Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Erand Llanaj | -- | 2058 | 2022-11-30 11:26:02 | | | |
| 2 | Vivi Li | -120 word(s) | 1938 | 2022-12-02 02:27:26 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Llanaj, E.; Ahanchi, N.S.; Dizdari, H.; Taneri, P.E.; Niehot, C.D.; Wehrli, F.; Khatami, F.; Raeisi-Dehkordi, H.; Kastrati, L.; Bano, A.; et al. Buckwheat and Cardiometabolic Health. Encyclopedia. Available online: https://encyclopedia.pub/entry/37329 (accessed on 08 February 2026).
Llanaj E, Ahanchi NS, Dizdari H, Taneri PE, Niehot CD, Wehrli F, et al. Buckwheat and Cardiometabolic Health. Encyclopedia. Available at: https://encyclopedia.pub/entry/37329. Accessed February 08, 2026.
Llanaj, Erand, Noushin Sadat Ahanchi, Helga Dizdari, Petek Eylul Taneri, Christa D. Niehot, Faina Wehrli, Farnaz Khatami, Hamidreza Raeisi-Dehkordi, Lum Kastrati, Arjola Bano, et al. "Buckwheat and Cardiometabolic Health" Encyclopedia, https://encyclopedia.pub/entry/37329 (accessed February 08, 2026).
Llanaj, E., Ahanchi, N.S., Dizdari, H., Taneri, P.E., Niehot, C.D., Wehrli, F., Khatami, F., Raeisi-Dehkordi, H., Kastrati, L., Bano, A., Glisic, M., & Muka, T. (2022, November 30). Buckwheat and Cardiometabolic Health. In Encyclopedia. https://encyclopedia.pub/entry/37329
Llanaj, Erand, et al. "Buckwheat and Cardiometabolic Health." Encyclopedia. Web. 30 November, 2022.
Copy Citation
Buckwheat (BW) is suggested to have beneficial effects, but evidence on how it affects cardiometabolic health (CMH) is not yet established. Buckwheat is a gluten-free pseudograin rich in fiber and bioactive compounds, and has been suggested to positively affect cardiometabolic health.
buckwheat
cardiometabolic health
diet
cardiovascular diseases
fagopyrum
1. Introduction
Buckwheat (BW) (referring mainly to Fagopyrum esculentum and F. tataricum), a gluten-free pseudograin rich in fiber and bioactive compounds, has been suggested to positively affect cardiometabolic health [1]. The non-grain portion of the BW plant represents an important source of concentrated phenolics [2]. Globally, buckwheat demand has steadily increased [3], reflected in a rise in production to almost four million tons in 2021 alone [4]. Studies in animals have indicated that the intake of BW or BW-rich foods can influence glucose homeostasis and lipid metabolism, by modulating serum total cholesterol (TC) and triglycerides [5][6]. Such effects in the cardiovascular system have been partially attributed to the well-balanced amino acid composition of its proteins, dietary fiber content in the seeds and/or the presence of polyphenols such as rutin and quercetin-3-glucoside (hereinafter referred to as ‘quercetin’), that may confer protective properties against cardiovascular diseases (CVDs) [5][7]. Contrary to animal models, studies in humans have not yet established BW’s role as a dietary component for prevention of CVDs. Some human studies have indicated that BW can reduce serum lipid levels and blood pressure (BP), as well as improve body morphology parameters, while other studies have failed to show any favorable modification of CVD risk [5][6][7].
Despite the growing body of evidence and attention gained in cardiometabolic research in recent years, to the best of researchers' knowledge, there is only one systematic review and meta-analysis on BW and its effects on CVD risk markers in humans [5], which has major methodological concerns that make interpretation of findings difficult. For example, data from different study designs, including cross-sectional with clinical trials, were pooled together without taking into account the correlation between pre- and post-intervention measures—something that can lead to seriously biased results and misleading conclusions [8]. Thus, it is not yet clear how and to what extent BW use and/or its bioactive compounds can influence CVD risk markers and exert cardiometabolic benefits.
