Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Shizuka Uchida | -- | 1227 | 2022-11-19 06:12:30 | | | |
| 2 | Dean Liu | Meta information modification | 1227 | 2022-11-21 03:22:31 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Ilieva, M.; Panella, R.; Uchida, S. AngiomiRs in Cancer. Encyclopedia. Available online: https://encyclopedia.pub/entry/35324 (accessed on 07 February 2026).
Ilieva M, Panella R, Uchida S. AngiomiRs in Cancer. Encyclopedia. Available at: https://encyclopedia.pub/entry/35324. Accessed February 07, 2026.
Ilieva, Mirolyuba, Riccardo Panella, Shizuka Uchida. "AngiomiRs in Cancer" Encyclopedia, https://encyclopedia.pub/entry/35324 (accessed February 07, 2026).
Ilieva, M., Panella, R., & Uchida, S. (2022, November 19). AngiomiRs in Cancer. In Encyclopedia. https://encyclopedia.pub/entry/35324
Ilieva, Mirolyuba, et al. "AngiomiRs in Cancer." Encyclopedia. Web. 19 November, 2022.
Copy Citation
Angiogenesis is the process of new blood vessel formation through the migration, growth, and differentiation of endothelial cells.
cancer
cardiovascular disease
miRNA
1. Introduction
Cancer and cardiovascular disease are the leading causes of death across the globe accounting for one in six deaths [1] and 32% of all deaths worldwide [2], respectively, according to World Health Organization (WHO). Both cancer and cardiovascular disease are the umbrella terms commonly used to describe several disease etiologies. Each etiology of cancer and cardiovascular disease (e.g., lung cancer and ischemic heart disease, respectively) has its own distinct cause and progression pattern. However, recent research suggests that many aspects of cancer and cardiovascular disease are similar in terms of pathogenesis [3][4][5], leading to the development of specific field of study called cardio-oncology [6][7]. For example, both diseases involve dysregulated functionalities in vasculature, where abnormal vasculature (called, tumor vasculature [8]) occurs in cancer, while coronary artery disease is a type of cardiovascular disease caused by the narrowing or blockage of coronary arteries [9]. Another example is the involvement of immune responses, where prolonged or chronic inflammation is a hallmark of cancer [10][11][12] as well as cardiovascular disease [13][14][15]. The activation of immune responses often leads to the deposition of excessive extracellular matrices [16][17], which are another hallmark of cancer [17] and cardiac fibrosis as the end-stage of heart failure [18].
MicroRNAs (miRNAs) are evolutionary-conserved, regulatory short [~22 nucleotides (nt)] non-protein-coding RNAs that function as translational inhibitors by binding to the 3′-untranslated regions (3′-UTRs) of messenger RNAs (mRNAs) [19][20]. As one miRNA is predicted to bind hundreds of mRNAs due to its very short seed sequence (~6 nt) [21][22][23], it is speculated and experimentally shown for some miRNAs to regulate cascades of signaling pathways and their downstream targets. Due to their versatilities, dysregulation in miRNAs is linked to a variety of diseases, including cancer [24][25] and cardiovascular disease [26][27][28]. As the regulatory importance of miRNAs is experimentally proven, the therapeutic silencing of miRNAs is being explored [29][30][31][32][33][34]. However, due to their biodistributions (e.g., including their presence in the circulation [35][36][37]) and the presence of many target mRNAs for one miRNA, the precise mechanistic elucidation of each miRNA is urgently needed to advance into clinics. Since a specific miRNA is highly dependent on which target mRNAs are present in a specific biological context, it must be taken into consideration that the same miRNA can yield different biological outcomes depending on the specific cell or tissue [38]. This is especially important when considering miRNAs as potential therapeutic targets.
As cancer and cardiovascular disease share several aspects of disease causes and progressions, it is no surprise that many miRNAs are shown to be involved in pathogeneses of both cancer and cardiovascular disease. Because the heart is the least likely organ to harbor tumor growth [39], the communication between researchers working in miRNAs for either cancer or cardiovascular biology is scarce, although many miRNAs are found to be dysregulated in both diseases.
