Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Yanfei Liu | -- | 1025 | 2022-12-06 07:17:57 | | | |
| 2 | Yanfei Liu | + 1050 word(s) | 2075 | 2022-12-12 01:13:57 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Liu, Y.; Fu, B.; Xu, Y.; Ren, B.; Qin, Z. Phenoxypyridine as an Active Scaffold for Pesticides. Encyclopedia. Available online: https://encyclopedia.pub/entry/38094 (accessed on 07 February 2026).
Liu Y, Fu B, Xu Y, Ren B, Qin Z. Phenoxypyridine as an Active Scaffold for Pesticides. Encyclopedia. Available at: https://encyclopedia.pub/entry/38094. Accessed February 07, 2026.
Liu, Yanfei, Bin Fu, Yanjun Xu, Bo Ren, Zhaohai Qin. "Phenoxypyridine as an Active Scaffold for Pesticides" Encyclopedia, https://encyclopedia.pub/entry/38094 (accessed February 07, 2026).
Liu, Y., Fu, B., Xu, Y., Ren, B., & Qin, Z. (2022, December 06). Phenoxypyridine as an Active Scaffold for Pesticides. In Encyclopedia. https://encyclopedia.pub/entry/38094
Liu, Yanfei, et al. "Phenoxypyridine as an Active Scaffold for Pesticides." Encyclopedia. Web. 06 December, 2022.
Copy Citation
Phenoxypyridine, the bioisostere of diaryl ethers, has been widely introduced into bioactive molecules as an active scaffold, which has different properties from diaryl ethers. In this paper, the bioactivities, structure-activity relationships, and mechanism of compounds containing phenoxypyridine were summarized, which may help to explore the lead compounds and discover novel pesticides with potential bioactivities.
phenoxypyridine
pesticide
structure-activity relationships
1. Introduction
Diaryl ether [1] is an important active fragment in pesticide molecules, which has good lipid solubility, metabolic stability, cell membrane penetration, sufficient molecular flexibility [2], and can improve biological activity and photostability. So far, the structure of diaryl ether has been widely studied and applied, such as aryloxyphenoxypropionate herbicides, pyrethroid insecticides [3], and triazole fungicides. Pyridine [4], as a nitrogen-containing heterocyclic ring, plays an important role in agrochemicals, and its derivatives have a wide range of biological activities. The hydrophobicity (one of key properties affecting biological activity) of pyridine is significantly higher than that of the benzene ring [5]. Meanwhile, pyridine is an ionizable polar aromatic compound, which can optimize solubility and bioavailability of the lead compound [6]. Replacing the benzene ring with a pyridine ring [7] can usually increase the π-π stacking probability of the target molecule [8] and improve the biological activity. Therefore, phenoxypyridine may have properties that are different from or even superior to those of diphenyl ether. The phenoxypyridine structure has been widely used in the molecular structure of pesticides.
2. Herbicides Containing Phenoxypyridine Scaffold
2.1. Acetyl CoA Carboxylase Inhibitors
Acetyl CoA carboxylase (ACCase) inhibitors [16,17] target ACCase [18] to inhibit fatty acid synthesis in gramineae plants. There are two classes of ACCase inhibitors: aryloxyphenoxypropionate [17] (AOPP or fop) and cyclohexanediones (CHD or dim). Aryloxyphenoxypropionate herbicides [19] occupy an important position in the world herbicide market which have characteristics of high efficiency, low toxicity, crop safety, and so on. In 1976, Ishihara discovered that the compound that was obtained by substituting the benzene ring on one side with a pyridine ring had higher herbicidal activity and launched the first aryloxyphenoxypropionate herbicide containing phenoxypyridine–pyrifenop [20]. Since then, extensive research on herbicides containing phenoxypyridine had been initiated.
The structure of aryloxyphenoxypropionate herbicides containing phenoxypyridine is shown in the Figure 2, in which part A is phenoxypyridine with different substitutions, most of which were electron withdrawing groups, such as F, Cl, Br, NO2, CN, and CF3; part Y is the linking arm, where conformation R [19] was the active ingredient of herbicide; and part Q are various heterocycles, both aromatic and non-aromatic (pyridine, thiazole, benzofuran, etc.).