Therefore, researchers systematically reviewed and meta-analyzed studies that investigated how dietary consumption or supplementation of BW, including bioactive compounds present in BW, was associated with a wide array of CVD risk markers, with the aim of understanding the association between BW intake and cardiometabolic health and translate its potential utility for clinical practice. Based on Population, Intervention, Comparison and Outcomes (PICO) criteria (see Table 1) researchers included only human adult subjects (≥18 years), exposed to a diet supplementation with buckwheat, rutin, quercetin and/or other related bioactives, compared to placebo, no buckwheat or other comparison. The outcomes researchers focused on were serum lipid profile, type 2 diabetes (T2D) and glucose homeostasis parameters, inflammatory markers, body morphology parameters, blood pressure, all-cause and CVD mortality, severity and/or clinical progression, markers of vasoconstriction/vasodilatation and/or markers of atherosclerosis, such as atherosclerotic plaque, arterial wall thickness, coronary artery calcification, intima media thickness, etc. With regards to study design, researchers considered all prospective cohort studies, case-cohort, nested-case control studies, randomized and non-randomized clinical trials.
Table 1. PICOS criteria for inclusion of studies.
| Parameter | Criterion |
|---|---|
| Population | Human adults (≥18 years) |
| Interventions/exposures | Diet supplementation with buckwheat, rutin, quercetin and/or other buckwheat related bioactives |
| Comparisons | Placebo, no buckwheat or other comparison |
| Outcomes | Serum lipid profile, type 2 diabetes and glucose homeostasis parameters, inflammatory and oxidative stress markers, body morphology parameters, blood pressure, all-cause and cardiovascular mortality, cardiovascular disease severity and/or clinical progression, markers of vasoconstriction/vasodilatation and/or markers of atherosclerosis, such as atherosclerotic plaque, arterial wall thickness, coronary artery calcification, intima media thickness, etc. |
| Study design | Prospective cohort studies, case-cohort, nested-case control studies, randomized and non-randomized clinical trials |
2. Researches on Buckwheat and Cardiometabolic Health
Evidence on exposure–outcome relationships can be inferred from many types of studies, including RCTs, cohort studies, case-control studies, cross-sectional analyses, ecological studies and animal studies. Each study type has characteristic strengths and weaknesses. For example, RCTs are the most robust method for dealing with confounding, but they are often conducted with strict inclusion and exclusion criteria, meaning that trial participants may be unlikely to be fully representative of the general population, as well as being carried out over relatively short durations.
Case-control studies are well suited for understanding the risks linked to rare outcomes, but they may be subject to recall bias for past exposure. Animal studies are widely used in evaluating the risks of consumer products and environmental risks but may not be generalizable to humans. Study design and analysis impacts causal interpretation and understanding of the results.
In their work, researchers took this fact into account and applied a stricter methodological rigor, differentiating study designs, as well as conducting a more structured evaluation of BW and cardiometabolic health. To the best of researchers' knowledge, this entry contains the largest number of human intervention studies to date, i.e., with three additional studies in the meta-analysis compared to the aforementioned review. Furthermore, it important to consider within-subject correlation in pre-post analysis, as failure to do so can lead to meta-analysis with misleading statistical results and inherent biases. To address this. researchers have used a coefficient of 0.8 in the calculations for the standard deviation of the mean difference [8].
Moreover, there are other reports that have attempted to ascertain the potential health benefits of consuming BW as a food, supplement, remedy or possible pharmaceutical agent. Recent work has focused on BW’s role in health and disease, especially investigating effects on lipid profile, BP, glucose and body weight, but the majority of these claimed effects in the literature are based on data extrapolated from in vitro studies or animal models [6][7].
Although animal models are vital in understanding physiological mechanisms and elucidating the potential health relevance, human intervention studies are scarce and inconsistent in supporting BW benefits identified in nonhuman studies. In the literature, a cross-sectional study with a questionnaire-based assessment of oat and buckwheat intakes showed a significant reduction in both systolic (−3.1 mm Hg, p < 0.001) and diastolic (−1.3 mm Hg, p < 0.01) BP [9]. Due to the study design (i.e., cross-sectional), this survey was not included in researchers' analysis. Nevertheless, it is worth mentioning that the authors highlighted that water-soluble fiber but not total dietary fiber was independently associated with BP. Hence, a possibility exists that there is an effect of BW on these parameters, since BW has higher levels of soluble than insoluble fiber. Only two cross-sectional studies [9][10] have suggested that consumption of BW seeds may be a preventative factor for hypertension, dyslipidemia and hyperglycemia. However, the inherent limitations of a cross-sectional design render these findings indicative.
There are some results on the role of Tatary BW in human nutrition, showing that foods made from grain components of Tartary BW may have preventive effects against several chronic diseases, including obesity, CVDs, gallstone formation and hypertension [11]. However, these findings are almost entirely based on in vitro and nonhuman models, with little relevance to human health. Additionally, such effects are hypothetically attributed to resistant starch, protein, and phenolic substances in the grain, and to the interactions among these constituents, without any methodological high-quality study design in humans supporting these claims. Of note, in the part of researchers' report, several studies used Tatary BW as intervention [12][13][14][15][16][17].
