. 2017, 6, 952-959, https://doi.org/ 10.1007/s00125-017-4272-9.
- Vegas, A.J.; Veiseh, O.; Gürtler, M.; Millman, J.R.; Pagliuca, F.W.; Bader, A.R.; Doloff, J.C.; Li, J.; Chen, M.; Olejnik, K.; Tam, H.H.; Jhunjhunwala, S.; Langan, E.; Aresta-Dasilva, S.; Gandham, S.; McGarrigle, J.J.; Bochenek, M.A.; Hollister-Lock, J.; Oberholzer, J.; Greiner, D.L.; Weir, G.C.; Melton, D.A.; Langer, R.; Anderson, D.G. Long-term glycemic control using polymer-encapsulated human stem cell-derived beta cells in immune-competent mice. Nat Med. 2016, 3, 306-11, https://doi.org/ 10.1038/nm.4030.
- Cao, H.; Lee, M.K.H.; Yang.; H, Sze, S.K.; Tan, N.S.; Tay, C.Y. Mechanoregulation of cancer-associated fibroblast phenotype in three-dimensional interpenetrating hydrogel n Langmuir. 2019, 23, 7487-7495, https://doi.org/ 10.1021/acs.langmuir.8b02649.
- Wang, X.; Yan, Y.; Zhang, R. Gelatin-based hydrogels for controlled cell assembly. In: Biomedical Applications of Hydrogels Handbook; Ottenbrite, R.M., Ed.; Springer: New York, NY, USA, 2010, pp, 269–284.
- Dufrane, D.; Gianello, P. Pig islet for xenotransplantation in human: structural and physiological compatibility for human clinical application. Transplant Rev (Orlando). 2012, 3, 183-8, https://doi.org/ 10.1016/j.trre.2011.07.004.
- Thompson, P.; Cardona, K.; Russell, M.; Badell, I.R.; Shaffer, V.; Korbutt, G.; Rayat, G.R.; Cano, J.; Song, M.; Jiang, W.; Strobert, E.; Rajotte, R.; Pearson, T.; Kirk, A.D.; Larsen, C.P. CD40-specific costimulation blockade enhances neonatal porcine islet survival in nonhuman primates. Am J Transplant. 2011, 5, 947-57, https://doi.org/ 10.1111/j.1600-6143.2011.03509.x.
- Zhang, C.; Aung, A.; Liao, L.; Varghese, S. A novel single precursor-based biodegradable hydrogel with enhanced mechanical properties. Soft Matter. 2009, 5, 3831–3834, https://doi.org/ 10.1039/b912102a.
- Lin, S.; Sangaj, N.; Razafiarison, T.; Zhang, C.; Varghese, S. Influence of physical properties of biomaterials on cellular behavior. Pharm Res. 2011, 6, 1422-30, https://doi.org/ 10.1007/s11095-011-0378-9.
- Marchioli, G.; Zellner, L.; Oliveira, C.; Engelse, M.; Koning, E.; Mano, J.; Karperien; Apeldoorn, A.V.; Moroni, L. Layered PEGDA hydrogel for islet of Langerhans encapsulation and improvement of vascularization. J Mater Sci Mater Med. 2017, 12, 195, https://doi.org/ 10.1007/s10856-017-6004-6.
- Wertz, I.E.; Newton, K.; Seshasayee, D.; Kusam, S.; Lam, C.; Zhang, J.; Popovych, N.; Helgason, E.; Schoeffler, A.; Jeet, S.; Ramamoorthi, N.; Kategaya, L.; Newman, R.J.; Horikawa, K.; Dugger, D.; Sandoval, W.; Mukund, S.; Zindal, A.; Martin, F.; Quan, C.; Tom, J.; Fairbrother, W.J.; Townsend, M.; Warming, S.; DeVoss, J.; Liu, J.; Dueber, E.; Caplazi, P.; Lee, W.P.; Goodnow, C.C.; Balazs, M.; Yu, K.; Kolumam, G.; Dixit, V.M. Phosphorylation and linear ubiquitin direct A20 inhibition of inflammation. 2015, 7582, 370-5, https://doi.org/ 10.1038/nature16165.
- Bai, X.; Pei, Q.; Pu, C.; Chen, Y.; He, S.; Wang, B. Multifunctional islet transplantation hydrogel encapsulating A20 high-expressing i Drug Des Devel Ther. 2020, 14, 4021-4027, https://doi.org/ 10.2147/DDDT.S273050.