2. Angiogenesis: AngiomiRs
Angiogenesis is the process of new blood vessel formation through the migration, growth, and differentiation of endothelial cells [40][41]. In cancer, angiogenesis allows for a tumor to grow as new vessels provide nutrients and oxygen to malignant cells [42][43]. In cardiovascular disease, therapeutic angiogenesis aims to provide the blood flow to the ischemic heart tissue [44][45]. Thus, in both diseases, angiogenesis is an important therapeutic target, although the opposite effects are observed. During angiogenesis, several miRNAs are functionally involved, which has created a specific term to describe these angiogenesis-related miRNAs called, angiomiRs (Figure 1). AngiomiRs include miR-15/16, miR-17~92 cluster, miR-18a, miR-19, miR-21, miR-23b, miR-27a/b, miR-29b, miR-30, miR-34a, miR-57, miR-125b, miR-126, miR-128, miR-143, miR-145, miR-155, miR-192, miR-194, miR-199a, miR-200 family, miR-204, miR-210, miR-217, miR-296, miR-378, miR-484, miR-494, miR-497, miR-542-3p, miR-573, miR-642, and let-7b [46][47], which some are discussed below.
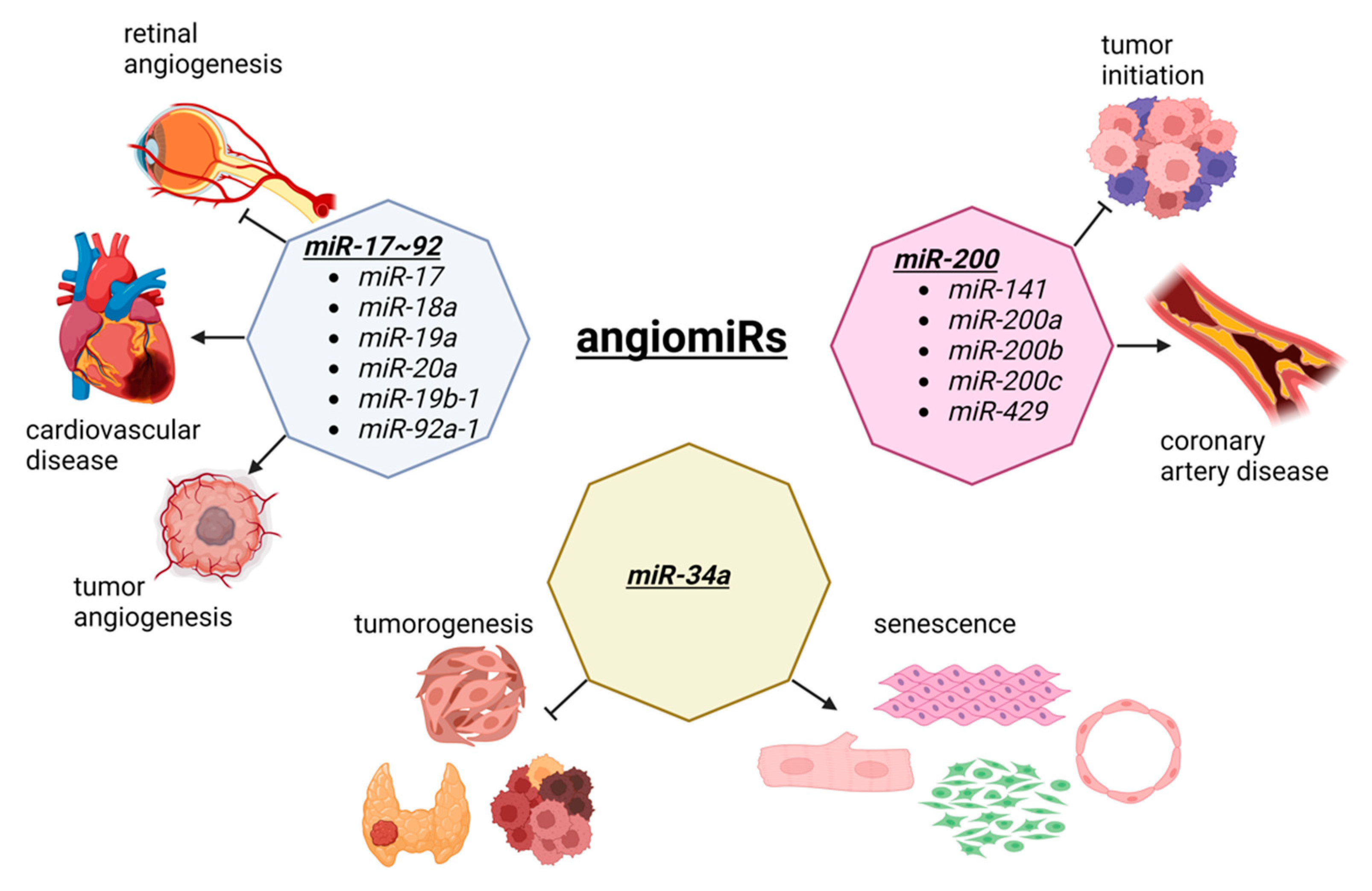
Figure 1. The dual role of angiomiRs in cancer and cardiac pathophysiology. The miR-17~92 cluster is involved in tumorigenesis and tumor vascularization. This cluster is also involved in retinal angiogenesis and the progression of cardiovascular disease. The members of the miR-200 family prevent the tumor initiation and malignant transformation, although they are upregulated in coronary artery disease. MiR-34a is a tumor suppressor involved in the development of thyroid cancer, head and neck squamous cell carcinoma, and cancer stem cells division. The overexpression of miR-34a suppress the proliferation and induces senescence in cardiomyocytes, fibroblasts, smooth muscle, and endothelial cells, by inhibiting sirtuin 1 (SIRT1). Figure created with BioRender.com, accessed on 24 October 2022.
