苯氧基吡啶是二芳基醚的生物等排体,已作为活性支架广泛引入生物活性分子中,其性质与二芳基醚不同。在农药应用中,苯氧基吡啶在先导化合物的开发中发挥了重要作用。通过将苯氧基吡啶连接到不同的活性片段或改变苯氧基吡啶的取代基而获得的化合物表现出广泛的生物活性,例如除草,杀菌,杀菌和杀虫活性。苯氧基吡啶不同位置的结构修饰可以提高其活性。
1. Introduction
Diaryl ether [
1] is an important active fragment in pesticide molecules, which has good lipid solubility, metabolic stability, cell membrane penetration, sufficient molecular flexibility [
2], and can improve biological activity and photostability. So far, the structure of diaryl ether has been widely studied and applied, such as aryloxyphenoxypropionate herbicides, pyrethroid insecticides [
3], and triazole fungicides. Pyridine [
4], as a nitrogen-containing heterocyclic ring, plays an important role in agrochemicals, and its derivatives have a wide range of biological activities. The hydrophobicity (one of key properties affecting biological activity) of pyridine is significantly higher than that of the benzene ring [
5]. Meanwhile, pyridine is an ionizable polar aromatic compound, which can optimize solubility and bioavailability of the lead compound [
6]. Replacing the benzene ring with a pyridine ring [
7] can usually increase the π-π stacking probability of the target molecule [
8] and improve the biological activity. Therefore, phenoxypyridine may have properties that are different from or even superior to those of diphenyl ether. The phenoxypyridine structure has been widely used in the molecular structure of pesticides.
2. Herbicides Containing Phenoxypyridine Scaffold
2.1. Acetyl CoA Carboxylase Inhibitors
Acetyl CoA carboxylase (ACCase) inhibitors [
16,
17] target ACCase [
18] to inhibit fatty acid synthesis in gramineae plants. There are two classes of ACCase inhibitors: aryloxyphenoxypropionate [
17] (AOPP or fop) and cyclohexanediones (CHD or dim). Aryloxyphenoxypropionate herbicides [
19] occupy an important position in the world herbicide market which have characteristics of high efficiency, low toxicity, crop safety, and so on. In 1976, Ishihara discovered that the compound that was obtained by substituting the benzene ring on one side with a pyridine ring had higher herbicidal activity and launched the first aryloxyphenoxypropionate herbicide containing phenoxypyridine–pyrifenop [
20]. Since then, extensive research on herbicides containing phenoxypyridine had been initiated.
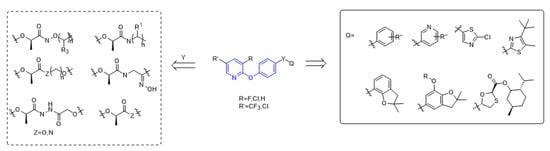
The structure of aryloxyphenoxypropionate herbicides containing phenoxypyridine is shown in the
Figure 2, in which part A is phenoxypyridine with different substitutions, most of which were electron withdrawing groups, such as F, Cl, Br, NO
2, CN, and CF
3; part Y is the linking arm, where conformation R [
19] was the active ingredient of herbicide; and part Q are various heterocycles, both aromatic and non-aromatic (pyridine, thiazole, benzofuran, etc.).
Figure 2. The general structural formula of ACCase inhibitors.
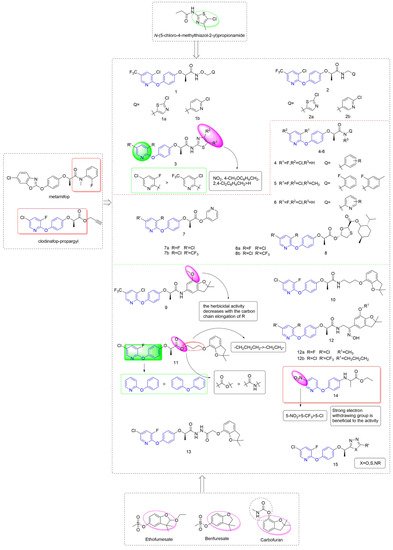
Taking metamifop and clodinafop as the leader, phenoxypyridine was linked to various aromatic rings through different linking arms to obtain active molecules with different structures, as shown in Figure 3.
Figure 3. ACCase inhibitors containing phenoxypyridine.