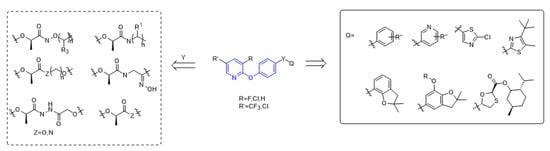
Figure 2. The general structural formula of ACCase inhibitors.
Taking metamifop and clodinafop as the leader, phenoxypyridine was linked to various aromatic rings through different linking arms to obtain active molecules with different structures, as shown in Figure 3.
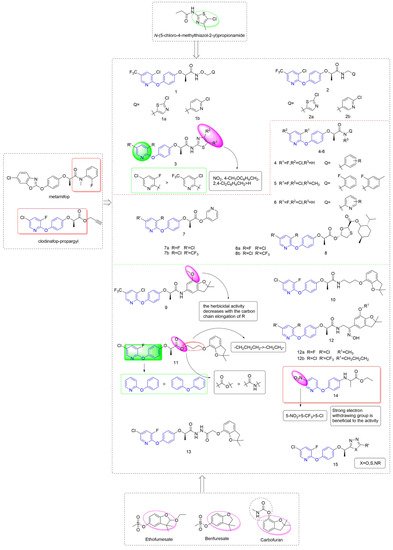
Figure 3. ACCase inhibitors containing phenoxypyridine.
2.2. Protoporphyrinogen IX Oxidase Inhibitors
Protoporphyrinogen oxidase (PPO) [38] is a key enzyme in the biosynthesis of chlorophyll and heme in plants and is one of the important targets for the creation of novel herbicides. At present, PPO-inhibiting herbicides mainly include diphenyl ethers, phenylpyrazoles, triazolinones, N-phenyl phthalimides, and diazoles [39]. Among these herbicides, diphenyl ethers (DPEs) [40] had been widely studied by researchers in the creation of novel pesticides due to their high efficiency, low toxicity, high selectivity, and simple synthesis process; Ye Fei’s team committed to the research and development of PPO inhibitors for a long time. Several series of compounds (Figure 4) containing phenoxypyridine had been designed, studied for greenhouse herbicidal activity, PPO inhibitory activity, crop selectivity, and structure-activity relationships (SARs). These studies fully confirmed that phenoxypyridine provided good herbicidal activity.
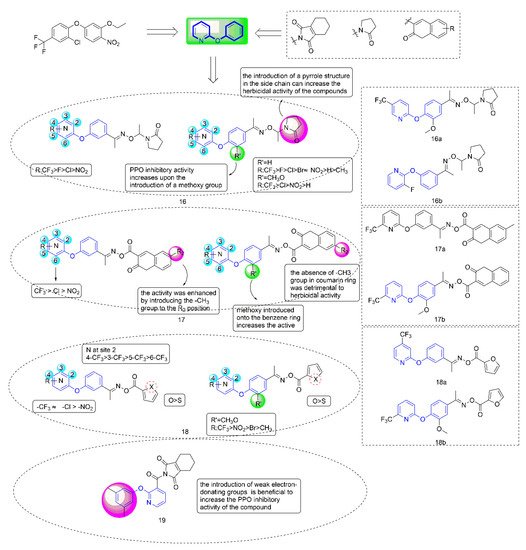
Figure 4. Protoporphyrinogen IX oxidase inhibitors containing phenoxypyridine.
2.3. Other Herbicides
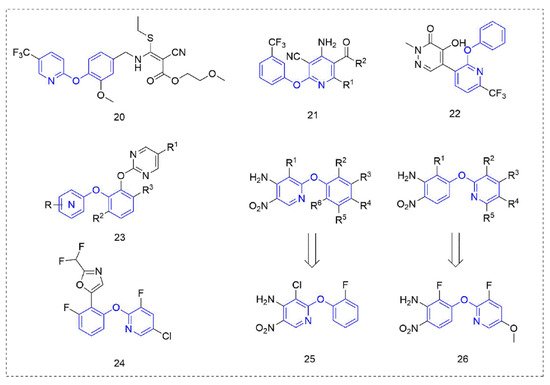
Cyanoacrylate derivatives [49] are photosystem II (PS II) inhibitors [50], which can control weeds by interfering with electron transfer in the photosynthetic system of the plant, preventing photosynthesis. This special mechanism makes cyanoacrylate extremely safe for animals, in line with the requirements of the current social market for new herbicides. The compounds (Figure 5) that were obtained by linking the trifluoromethyl-substituted phenoxypyridine unit with cyanoacrylate skeleton showed good herbicidal activity.