Results were not consistent with regards to effect of Tatary BW on CVD markers and study designs were very heterogeneous, with little, if any, considerations on compliance with the BW intervention, including no control arm or head-to-head comparison drugs. In researchers' entry, a consistent finding that aligns with previous reviews on BW [2][18], is the fact that many of the studies on BW were published before 2010, indicating an additional complexity with regards to methodological and technical procedures used. This aspect warrants further exploration, but it is a reminder that additional caution should be exercised when interpreting the literature on BW with considerations of separation, extraction, formulation, and processing methods.
Another observation is that BW is commonly a basis for noodle recipes in Asia. However, in Europe, BW flour is used in pancakes and crepes, as a common ingredient in gluten-free products, to which coeliac patients are particularly exposed. With such a wide use of BW at population level, it is reasonable to assume that the borderline effect of BW interventions on total and HDL cholesterol warrants further investigation, regardless of the questionable methodological rigor of evidence so far. According to BW’s degree of processing and food matrix, the primary mechanism of action may differ, but researchers speculate that this mechanism may include slower gastric emptying, the inhibition of hepatic cholesterol synthesis and/or enhanced fecal excretion of cholesterol and bile salts (see Figure 1).
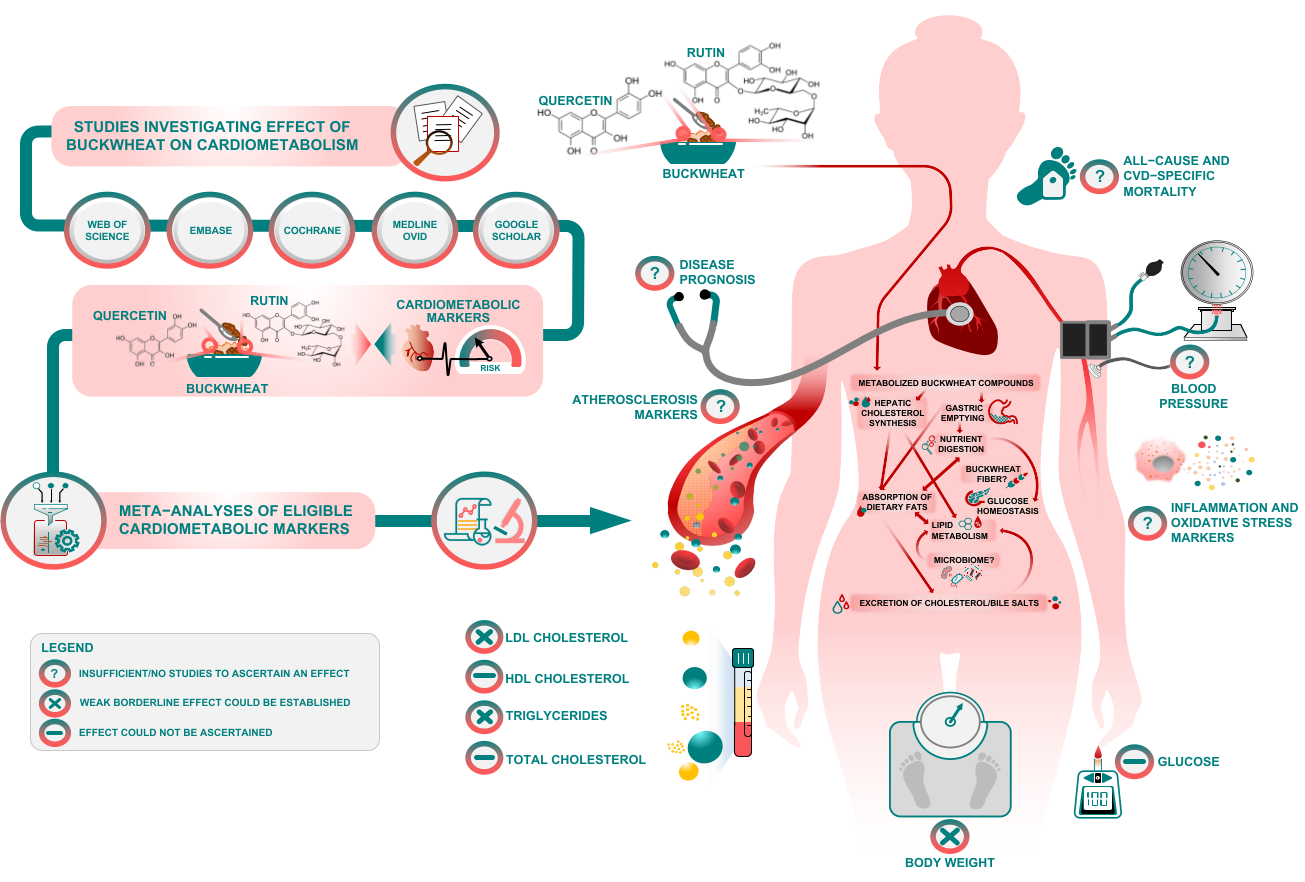 Figure 1. Visual summary, mechanisms and potential avenues for future research.