2.2. Protoporphyrinogen IX Oxidase Inhibitors
Protoporphyrinogen oxidase (PPO) [
38] is a key enzyme in the biosynthesis of chlorophyll and heme in plants and is one of the important targets for the creation of novel herbicides. At present, PPO-inhibiting herbicides mainly include diphenyl ethers, phenylpyrazoles, triazolinones,
N-phenyl phthalimides, and diazoles [
39]. Among these herbicides, diphenyl ethers (DPEs) [
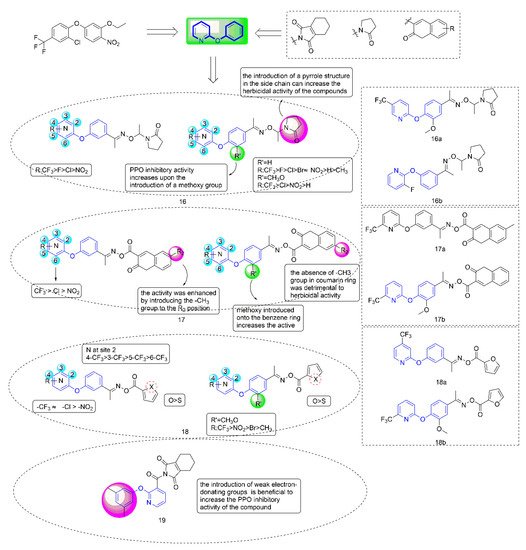
40] had been widely studied by researchers in the creation of novel pesticides due to their high efficiency, low toxicity, high selectivity, and simple synthesis process; Ye Fei’s team committed to the research and development of PPO inhibitors for a long time. Several series of compounds (
Figure 4) containing phenoxypyridine had been designed, studied for greenhouse herbicidal activity, PPO inhibitory activity, crop selectivity, and structure-activity relationships (SARs). These studies fully confirmed that phenoxypyridine provided good herbicidal activity.
Figure 4. Protoporphyrinogen IX oxidase inhibitors containing phenoxypyridine.
2.3. Other Herbicides
Cyanoacrylate derivatives [
49] are photosystem II (PS II) inhibitors [
50], which can control weeds by interfering with electron transfer in the photosynthetic system of the plant, preventing photosynthesis. This special mechanism makes cyanoacrylate extremely safe for animals, in line with the requirements of the current social market for new herbicides. The compounds (
Figure 5) that were obtained by linking the trifluoromethyl-substituted phenoxypyridine unit with cyanoacrylate skeleton showed good herbicidal activity.
Figure 5. Other compounds with herbicidal activity containing phenoxypyridine.
3. Fungicides and Bactericides Containing Phenoxypyridine Scaffold
3.1. Complex I Inhibitors
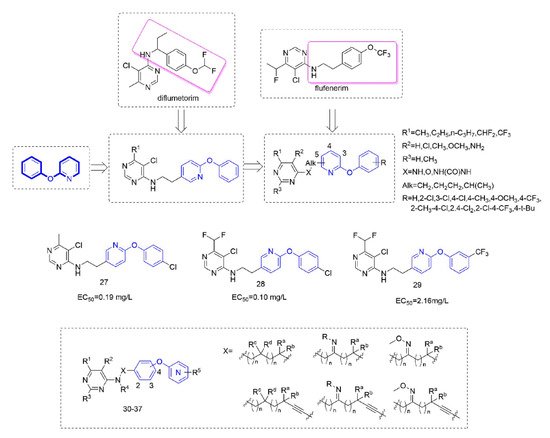
Diflumetorim is a member of aminoalkylpyrimidines [
60] targeting mitochondrial complex I (MET I) [
61] which has a unique mode of action that is different from the MET I inhibitor acting as insecticide [
62]. Therefore, it has no cross-resistance with existing traditional fungicides and is safe for non-target organisms. Liu and co-workers devoted to the research of pyrimidine amine compounds (
Figure 6), and the fungicidal activity of the compounds that were synthesized by introducing a phenoxypyridine structure was significantly improved.
Figure 6. Complex I inhibitors containing phenoxypyridine.
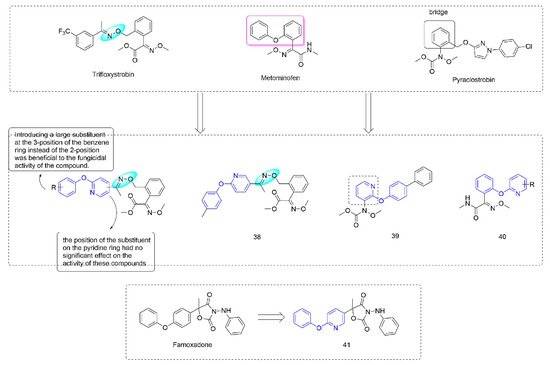
3.2. Complex III Inhibitors
Strobilurin [
73] were derived from strobilurin A [
74], a natural antibiotic with bactericidal activity, and were a kind of agricultural fungicide with great development potential and market vitality [
75,
76]. Strobilurins act on the Qo site of mitochondrial electron transport chain complex III and are also known as Qo site inhibitors. Some strobilurin derivatives containing phenoxypyridine are shown in
Figure 7.
Figure 7. Complex III inhibitors containing phenoxypyridine.