- Su, J.; Hu, B.H.; Lowe, W.L. Jr; Kaufman, D.B.; Messersmith, P.B. Anti-inflammatory peptide-functionalized hydrogels for insulin-secreting cell encapsulation. 2010, 2, 308-14, https://doi.org/ 10.1016/j.biomaterials.2009.09.045.
- Soleymaninejadian, E.; Pramanik, K.; Samadian, E. Immunomodulatory properties of mesenchymal stem cells: cytokines and factors. Am J Reprod Immunol. 2012, 1, 1-8, https://doi.org/ 10.1111/j.1600-0897.2011.01069.x.
- Vaithilingam, V.; Evans, MDM.; Lewy, D.M.; Bean, P.A.; Bal, S.; Tuch, B.E. Co-encapsulation and co-transplantation of mesenchymal stem cells reduces pericapsular fibrosis and improves encapsulated islet survival and function when allografted. Sci Rep. 2017, 1, 10059.
- Mochizuki, Y.; Kogawa, R.; Takegami, R.; Nakamura, K.; Wakabayashi, A.; Ito, T.; Yoshioka, Y. Co-microencapsulation of islets and MSC CellSaics, Mosaic-like aggregates of MSCs and recombinant peptide pieces, and therapeutic effects of their subcutaneous transplantation on d Biomedicines. 2020, 9, 318, https://doi.org/ 10.3390/biomedicines8090318.
- Weaver, J.D.; Headen, D.M.; Hunckler, M.D.; Coronel, M.M.; Stabler, C.L.; García, A.J. Design of a vascularized synthetic poly(ethylene glycol) macroencapsulation device for islet transplantation. 2018, 172, 54-65, https://doi.org/ 10.1016/j. biomaterials.2018.04.047.
- Weaver, J.D.; Headen, D.M.; Coronel, M.M.; Hunckler, M.D.; Shirwan, H.; García, A.J. Synthetic poly(ethylene glycol)-based microfluidic islet encapsulation reduces graft volume for delivery to highly vascularized and retrievable transplant site. Am. J. 2019, 5, 1315-1327, https://doi.org/ 10.1111/ajt.15168.
- Pham, T.T.; Nguyen, T.T.; Pathak, S.; Regmi, S.; Nguyen, H.T.; Tran, T.H.; Yong, C.S.; Kim, J.O.; Park, P.H.; Park, M.H.; Bae, Y.K.; Choi, J.U.; Byun, Y.; Ahn, C.H.; Yook, S.; Jeong, J.H. Tissue adhesive FK506-loaded polymeric nanoparticles for multi-layered nano-shielding of pancreatic islets to enhance xenograft survival in a diabetic mouse model. 2018, 154, 182-196, https://doi.org/ 10.1016/j.biomaterials.2017.10.049.
- Ullah, I.; Busch, J.F.; Rabien, A.; Ergün, B.; Stamm, C.; Knosalla, C. Adult tissue extracellular matrix determines tissue specification of human iPSC-derived embryonic stage mesodermal precursor cells. Adv. 2020, 7, 1901198.
- Raza, F.; Zafar, H.; Zhu, Y.; Ren, Y.; Ullah, A.; Khan, A.U.; He, X.; Han, H.; Aquib, M.; Boakye-Yiadom, K.O.; Ge, L. A Review on recent advances in stabilizing peptides/proteins upon fabrication in hydrogels from biodegradable p Pharmaceutics. 2018, 1, 16, https://doi.org/ 10.3390/pharmaceutics10010016.
- Kim, H.; Kim, S.W.; Lee, S.; Kim, S.C.; Cho, D.W.; Jang, J. 3D cell printing of islet-laden pancreatic tissue-derived extracellular matrix bioink constructs for enhancing pancreatic functions. J. Mater. Chem. 2019, 10, 1773-1781, https://doi.org/ 10.1039/c8tb02787k.
- Czerwinski, M.; Spence, J.R. Hacking the Matrix. Cell Stem Cell. 2017, 1, 9-10, https://doi.org/ 10.1016/j.stem.2016.12.010.