Figure 1. Visual summary, mechanisms and potential avenues for future research.Similar effects have been observed from dietary fibers in general [19] and it is not possible to differentiate the effect of quercetin or rutin from the effects of fibers present in BW. Nevertheless, the gel-forming attributes of soluble fibers in BW may be a basis for the borderline effect on some of lipids and glucose [20][21][22]. It is not clear whether different types of BW supplementation (e.g., grain vs. non-grain components) may have physiologically different effects on health. Herbal supplementation from non-grain components could be a richer source of natural phytochemicals, but this area of BW research remains speculative and to be elucidated in the future.
Processing can also influence the BW matrix and its composition. It may be the case that a single compound (i.e., rutin or quercetin) can influence several physiological functions, but also several BW compounds may affect a single defined physiological mechanism.
Processing, such as the type that disrupts the food matrix, may indirectly influence digestibility and/or bioavailability of BW nutrients, but can also degrade functionality by altering the structure of its components (e.g., depolymerisation of rutin or quercetin, lipid coalescence or protein denaturation) and/or their interaction. This warrants further research to confirm the specific effects and the mechanisms involved.
Over the past five decades, there have been efforts to document and establish health benefits from BW [5][6][7][11]. Despite its potential to improve human health, BW remains understudied in nutrition and clinical settings. Although the bioactive components present in BW are implicated in beneficial human health effects, future studies should focus on how insufficiently studied BW phytocompounds (such as phenolic acids and polyphenols) are metabolized in humans and influence cardiometabolic health. Thus, findings from this entry and meta-analysis might help guide future research to explore the potential health-promoting components of BW, which in turn will shed light on any health benefit this crop may deliver and its potential to be incorporated into human diet for optimal health.
Although researchers' report is the largest review of RCTs and human interventional studies to date, concerns about the scarcity of studies, heterogeneity and methodological rigor concerns undermine establishment of causal inferences. The available interventional studies on BW have multiple limitations and flaws regarding sample size, intervention/follow-up time, confounders, blinding, randomization and allocation issues, which reinforce the need for more and better trials on the topic. In spite of the sensitivity analyses researchers performed to address limitations of the available evidence base, caution should be paid in interpreting some results as pooled studies had differing health characteristics. This constrains strong casual inferences and generalizability of findings, but researchers believe it can further stimulate the exploration of BW phytochemicals and their role(s) in human health. In addition, for crossover designs researchers used data collected from the first period only. Future studies should explore further interactions of BW bioactive components with health (Figure 1).
References
- Li, S.; Zhang, Q. Advances in the Development of Functional Foods from Buckwheat. Crit. Rev. Food Sci. Nutr. 2001, 41, 451–464.
- Raguindin, P.F.; Itodo, O.A.; Stoyanov, J.; Dejanovic, G.M.; Gamba, M.; Asllanaj, E.; Minder, B.; Bussler, W.; Metzger, B.; Muka, T.; et al. A systematic review of phytochemicals in oat and buckwheat. Food Chem. 2020, 338, 127982.
- Su Jeong, K.; Hwang Bae, S.; Jong Taek, S.; Geum Hee, K.; Su Young, H.; Dong Chil, C.; Kim, K.D.; Koo, B.C.; Kim, Y.H. Domestic and Overseas Status of Buckwheat Production and Future Trends. J. Korean Soc. Int. Agric. 2017, 29, 226–233.
- Zou, L.; Wu, D.; Ren, G.; Hu, Y.; Peng, L.; Zhao, J.; Garcia-Perez, P.; Carpena, M.; Prieto, M.A.; Cao, H.; et al. Bioactive compounds, health benefits, and industrial applications of Tartary buckwheat (Fagopyrum tataricum). Crit. Rev. Food Sci. Nutr. 2021, 1–17.
- Li, L.; Lietz, G.; Seal, C. Buckwheat and CVD risk markers: A systematic review and meta-analysis. Nutrients 2018, 10, 619.