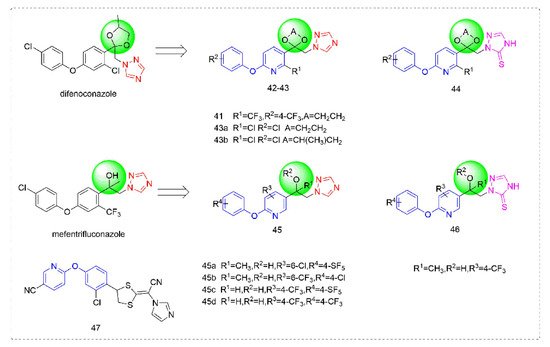
3.3. Sterol Biosynthesis Inhibitors
Triazole fungicides are a new type of fungicide with broad spectrum, high efficiency, low residue, long effect, good systemic translocation, and both protective and curative effects. Triazole fungicides belong to ergosterol biosynthesis inhibitors, which mainly inhibit the activity of sterol 14α-demethylase in sterol biosynthesis to achieve fungicidal effects [
82,
83]. The triazole derivatives (
Figure 8) that were synthesized by Bayer exhibited good protective activity against a variety of pathogenic fungi (
Puccinia recondite,
Sphaerotheca fuliginea,
Uromyces appendiculatus, and
Blumeria).
Figure 8. Sterol biosynthesis inhibitors containing phenoxypyridine.
3.4. Succinate Dehydrogenase Inhibitors
Succinate dehydrogenase inhibitors are a class of fungicides with a long history of development, accounting for a considerable proportion of fungicides. Succinate dehydrogenase inhibitors mainly bind to the ubiquinone pocket of SDH and mainly affect the electron transfer of the respiratory chain, to inhibit the growth of pathogenic fungi and eventually lead to death.
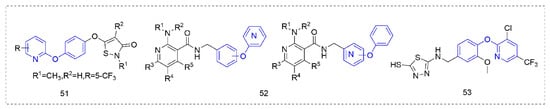
3.5. Other Fungicides and Bactericides
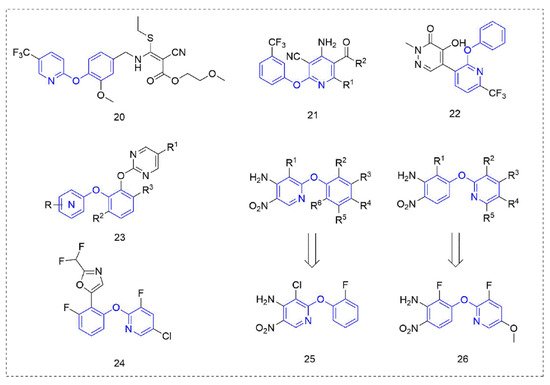
Some other types of compounds containing phenoxypyridine structures with fungicidal or bactericidal activity are summarized in
Figure 10.
Figure 10. Other compounds with fungicidal or bactericidal activity containing phenoxypyridine.
4. Insecticides Containing Phenoxypyridine Scaffold
Pymetrozine [97] is a triazinone insecticide [98] that acts on the specific insecticide target protein transient receptor potential vanilloid (TRPV) ion channel, and showed no cross-resistance with other insecticides [99].
Some insecticides and acaricides (flufenerim, purimidifen, tebufenpyrad, and tolfenpyrad [103]) worked by inhibiting the mitochondrial electron transport (MET) at complex I to disrupt respiration, known as complex I inhibitors [104].
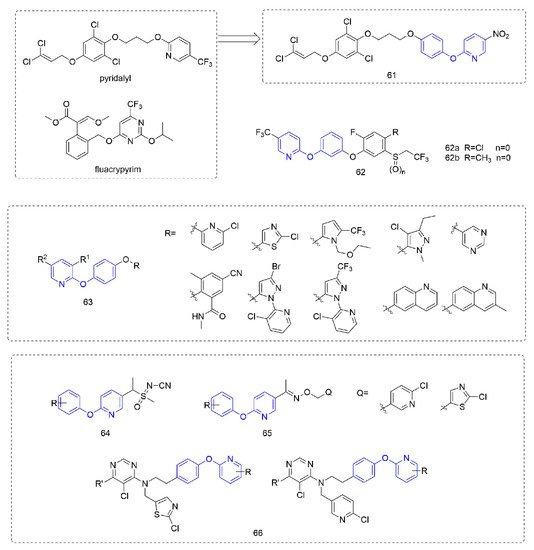
Phenoxypyridine-containing compounds with insecticidal activity are summarized in Figure 14. Pyridalyl [111] inhibited cellular protein synthesis in insect cell lines but not mammalian cell lines. The novel dihalopropene ether insecticides that were synthesized by Liu et al. [112] exhibited good insecticidal activity.
图 14.其它含有杀虫活性的化合物含有苯氧基吡啶。
This entry is adapted from the peer-reviewed paper 10.3390/molecules27206803