- Freytes, D.O.; Martin, J.; Velankar, S.S.; Lee, A.S.; Badylak, S.F. Preparation and rheological characterization of a gel form of the porcine urinary bladder matrix. 2008, 11, 1630-7, https://doi.org/ 10.1016/j.biomaterials.2007.12.014.
- Giobbe, G.G.; Crowley, C.; Luni, C.; Campinoti, S.; Khedr, M.; Kretzschmar, K.; De Santis, M.M.; Zambaiti, E.; Michielin, F.; Meran, L.; Hu, Q.; van Son, G.; Urbani, L.; Manfredi, A.; Giomo, M.; Eaton, S.; Cacchiarelli, D.; Li, V.S.W.; Clevers, H.; Bonfanti, P.; Elvassore, N.; De Coppi, P. Extracellular matrix hydrogel derived from decellularized tissues enables endodermal organoid culture. Nat. 2019, 1, 5658, https://doi.org/ 10.1038/s41467-019-13605-4.
- Wang, J.K.; Cheam, N.M.J.; Irvine, S.A.; Tan, N.S.; Venkatraman, S.; Tay, C.Y. Interpenetrating network of alginate-human adipose extracellular matrix hydrogel for islet cells e Macromol. Rapid Commun. 2020, 21, e2000275, https://doi.org/ 10.1002/marc.202000275.
- Yang, F.; Williams, C.G.; Wang, D.A.; Lee, H.; Manson, P.N.; Elisseeff J. The effect of incorporating RGD adhesive peptide in polyethylene glycol diacrylate hydrogel on osteogenesis of bone marrow stromal cells. 2005, 30, 5991-8, https://doi.org/ 10.1016/j.biomaterials.2005.03.018.
- Wang, X.; He, K.; Zhang, W. Optimizing the fabrication processes for manufacturing a hybrid hierarchical polyurethane-cell/hydrogel construct. Bioact. Compat. Polym. 2013, 28, 303–319.
- Wang, X.; Liu, C. 3D Bioprinting of adipose-derived stem cells for organ m Adv Exp Med Biol. 2018, 1078, 3-14, https://doi.org/ 10.1007/978-981-13-0950-2_1.
- Wang, X.; Ao, Q.; Tian, X.; Fan, J.; Tong, H.; Hou, W.; Bai, S. Gelatin-based hydrogels for organ 3D b Polymers (Basel). 2017, 9, 401, https://doi.org/ 10.3390/polym9090401.
- Zhu, W.; Qu, X.;, Zhu, J.; Ma, X.; Patel, S.; Liu, J.; Wang, P.; Lai, C.S.; Gou, M.; Xu, Y.; Zhang, K.; Chen, S. Direct 3D bioprinting of prevascularized tissue constructs with complex microarchitecture. 2017, 124, 106-115, https://doi.org/ 10.1016/j.biomaterials.2017.01.042.
- Baltazar, T.; Merola, J.; Catarino, C.; Xie, C.B.; Kirkiles-Smith, N.C.; Lee, V.; Hotta, S.; Dai, G.; Xu, X.; Ferreira, F.C.; Saltzman, W.M.; Pober, J.S.; Karande, P. Three dimensional bioprinting of a vascularized and perfusable skin graft using human keratinocytes, fibroblasts, pericytes, and endothelial c Tissue Eng. Part A. 2020, 5-6, 227-238, https://doi.org/ 10.1089/ten.TEA.2019.0201.
- Li, S.; Tian, X.; Fan, J.; Tong, H.; Ao, Q.; Wang, X. Chitosans for tissue repair and organ three-dimensional (3D) bioprinting. Micromachines 2019, 10, 765, https://doi.org/10.3390/mi10110765.
- Lam, T.; Dehne, T.; Krüger, J.P.; Hondke, S.; Endres, M.; Thomas, A.; Lauster, R.; Sittinger, M.; Kloke, L. Photopolymerizable gelatin and hyaluronic acid for stereolithographic 3D bioprinting of tissue-engineered cartilage. J Biomed Mater Res B Appl Biomater. 2019, 8, 2649-2657, https://doi.org/ 10.1002/jbm.b.34354.
- Lee, A.; Hudson, A.R.; Shiwarski, D.J.; Tashman, J.W.; Hinton, T.J.; Yerneni, S.; Bliley, J.M.; Campbell, P.G.; Feinberg, A.W. 3D bioprinting of collagen to rebuild components of the human heart. 2019, 6452, 482-487, https://doi.org/ 10.1126/science.aav9051.
