Diabetes is the most common chronic disease in the world, and it brings a heavy burden to people’s health. Against this background, diabetic research, including islet functionalization has become a hot topic in medical institutions all over the world. Especially with the rapid development of microencapsulation and three-dimensional (3D) bioprinting technologies, organ engineering and manufacturing have become the main trends for disease modeling and drug screening. Especially the advanced 3D models of pancreatic islets have shown better physiological functions than monolayer cultures, suggesting their potential in elucidating the behaviors of cells under different growth environments.
- 3D bioprinting
- organ engineering/manufacturing
- vascularization
1. Natural Polymers for Pancreas 3D Printing
2. Synthetic Polymers for Pancreas 3D Printing

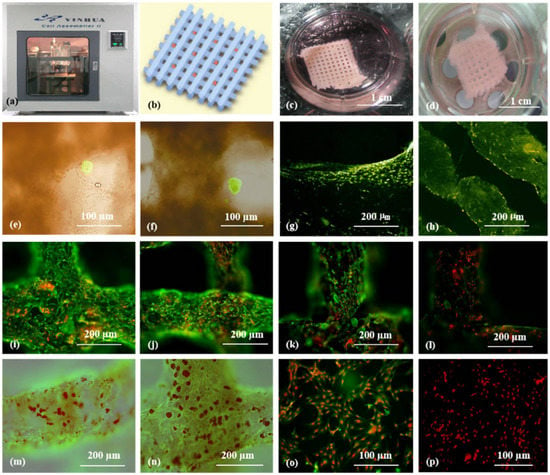
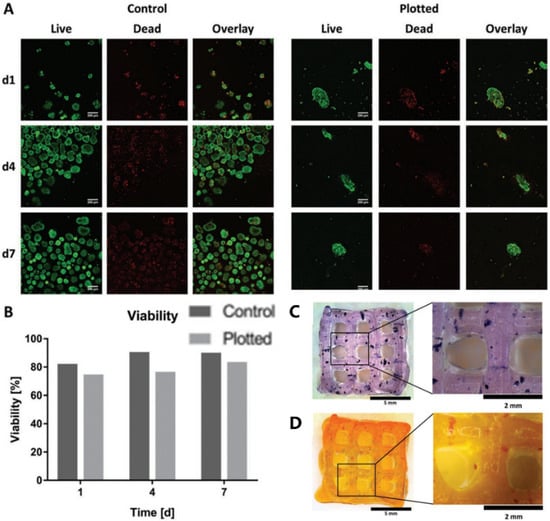
3. ECM and dECM for Pancreas 3D Printing
This entry is adapted from the peer-reviewed paper 10.3390/polym14235143
References
- Li, S.; Yan, Y.; Xiong, Z.; Weng, C.; Zhang, R.; Wang, X. Gradient hydrogel construct based on an improved cell assembling system. J. Bioact. Compat. Polym. 2009, 24, 84–99.
- Mahendiran, B.; Muthusamy, S.; Sampath, S.; Jaisankar, S.N.; Popat, K.C.; Selvakumar, R.; Krishnakumar, G.S. Recent trends in natural polysaccharide based bioinks for multiscale 3D printing in tissue regeneration: A review. Int. J. Biol. Macromol. 2021, 183, 564–588.
- Hong, H.; Seo, Y.B.; Kim, D.Y.; Lee, J.S.; Lee, Y.J.; Lee, H.; Ajiteru, O.; Sultan, M.T.; Lee, O.J.; Kim, S.H.; et al. Digital light processing 3D printed silk fibroin hydrogel for cartilage tissue engineering. Biomaterials 2020, 232, 119679.
- Wang, X. Spatial effects of stem cell engagement in 3D printing constructs. J. Stem Cells Res. Rev. Rep. 2014, 1, 5–9.