Figure 5. Other compounds with herbicidal activity containing phenoxypyridine.
3. Fungicides and Bactericides Containing Phenoxypyridine Scaffold
3.1. Complex I Inhibitors
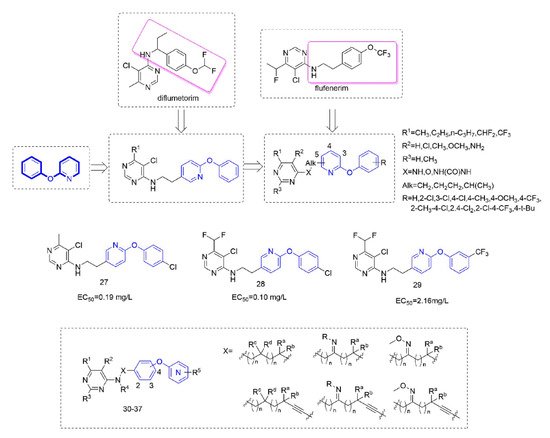
Diflumetorim is a member of aminoalkylpyrimidines [60] targeting mitochondrial complex I (MET I) [61] which has a unique mode of action that is different from the MET I inhibitor acting as insecticide [62]. Therefore, it has no cross-resistance with existing traditional fungicides and is safe for non-target organisms. Liu and co-workers devoted to the research of pyrimidine amine compounds (Figure 6), and the fungicidal activity of the compounds that were synthesized by introducing a phenoxypyridine structure was significantly improved.

Figure 6. Complex I inhibitors containing phenoxypyridine.
3.2. Complex III Inhibitors
Strobilurin [73] were derived from strobilurin A [74], a natural antibiotic with bactericidal activity, and were a kind of agricultural fungicide with great development potential and market vitality [75,76]. Strobilurins act on the Qo site of mitochondrial electron transport chain complex III and are also known as Qo site inhibitors. Some strobilurin derivatives containing phenoxypyridine are shown in Figure 7.
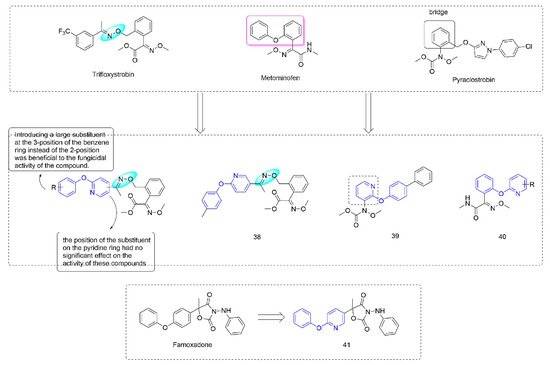

Figure 7. Complex III inhibitors containing phenoxypyridine.
3.3. Sterol Biosynthesis Inhibitors
Triazole fungicides are a new type of fungicide with broad spectrum, high efficiency, low residue, long effect, good systemic translocation, and both protective and curative effects. Triazole fungicides belong to ergosterol biosynthesis inhibitors, which mainly inhibit the activity of sterol 14α-demethylase in sterol biosynthesis to achieve fungicidal effects [82,83]. The triazole derivatives (Figure 8) that were synthesized by Bayer exhibited good protective activity against a variety of pathogenic fungi (Puccinia recondite, Sphaerotheca fuliginea, Uromyces appendiculatus, and Blumeria).