- Wang, X.; Yan, Y.; Pan, Y.; Xiong, Z.; Liu, H.; Cheng, J.; Liu, F.; Lin, F.; Wu, R.; Zhang, R.; Lu, Q. Generation of three-dimensional hepatocyte/gelatin structures with rapid prototyping system. Tissue Eng. 2006, 1, 83-90, https://doi.org/ 10.1089/ten.2006.12.83.
- Gao, G.; Hubbell, K.; Schilling, A.F.; Dai, G.; Cui, X. Bioprinting cartilage tissue from mesenchymal stem cells and PEG hydrogel. Methods Mol. 2017, 1612, 391–398, https://doi.org/10.1007/978-1-4939-7021-6_28.
- Wickramasinghe, S.; Do, T.; Tran, P. FDM-based 3D printing of polymer and associated composite: A review on mechanical properties, defects and treatments. Polymers 2020, 12, 1529, https://doi.org/10.3390/polym12071529.
- Ding, L.; Lu, W.; Zhang, J.; Yang, C.; Wu, G. Preparation and performance evaluation of duotone 3D-printed polyetheretherketone as oral prosthetic materials: A proof-of-concept study. Polymers 2021, 13, 1949, https://doi.org/10.3390/polym13121949.
- Rutz, A.L.; Gargus, E.S.; Hyland, K.E.; Lewis, P.L.; Setty, A.; Burghardt, W.R.; Shah, R.N. Employing PEG crosslinkers to optimize cell viability in gel phase bioinks and tailor post printing mechanical properties. Acta Biomater. 2019, 99, 121–132, https://doi.org/10.1016/j.actbio.2019.09.007.
- Gu, Y.; Zhang, L.; Du, X.; Fan, Z.; Wang, L.; Sun, W.; Cheng, Y.; Zhu, Y.; Chen, C. Reversible physical crosslinking strategy with optimal temperature for 3D bioprinting of human chondrocyte-laden gelatin methacryloyl bioink. Biomater. Appl. 2018, 33, 609–618, https://doi.org/10.1177/0885328218805864.
- Quan, H.; Zhang, T.; Xu, H.; Luo, S.; Nie, J.; Zhu, X. Photo-curing 3D printing technique and its challenges. Mater. 2020, 22, 110–115, https://doi.org/10.1016/j.bioactmat.2019.12.003.
- Taghipour, Y.D.; Hokmabad, V.R.; Del Bakhshayesh, A.R.; Asadi, N.; Salehi, R.; Nasrabadi, H.T. The application of hydrogels based on natural polymers for tissue engineering. Med. Chem. 2020, 27, 2658–2680, https://doi.org/10.2174/0929867326666190711103956.
- Hung, B.P.; Naved, B.A.; Nyberg, E.L.; Dias, M.; Holmes, C.; Elisseeff, J.H.; Dorafshar, A.; Grayson, W.L. Three-dimensional printing of bone extracellular matrix for craniofacial regeneration. ACS Biomater. Eng. 2016, 2, 1806–1816, https://doi.org/10.1021/acsbiomaterials.6b00101.
- Mott, E.J.; Busso, M.; Luo, X.; Dolder, C.; Wang, M.O.; Fisher, J.P.; Dean, D. Digital micromirror device (DMD)-based 3D printing of poly(propylene fumarate) scaffolds. Sci. Eng. C Mater. Biol. Appl. 2016, 61, 301–311, https://doi.org/10.1016/j.msec.2015.11.071.
- Wang, X.; Yan, Y.; Zhang, R. Rapid prototyping as a tool for manufacturing bioartificial livers. Trends Biotechnol. 2007, 25, 505–513, https://doi.org/10.1016/j.tibtech.2007.08.010.
- Li, S.; Yan, Y.; Xiong, Z.; Weng, C.; Zhang, R.; Wang, X. Gradient hydrogel construct based on an improved cell assembling system. Bioact. Compat. Polym. 2009, 24, 84–99.
- Mahendiran, B.; Muthusamy, S.; Sampath, S.; Jaisankar, S.N.; Popat, K.C.; Selvakumar, R.; Krishnakumar, G.S. Recent trends in natural polysaccharide based bioinks for multiscale 3D printing in tissue regeneration: A review. Int J Biol Macromol. 2021, 183, 564-588, https://doi.org/ 10.1016/j.ijbiomac.2021.04.179.