- Wang, X. 3D printing of tissue/organ analogues for regenerative medicine. In Handbook of Intelligent Scaffolds for Regenerative Medicine, 2nd ed.; Pan Stanford Publishing: Palo Alto, CA, USA, 2016; pp. 557–570.
- Erkoc, P.; Uvak, I.; Nazeer, M.A.; Batool, S.R.; Odeh, Y.N.; Akdogan, O.; Kizilel, S. 3D Printing of cytocompatible gelatin-cellulose-alginate blend hydrogels. Macromol. Biosci. 2020, 10, e2000106.
- Jose, G.; Shalumon, K.T.; Chen, J.P. Natural polymers based hydrogels for cell culture applications. Curr. Med. Chem. 2020, 16, 2734–2776.
- Luetchford, K.A.; Chaudhuri, J.B.; De Bank, P.A. Silk fibroin/gelatin microcarriers as scaffolds for bone tissue engineering. Mater. Sci. Eng. C 2020, 106, 110116.
- Xu, M.; Yan, Y.; Liu, H.; Yao, Y.; Wang, X. Control adipose-derived stromal cells differentiation into adipose and endothelial cells in a 3D structure established by cell-assembly technique. J. Bioact. Compat. Polym. 2009, 24, 31–47.
- Wang, X. Advanced polymers for three-dimensional (3D) organ bioprinting. Micromachines 2019, 12, 814.
- Duin, S.; Schütz, K.; Ahlfeld, T.; Lehmann, S.; Lode, A.; Ludwig, B.; Gelinsky, M. 3D bioprinting of functional islets of langerhans in an alginate/methylcellulose hydrogel blend. Adv. Healthc. Mater. 2019, 7, e1801631.
- Hu, S.; Martinez-Garcia, F.D.; Moeun, B.N.; Burgess, J.K.; Harmsen, M.C.; Hoesli, C.; de Vos, P. An immune regulatory 3D-printed alginate-pectin construct for immunoisolation of insulin producing β-cells. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 123, 112009.
- Huang, Y.; He, K.; Wang, X. Rapid Prototyping of a hybrid hierarchical polyurethane-cell/hydrogel construct for regenerative medicine. Mater. Sci. Eng. C 2013, 33, 3220–3229.
- Xu, W.; Wang, X.; Yan, Y.; Zhang, R. Rapid prototyping of polyurethane for the creation of vascular systems. J. Bioact. Compat. Polym. 2008, 23, 103–114.
- Ashwin, B.; Abinaya, B.; Prasith, T.P.; Chandran, S.V.; Yadav, L.R.; Vairamani, M.; Patil, S.; Selvamurugan, N. 3D-poly (lactic acid) scaffolds coated with gelatin and mucic acid for bone tissue engineering. Int. J. Biol. Macromol. 2020, 162, 523–532.
- Babilotte, J.; Martin, B.; Guduric, V.; Bareille, R.; Agniel, R.; Roques, S.; Héroguez, V.; Dussauze, M.; Gaudon, M.; Le Nihouannen, D.; et al. Development and characterization of a PLGA-HA composite material to fabricate 3D-printed scaffolds for bone tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 118, 111334.
- Hung, K.C.; Tseng, C.S.; Dai, L.G.; Hsu, S.H. Water-based polyurethane 3D printed scaffolds with controlled release function for customized cartilage tissue engineering. Biomaterials 2016, 83, 156–168.
- Liu, F.; Chen, Q.; Liu, C.; Ao, Q.; Tian, X.; Fan, J.; Tong, H.; Wang, X. Natural polymers for organ 3D bioprinting. Polymers 2018, 11, 1278.
- Lei, M.; Wang, X. Biodegradable polymers and stem cells for bioprinting. Molecules 2016, 21, 539.
- Farina, M.; Ballerini, A.; Fraga, D.W.; Nicolov, E.; Hogan, M.; Demarchi, D.; Scaglione, F.; Sabek, O.M.; Horner, P.; Thekkedath, U.; et al. 3D printed vascularized device for subcutaneous transplantation of human islets. Biotechnol. J. 2017, 9, 1700169.