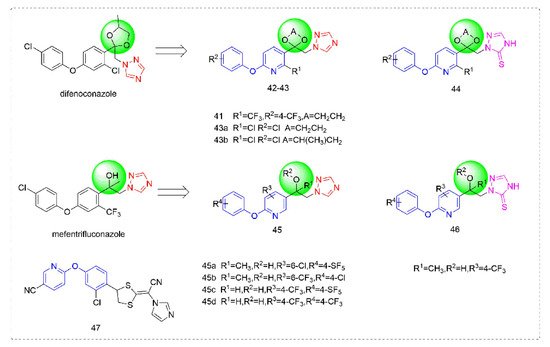

Figure 8. Sterol biosynthesis inhibitors containing phenoxypyridine.
3.4. Succinate Dehydrogenase Inhibitors
Succinate dehydrogenase inhibitors are a class of fungicides with a long history of development, accounting for a considerable proportion of fungicides. Succinate dehydrogenase inhibitors mainly bind to the ubiquinone pocket of SDH and mainly affect the electron transfer of the respiratory chain, to inhibit the growth of pathogenic fungi and eventually lead to death. Most of the pyrazole amide, such as Compounds 48 (Figure 9), that were designed and synthesized by Guan et al. [89] showed good protective activity against Pseudoperonospora cubensis (Berk.et Curt.) Rostov., Blumeria graminis, and Puccinia sorghi in addition to certain insecticidal activity. The control effect of Compound 49 [90] against Pseudoperonospora cubensis (Berk.et Curt.) Rostov. was 100% at 12.5 ppm, and the control effect in the field was also better than that of dimethomorph. The activity of Compound 50 which was synthesized by Sun et al. [91] against Pyricularia grisea was significantly better than that of diphenyl ether and other skeleton compounds, with an EC50 value of 2.286 μg/mL, similar to fluxapyroxad (2.101 μg/mL), more than eight-fold higher than isopyrazam and more than 15-fold higher than the aminopyralid boscalid. Preliminary mechanistic studies suggested that these compounds may not be SDH inhibitors, but inhibited fungal growth by inducing plant defense responses.
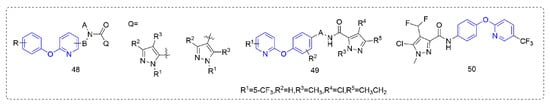

Figure 9. Succinate dehydrogenase inhibitors containing phenoxypyridine.
3.5. Other Fungicides and Bactericides
Some other types of compounds containing phenoxypyridine structures with fungicidal or bactericidal activity are summarized in Figure 10. Phenoxypyridine was linked to isothiazolinone, resulting in Compound 51 [92] with good control effects on Blumeria graminis, Botrytis cinereal, and Pyricularia grisea at low doses. The introduction of chlorine at 4-position of isothiazolinone made the compound lose its inhibitory effect on Botrytis cinereal [93]. The Compounds 52 that were synthesized by Nippon Soda Co., Ltd. (Tokyo, Japan) [94] showed more than a 75% control effect against cucumber gray mold at 500 mg/L and did not cause any damage to the plant.