- Hong, H.; Seo, Y.B.; Kim, D.Y.; Lee, J.S.; Lee, Y.J.; Lee, H.; Ajiteru, O.; Sultan, M.T.; Lee, O.J.; Kim, S.H.; Park, C.H. Digital light processing 3D printed silk fibroin hydrogel for cartilage tissue engineering. 2020, 232, 119679, https://doi.org/ 10.1016/j.biomaterials.2019.119679.
- Wang, X. Spatial effects of stem cell engagement in 3D printing constructs. Stem. Cells Res. Rev. Rep. 2014, 1, 5–9.
- Wang, X. 3D printing of tissue/organ analogues for regenerative medicine. In: Handbook of Intelligent Scaffolds for Regenerative Medicine, 2nd ed.; Pan Stanford Publishing: Palo Alto, CA, USA, 2016, pp, 557–570.
- Erkoc, P.; Uvak, I.; Nazeer, M.A.; Batool, S.R.; Odeh, Y.N.; Akdogan, O.; Kizilel, S. 3D Printing of cytocompatible gelatin-cellulose-alginate blend h Macromol Biosci. 2020, 10, e2000106, https://doi.org/ 10.1002/mabi.202000106.
- Jose, G.; Shalumon, K.T.; Chen, J.P. Natural polymers based hydrogels for cell culture a Curr Med Chem. 2020, 16, 2734-2776, https://doi.org/ 10.2174/0929867326666190903113004.
- Luetchford, K.A.; Chaudhuri, J.B.; De Bank, P.A. Silk fibroin/gelatin microcarriers as scaffolds for bone tissue engineering. Sci. Eng. C 2020, 106, 110116.
- Xu, M.; Yan, Y.; Liu, H.; Yao, Y.; Wang, X. Control adipose-derived stromal cells differentiation into adipose and endothelial cells in a 3D structure established by cell-assembly technique. Bioact. Compat. Polym. 2009, 24, 31–47.
- Wang X. Advanced polymers for three-dimensional (3D) organ b Micromachines (Basel). 2019, 12, 814, https://doi.org/ 10.3390/mi10120814.
- Duin, S.; Schütz, K.; Ahlfeld, T.; Lehmann, S.; Lode, A.; Ludwig, B.; Gelinsky, M. 3D bioprinting of functional islets of langerhans in an alginate/methylcellulose hydrogel b Adv. Healthc. Mater. 2019, 7, e1801631, https://doi.org/ 10.1002/adhm.201801631.
- Hu, S.; Martinez-Garcia, F.D.; Moeun, B.N.; Burgess, J.K.; Harmsen, M.C.; Hoesli, C.; de Vos, P. An immune regulatory 3D-printed alginate-pectin construct for immunoisolation of insulin producing β-cells. Mater. Sci. Eng. C Mater. Biol. 2021, 123, 112009, https://doi.org/ 10.1016/j.msec.2021.112009.
- Huang, Y.; He, K.; Wang, X. Rapid Prototyping of a hybrid hierarchical polyurethane-cell/hydrogel construct for regenerative medicine. Sci. Eng. C 2013, 33, 3220–3229.
- Xu, W.; Wang, X.; Yan, Y.; Zhang, R. Rapid prototyping of polyurethane for the creation of vascular systems. Bioact. Compat. Polym. 2008, 23, 103–114.
- Ashwin, B.; Abinaya, B.; Prasith, T.P.; Chandran, S.V.; Yadav, L.R.; Vairamani, M.; Patil, S.; Selvamurugan, N. 3D-poly (lactic acid) scaffolds coated with gelatin and mucic acid for bone tissue engineering. Int. J. Biol. 2020, 162, 523-532, https://doi.org/ 10.1016/j.ijbiomac.2020.06.157.
- Babilotte, J.; Martin, B.; Guduric, V.; Bareille, R.; Agniel, R.; Roques, S.; Héroguez, V.; Dussauze, M.; Gaudon, M.; Le Nihouannen, D.; Catros, S. Development and characterization of a PLGA-HA composite material to fabricate 3D-printed scaffolds for bone tissue engineering. Mater Sci Eng C Mater Biol Appl. 2021, 118, 111334, https://doi.org/ 10.1016/j.msec.2020.111334.