- Giménez-Bastida, J.A.; Zieliński, H. Buckwheat as a Functional Food and Its Effects on Health. J. Agric. Food Chem. 2015, 63, 7896–7913.
- Kreft, M. Buckwheat phenolic metabolites in health and disease. Nutr. Res. Rev. 2016, 29, 30–39.
- Riley, R.D. Multivariate meta-analysis: The effect of ignoring within-study correlation. J. R. Stat. Soc. Ser. A Stat. Soc. 2009, 172, 789–811.
- He, J.; Klag, M.J.; Whelton, P.K.; Mo, J.P.; Chen, J.Y.; Qian, M.C.; Mo, P.S.; He, G.Q. Oats and buckwheat intakes and cardiovascular disease risk factors in an ethnic minority of China. Am. J. Clin. Nutr. 1995, 61, 366–372.
- Zhang, H.-W.; Zhang, Y.-H.; Lu, M.-J.; Tong, W.-J.; Cao, G.-W. Comparison of hypertension, dyslipidaemia and hyperglycaemia between buckwheat seed-consuming and non-consuming Mongolian-Chinese populations in Inner Mongolia, China. Clin. Exp. Pharmacol. Physiol. 2007, 34, 838–844.
- Luthar, Z.; Golob, A.; Germ, M.; Vombergar, B.; Kreft, I. Tartary Buckwheat in Human Nutrition. Plants 2021, 10, 700.
- Wieslander, G.; Fabjan, N.; Vogrincic, M.; Kreft, I.; Janson, C.; Spetz-Nyström, U.; Vombergar, B.; Tagesson, C.; Leanderson, P.; Norbäck, D.; et al. Eating buckwheat cookies is associated with the reduction in serum levels of myeloperoxidase and cholesterol: A double blind crossover study in day-care centre staffs. Tohoku J. Exp. Med. 2011, 225, 123–130.
- Qiu, J.; Li, Z.; Qin, Y.; Yue, Y.; Liu, Y. Protective effect of tartary buckwheat on renal function in type 2 diabetics: A randomized controlled trial. Clin. Risk Manag. 2016, 12, 1721–1727.
- Qiu, J.; Liu, Y.; Yue, Y.; Qin, Y.; Li, Z. Dietary tartary buckwheat intake attenuates insulin resistance and improves lipid profiles in patients with type 2 diabetes: A randomized controlled trial. Nutr. Res. 2016, 36, 1392–1401.
- Nishimura, M.; Ohkawara, T.; Sato, Y.; Satoh, H.; Suzuki, T.; Ishiguro, K.; Noda, T.; Morishita, T.; Nishihira, J. Effectiveness of rutin-rich Tartary buckwheat (Fagopyrum tataricum Gaertn.) ‘Manten-Kirari’ in body weight reduction related to its antioxidant properties: A randomised, double-blind, placebo-controlled study. J. Funct. Foods 2016, 26, 460–469.
- Huang, G.; Huang, M.; Chen, W.; Huang, Y.; Yang, Z.; You, Y. Clinical effects of tartary buckwheat mixture on the treatment of early diabetic and nephropathy. J. Chin. Med. Mater. 2009, 32, 1932–1935.
- Zheng, G.; Pan, X.; An, Z.; Wang, Y. Preliminary observation of lipid-decreasing effects of compound Tartary buckwheat flour on niddm patients. Beijing Med. J. 1991, 5, 280–281.
- Zhu, F. Chemical composition and health effects of Tartary buckwheat. Food Chem. 2016, 203, 231–245.
- Brown, L.; Rosner, B.; Willett, W.W.; Sacks, F.M. Cholesterol-lowering effects of dietary fiber: A meta-analysis. Am. J. Clin. Nutr. 1999, 69, 30–42.
- Fernandez, M.-L. Soluble fiber and nondigestible carbohydrate effects on plasma lipids and cardiovascular risk. Curr. Opin. Lipidol. 2001, 12, 35–40.
- Roy, S.; Vega-Lopez, S.; Fernandez, M.L. Gender and Hormonal Status Affect the Hypolipidemic Mechanisms of Dietary Soluble Fiber in Guinea Pigs. J. Nutr. 2000, 130, 600–607.
- Gill, S.K.; Rossi, M.; Bajka, B.; Whelan, K. Dietary fibre in gastrointestinal health and disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 101–116.
More
Information
Subjects:
Cardiac & Cardiovascular Systems
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Entry Collection:
Hypertension and Cardiovascular Diseases
Revisions:
2 times
(View History)
Update Date:
02 Dec 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