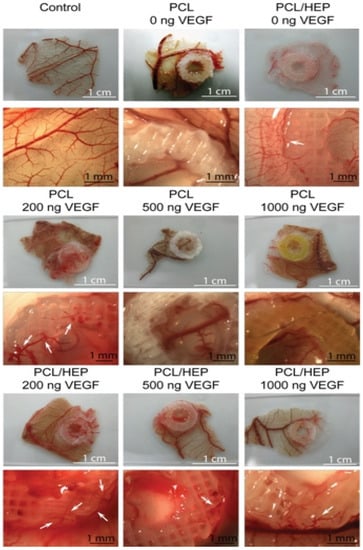
- Marchioli, G.; Luca, A.D.; de Koning, E.; Engelse, M.; Van Blitterswijk, C.A.; Karperien, M.; Van Apeldoorn, A.A.; Moroni, L. Hybrid polycaprolactone/alginate scaffolds functionalized with VEGF to promote de Novo vessel formation for the transplantation of islets of Langerhans. Adv. Healthc. Mater. 2016, 13, 1606–1616.
- Foyt, D.A.; Norman, M.D.A.; Yu, T.T.L.; Gentleman, E. Exploiting Advanced Hydrogel Technologies to Address Key Challenges in Regenerative Medicine. Adv. Healthc. Mater. 2018, 8, e1700939.
- Ge, F.; Lu, Y.; Li, Q.; Zhang, X. Decellularized extracellular matrices for tissue engineering and regeneration. Adv. Exp. Med. Biol. 2020, 1250, 15–31.
- Zhao, X.; Liu, L.; Wang, J.; Xu, Y.F.; Zhang, W.M.; Khang, G.; Wang, X. In vitro vascularization of a combined system based on a 3D bioprinting technique. J. Tissue Eng. Regen. Med. 2016, 10, 833–842.
- Yao, Q.; Zheng, Y.W.; Lan, Q.H.; Kou, L.; Xu, H.L.; Zhao, Y.Z. Recent development and biomedical applications of decellularized extracellular matrix biomaterials. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 104, 109942.
- Kim, B.S.; Kwon, Y.W.; Kong, J.S.; Park, G.T.; Gao, G.; Han, W.; Kim, M.B.; Lee, H.; Kim, J.H.; Cho, D.W. 3D cell printing of in vitro stabilized skin model and in vivo pre-vascularized skin patch using tissue-specific extracellular matrix bioink: A step towards advanced skin tissue engineering. Biomaterials 2018, 168, 38–53.
- Taylor, D.A.; Sampaio, L.C.; Ferdous, Z.; Gobin, A.S.; Taite, L.J. Decellularized matrices in regenerative medicine. Acta Biomater. 2018, 74, 74–89.
- Kim, J.; Kim, M.; Hwang, D.G.; Shim, I.K.; Kim, S.C.; Jang, J. Pancreatic tissue-derived extracellular matrix bioink for printing 3D cell-laden pancreatic tissue constructs. J. Vis. Exp. 2019, 154, e60434.
- Wang, X.; Tuomi, J.; Mäkitie, A.A.; Poloheimo, K.-S.; Partanen, J.; Yliperttula, M. The integrations of biomaterials and rapid prototyping techniques for intelligent manufacturing of complex organs. In Advances in Biomaterials Science and Applications in Biomedicine; Lazinica, R., Ed.; InTech: Rijeka, Croatia, 2013; pp. 437–463.
- Wang, D.; Guo, Y.; Zhu, J.; Liu, F.; Xue, Y.; Huang, Y.; Zhu, B.; Wu, D.; Pan, H.; Gong, T.; et al. Hyaluronic acid methacrylate/pancreatic extracellular matrix as a potential 3D printing bioink for constructing islet organoids. Acta Biomater. 2022, 22, 00375.