- Hung, K.C.; Tseng, C.S.; Dai, L.G.; Hsu, S.H. Water-based polyurethane 3D printed scaffolds with controlled release function for customized cartilage tissue engineering. 2016, 83, 156-68, https://doi.org/ 10.1016/j.biomaterials.2016.01.019.
- Liu, F.; Chen, Q.; Liu, C.; Ao, Q.; Tian, X.; Fan, J.; Tong, H.; Wang, X. Natural polymers for organ 3D b Polymers (Basel). 2018, 11, 1278, https://doi.org/ 10.3390/polym10111278.
- Lei, M.; Wang, X. Biodegradable polymers and stem cells for b Molecules 2016, 21, 539, https://doi.org/10.3390/molecules21050539.
- Farina, M.; Ballerini, A.; Fraga, D.W.; Nicolov, E.; Hogan, M.; Demarchi, D.; Scaglione, F.; Sabek, O.M.; Horner, P.; Thekkedath, U.; Gaber, O.A.; Grattoni, A. 3D printed vascularized device for subcutaneous transplantation of human i Biotechnol J. 2017, 9, https://doi.org/ 10.1002/biot.201700169.
- Marchioli, G.; Luca, A.D.; de Koning, E.; Engelse, M.; Van Blitterswijk, C.A.; Karperien, M.; Van Apeldoorn, A.A.; Moroni, L. Hybrid polycaprolactone/alginate scaffolds functionalized with VEGF to promote de Novo vessel formation for the transplantation of islets of Langerhans. Adv Healthc Mater. 2016, 13, 1606-16, https://doi.org/ 10.1002/adhm.201600058.
- Foyt, D.A.; Norman, M.D.A.; Yu, T.T.L.; Gentleman, E. Exploiting Advanced Hydrogel Technologies to Address Key Challenges in Regenerative Medicine. Adv Healthc Mater. 2018, 8, e1700939, https://doi.org/ 10.1002/adhm.201700939.
- Ge, F.; Lu ,Y.; Li, Q.; Zhang, X. Decellularized extracellular matrices for tissue engineering and r Adv Exp Med Biol. 2020, 1250, 15-31, https://doi.org/ 10.1007/978-981-15-3262-7_2.
- Zhao, X.; Liu, L.; Wang, J.; Xu, Y.F.; Zhang, W.M.; Khang, G.; Wang, X. In vitro vascularization of a combined system based on a 3D bioprinting technique. Tissue Eng. Regen. Med. 2016, 10, 833–842.
- Yao, Q.; Zheng, Y.W.; Lan, Q.H.; Kou, L.; Xu, H.L.; Zhao, Y.Z. Recent development and biomedical applications of decellularized extracellular matrix biomaterials. Mater. Sci. Eng. C Mater. Biol. 2019, 104, 109942, https://doi.org/ 10.1016/j.msec.2019.109942.
- Kim, B.S.; Kwon, Y.W.; Kong, J.S.; Park, G.T.; Gao, G.; Han, W.; Kim, M.B.; Lee, H.; Kim, J.H.; Cho, D.W. 3D cell printing of in vitro stabilized skin model and in vivo pre-vascularized skin patch using tissue-specific extracellular matrix bioink: A step towards advanced skin tissue engineering. 2018, 168, 38-53, https://doi.org/ 10.1016/j.biomaterials.2018.03.040.
- Taylor, D.A.; Sampaio, L.C.; Ferdous, Z.; Gobin, A.S.; Taite, L.J. Decellularized matrices in regenerative medicine. Acta Biomater. 2018, 74, 74-89, https://doi.org/ 10.1016/j.actbio.2018.04.044.
- Kim, J.; Kim, M.; Hwang, D.G.; Shim, I.K.; Kim, S.C.; Jang, J. Pancreatic tissue-derived extracellular matrix bioink for printing 3D cell-laden pancreatic tissue c J. Vis. Exp. 2019, 154, https://doi.org/ 10.3791/60434.
- Wang, X.; Tuomi, J.; Mäkitie, A.A.; Poloheimo, K.-S.; Partanen, J.; Yliperttula, M. The integrations of biomaterials and rapid prototyping techniques for intelligent manufacturing of complex organs. In: Advances in Biomaterials Science and Applications in Biomedicine; Lazinica, R., Ed.; InTech: Rijeka, Croatia:2013; pp. 437–463.