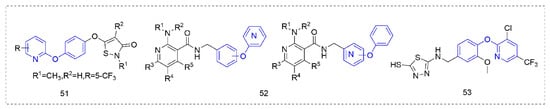
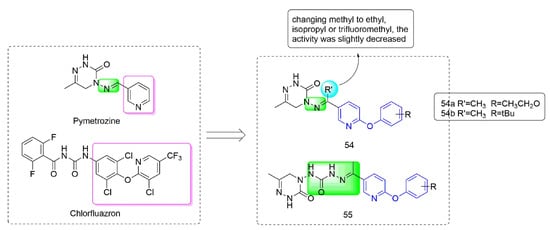
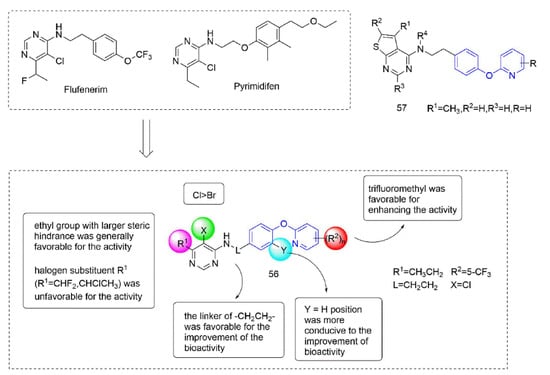
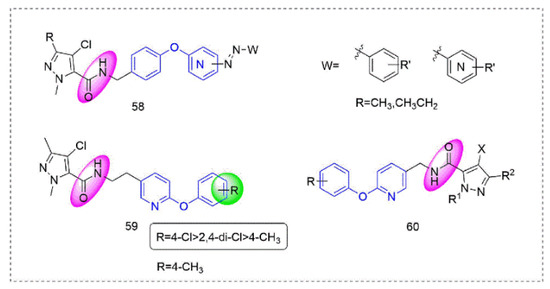
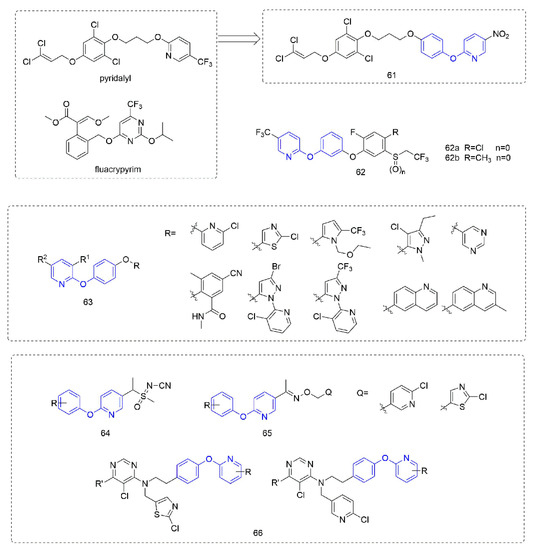

Figure 10. Other compounds with fungicidal or bactericidal activity containing phenoxypyridine.
Phenoxypyridine was linked to isothiazolinone, resulting in Compound 51 [92] with good control effects on Blumeria graminis, Botrytis cinereal, and Pyricularia grisea at low doses. The introduction of chlorine at 4-position of isothiazolinone made the compound lose its inhibitory effect on Botrytis cinereal [93]. The Compounds 52 that were synthesized by Nippon Soda Co., Ltd. (Tokyo, Japan) [94] showed more than a 75% control effect against cucumber gray mold at 500 mg/L and did not cause any damage to the plant.
4. Insecticides Containing Phenoxypyridine Scaffold
4.1. Transient Receptor Potential Vanilloid Channel Blockers
Pymetrozine [97] is a triazinone insecticide [98] that acts on the specific insecticide target protein transient receptor potential vanilloid (TRPV) ion channel, and showed no cross-resistance with other insecticides [99]. Compounds 54–55 (Figure 11) were developed by Nankai University with both phenoxypyridine groups and triazinone groups. The activity against aphids of Compound 54, which was synthesized by Yang et al. [100] by constructing phenoxypyridine structure and introducing a methyl group to the imino group, was significantly improved. At the concentration of 5 mg/kg, the activities against aphids of 54a (80%) and 54b (80%) were both higher than those of pymetrozine (30%). Meanwhile, 54 also exhibited significant insecticidal activity against mosquitoes and lepidopteran pests (cotton bollworm, corn borer, and oriental stick insect). By modifying the linker arm, Wang et al. [101,102] designed and synthesized a series of triazinone derivatives 55 containing an acylhydrazone structure. These compounds had certain activities against aphids, cotton bollworm, corn borer, and armyworm.

Figure 11. Transient receptor potential vanilloid channel blockers containing phenoxypyridine.