- Wang, D.; Guo, Y.; Zhu, J.; Liu, F.; Xue, Y.; Huang, Y.; Zhu, B.; Wu, D.; Pan, H.; Gong, T.; Lu, Y.; Yang, Y.; Wang, Z. Hyaluronic acid methacrylate/pancreatic extracellular matrix as a potential 3D printing bioink for constructing islet organoids. Acta Biomater. 2022, 22, 00375-0, https://doi.org/ 10.1016/j.actbio.2022.06.036.
- Yan, Y.; Wang, X.; Pan, Y.; Liu, H.; Cheng, J.; Xiong, Z.; Lin, F.; Wu, R.; Zhang, R.; Lu, Q. Fabrication of viable tissue-engineered constructs with 3D cell-assembly technique. Biomaterials 2005, 26, 5864–5871.
- Yan, Y.; Wang, X.; Xiong, Z.; Liu, H.; Liu, F.; Lin, F.; Wu, R.; Zhang, R.; Lu, Q. Direct construction of a three-dimensional structure with cells and hydrogel. Bioact. Compat. Polym. 2005, 20, 259–269.
- Xu, W.; Wang, X.; Yan, Y.; Zheng, W.; Xiong, Z.; Lin, F.; Wu, R.; Zhang, R. Rapid prototyping three-dimensional cell/gelatin/fibrinogen constructs for medical regeneration. Bioact. Compat. Polym. 2007, 22, 363–377.
- Zhang, T.; Yan, Y.; Wang, X.; Xiong, Z.; Lin, F.; Wu, R.; Zhang, R. Three-dimensional gelatin and gelatin/hyaluronan hydrogel structures for traumatic brain injury. Bioact. Compat. Polym. 2007, 22, 19–29.
- Xu, W.; Wang, X.; Yan, Y.; Zhang, R. A polyurethane-gelatin hybrid construct for the manufacturing of implantable bioartificial livers. Bioact. Compat. Polym. 2008, 23, 409–422.
- Li, S.; Xiong, Z.; Wang, X.; Yan, Y.; Liu, H.; Zhang, R. Direct fabrication of a hybrid cell/hydrogel construct by a double-nozzle assembling technology. Bioact. Compat. Polym. 2009, 24, 249–265.
- Xu, M.; Wang, X.; Yan, Y.; Yao, R.; Ge, Y. A cell-assembly derived physiological 3D model of the metabolic syndrome, based on adipose-derived stromal cells and a gelatin/alginate/fibrinogen matrix. Biomaterials 2010, 31, 3868–3877.
- Sui, S.; Wang, X.; Liu, P.; Yan, Y.; Zhang, R. Cryopreservation of cells in 3D constructs based on controlled cell assembly processes. Bioact. Compat. Polym. 2009, 24, 473–48.
- Wang, X.; Xu, H. Incorporation of DMSO and dextran-40 into a gelatin/alginate hydrogel for controlled assembled cell cryopreservation. Cryobiology 2010, 61, 345–351.
- Cui, T.; Yan, Y.; Zhang, R.; Liu, L.; Xu, W.; Wang, X. Rapid prototyping of a double layer polyurethane-collagen conduit for peripheral nerve regeneration. Tissue Eng. C 2009, 15, 1–9.
- Wang, X.; Cui, T.; Yan, Y.; Zhang, R. Peroneal nerve regeneration along a new polyurethane-collagen guide conduit. Bioact. Compat. Polym. 2009, 24, 109–127.
- He, K.; Wang, X. Rapid prototyping of tubular polyurethane and cell/hydrogel construct. Bioact. Compat. Polym. 2011, 26, 363–374.
- Huang, Y.; He, K.; Wang, X. Rapid Prototyping of a hybrid hierarchical polyurethane-cell/hydrogel construct for regenerative medicine. M Sci. Eng. C 2013, 33, 3220–3229.
- Zhao, X.; Du, S.; Chai, L.; Xu, Y.; Liu, L.; Zhou, X.; Wang, J.; Zhang, W.; Liu, C.-H.; Wang, X. Anti-cancer drug screening based on an adipose-derived stem cell/hepatocyte 3D printing technique. Stem Cell Res. Ther. 2015, 5, 273.
- Zhou,; Liu, C.; Zhao, X.; Wang, X. A 3D bioprinting liver tumor model for drug screening. World J. Pharm. Pharm. Sci. 2016, 5, 196–213.
- Wang X. Bioartificial organ manufacturing t Cell Transplant. 2019, 1, 5-17, https://doi.org/ 10.1177/0963689718809918.
- Pati, F.; Gantelius, J.; Svahn, H.A. 3D bioprinting of tissue/organ m Angew Chem. Int. Ed. Engl. 2016, 15, 4650-65, https://doi.org/ 10.1002/anie.201505062.
- Wang, X. Overview on biocompatibilities of implantable biomaterials. In: Advances in Biomaterials Science and Biomedical Applications in Biomedicine; Lazinica, R., Ed.; InTech: Rijeka, Croatia, 2013, pp, 111–15.
- Dey, M.; Ozbolat, I.T. 3D bioprinting of cells, tissues and organs. Sci Rep. 2020, 1, 14023, https://doi.org/ 10.1038/s41598-020-70086-y. PMID: 32811864; PMCID: PMC7434768.
- Liu, F.; Wang X. Synthetic polymers for organ 3D p Polymers (Basel). 2020, 8, 1765, https://doi.org/ 10.3390/polym12081765.
- Hann, S.Y.; Cui, H.; Esworthy, T.; Miao, S.; Zhou, X.; Lee, S.J.; Fisher, J.P.; Zhang, L.G. Recent advances in 3D printing: vascular network for tissue and organ regeneration. Transl. 2019, 211 ,46-63, https://doi.org/ 10.1016/j.trsl.2019.04.002.
- Chen, E.P.; Toksoy, Z.; Davis, B.A.; Geibel, J.P. 3D Bioprinting of vascularized tissues for in vitro and in vivo a Front. Bioeng. Biotechnol. 2021, 9 ,664188, https://doi.org/ 10.3389/fbioe.2021.664188.
- Wang, Z.; Wang, L.; Li, T.; Liu, S.; Guo, B.; Huang, W.; Wu, Y. 3D bioprinting in cardiac tissue engineering. Theranostics 2021, 16, 7948–7969. https://doi.org/10.7150/thno.61621.
- Mandrycky, C.; Wang, Z.; Kim, K.; Kim, D.H. 3D bioprinting for engineering complex tissues. Biotech. Adv. 2016, 4, 422–434. https://doi.org/10.1016/j.biotechadv.2015.12.011.
- Matai, I.; Kaur, G.; Seyedsalehi, A.; McClinton, A.; Laurencin, C.T. Progress in 3D bioprinting technology for tissue/organ regenerative engineering. Biomaterials 2020, 226, 119536. https://doi.org/10.1016/j.biomaterials.2019.119536.
- Berg, J.; Kurreck, J. Clean bioprinting-fabrication of 3D organ models devoid of animal components. ALTEX, 2021, 2, 269–288. https://doi.org/10.14573/altex.2009151.
- Deo, K.A.; Singh, K.A.; Peak, C.W.; Alge, D.L.; Gaharwar, A.K. Bioprinting 101: design, fabrication, and evaluation of cell-laden 3D bioprinted s Tissue Eng. Part A, 2020, 5-6, 318–338. https://doi.org/10.1089/ten.TEA.2019.0298.
- Sorkio, A.; Koch, L.; Koivusalo, L.; Deiwick, A.; Miettinen, S.; Chichkov, B.; Skottman, H. Human stem cell based corneal tissue mimicking structures using laser-assisted 3D bioprinting and functional bioinks. Biomaterials. 2018, 171, 57–71. https://doi.org/10.1016/j.biomaterials.2018.04.034.
- Huh, D.; Hamilton, G.A.; Ingber, D.E. From 3D cell culture to organs-on-chips. Trends Cell Biol. 2011, 12, 745–754. https://doi.org/10.1016/j.tcb.2011.09.005.
- Zhu, J.; Wang, Y.; Zhong, L.; Pan, F.; Wang, J. Advances in tissue engineering of vasculature through three-dimensional bioprinting. Developmental dynamics: an official publication of the American Association of Anatomists, 2021, 12, 1717–1738. https://doi.org/10.1002/dvdy.385.
This entry is adapted from the peer-reviewed paper 10.3390/polym14235143