4.2. Complex I Inhibitors
Some insecticides and acaricides (flufenerim, purimidifen, tebufenpyrad, and tolfenpyrad [103]) worked by inhibiting the mitochondrial electron transport (MET) at complex I to disrupt respiration, known as complex I inhibitors [104]. Most of the 4-aminopyrimidine [105] derivatives that were synthesized by Wang et al. [106] through intermediate derivatization methods showed good activity against Myzus persicae, among which 56 (Figure 12) had the highest activity and the lowest LC50 value of 0.34 mg/L. The structure-activity relationships suggested that the linker of -CH2CH2- was favorable for bioactivity; the halogen substituent at the X position (X = Cl, Br) was more beneficial to the activity; for R1, the ethyl group with large steric resistance was generally conducive to improve the activity. The substituted thienopyrimidine amines 57 (Figure 12) that were synthesized by Chai et al. [107] had broad-spectrum insecticidal and acaricidal activity, which were very effective against lepidoptera pests, homoptera, and mites even at a very low dose, especially against aphids, Tetranychus cinarcini, Plutella xylodes, and armyworm.

Figure 12. Complex I inhibitors (4-aminopyrimidine) containing phenoxypyridine.
Pyrazole-5-carboxamide insecticides 58 (Figure 13) containing an azo structure were synthesized by Shao et al. [108], many of which had 100% activity against Aphis craccivora Koch and Tetranychus cinnabarinus. Compound 59 [109] showed broad-spectrum insecticidal activity and a 100% mortality rate against Plutella xylostella and Myzus persicae at 600 mg/L. At the same time, several compounds had good activity against Blumeria graminis and southern corn rust. Pyrazole derivatives 60 that were designed and synthesized by Okada et al. [110] had good insecticidal activity against various insect pests (Plutella xylostella, Nilaparvata lugens, and the eggs and adults of Tetranychus urticae).

Figure 13. Complex I inhibitors (Pyrazole-5-carboxamide) containing phenoxypyridine.
4.3. Other Insecticides
Phenoxypyridine-containing compounds with insecticidal activity are summarized in Figure 14. Pyridalyl [111] inhibited cellular protein synthesis in insect cell lines but not mammalian cell lines. The novel dihalopropene ether insecticides that were synthesized by Liu et al. [112] exhibited good insecticidal activity. The LC50 of Compound 61, which introduced phenoxypyridine, was 4.05 mg/L and 9.82 mg/L against M. separate and P. litura, respectively, was better than the control pyridalyl (LC50 = 4.81 mg/L and 10.07 mg/L) and better than the compounds with other aromatic ring substitutions. Alkylphenyl sulfide derivatives 62 that was reported by Kumiai Chemical Industry Co., Ltd. [113] had more than 90% control of Tetranychus urticae (Koch) at a concentration of 4 mg/L. Inspired by juvenile hormone, the analogues 63 that were prepared by Li et al. [114] with the introduction of phenoxypyridine were more than 85% effective against Nilaparvata lugens at a concentration of 200 mg/L. Using phenoxypyridine molecular plug-ins, the sulfoximine and oxime ether, Compounds 64 and 65 with insecticidal activity were synthesized by Liu et al. [115] and Du et al. [116]. The neonicotinoids 66 that was designed and synthesized by Tang et al. [117] had certain activities against lepidoptera, homoptera, coleoptera, and the larvae and adults of orthoptera.

Figure 14. Other compounds with insecticidal activity containing phenoxypyridine.
5. Conclusions
In pesticide applications, phenoxypyridine played an important role in the development of lead compounds. Compounds that were derived by linking phenoxypyridine to different active fragments or changing the substituents of phenoxypyridine exhibited a wide range of biological activity, such as herbicidal, fungicidal, bactericidal, and insecticidal activities. In this paper, the derivatives with different activities were classified. The summary of the structure-activity relationship of the derivatives indicated that structural modifications at different positions of phenoxypyridine could improve its activity. Previous studies had focused on compounds that were linked to the phenoxy group at position 2 of pyridine, possibly due to the difficulty of synthesis, so the relationship between the position of the N atom on pyridine and biological activity was unclear. The inhibitory effects of these compounds may be performed by different mechanisms and, therefore, further studies on the mechanism (or targets) are necessary for better evaluations. Still, a lot of activity of phenoxypyridine needs to be prospected in bactericides. In conclusion, phenoxypyridine could be considered as the promising active scaffold for pesticides.
Information
Subjects:
Chemistry, Applied
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Entry Collection:
Organic Synthesis
Revisions:
2 times
(View History)
Update Date:
12 Dec 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