The miR-17~92 cluster was first reported in tumorigenesis [48] and is one of the most well-studied miRNA clusters [49][50]. By crossing miR-17~92 floxed mice with an inducible vascular endothelial cell specific Cre driver (Cdh5-cre/ERT2), Chamorro-Jorganes et al. demonstrated that retinal angiogenesis was reduced during the development of these mice [51]. Furthermore, the vascular endothelial growth factor (VEGF)-induced ear and tumor angiogenesis were reduced, suggesting that VEGF regulates miR-17~92 cluster expression leading to the regulation of angiogenesis. The involvement of the miR-17~92 cluster is well documented in various diseases, including cardiovascular disease [52][53]. Since the miR-17~92 cluster consists of miR-17, miR-18a, miR-19a, miR-20a, miR-19b-1, and miR-92a-1, each miRNA in this cluster is also shown to be important for angiogenesis, including tumorigenesis and cardiovascular disease. For example, miR-92a is dysregulated in many forms of cancer, suggesting it is a potential diagnostic biomarker as well as a therapeutic target [54]. In the cardiovascular system, Bonauer et al. demonstrated that overexpression of miR-92a in endothelial cells inhibited angiogenesis in murine models of limb ischemia and myocardial infarction, while the silencing of miR-92a via antagomiR resulted in enhanced angiogensis and the functional recovery of the damaged tissues in murine disease models, suggesting miR-92a as a potential therapeutic target for ischemia diseases [55].
The miR-200 family is another well studied miRNA family that includes miR-141, miR-200a, miR-200b, miR-200c, and miR-429 [56]. In cancer, the miR-200 family is shown to play functional roles in cell malignant transformation and preventing tumor initiation [57]. By profiling epicardial adipose tissue from coronary artery disease (CAD) patients and non-CAD atherosclerotic patients, Zhang et al. demonstrated that the expressions of miR-141-3p, miR-200b, miR-200c-3p, and miR-429 are up-regulated in CAD patients compared to non-CAD patients [58]. By performing a series of experiments in vitro, the authors demonstrated that the overexpression of miR-200b-3p in human umbilical vein endothelial cells (HUVECs) resulted in increased apoptosis under oxidative stress. Mechanistically, miR-300b-3p targets histone deacetylase 4 (HDAC4) as the overexpression of HDAC4 reduced the increased apoptosis induced by inhibiting miR-200b-3p, suggesting that miR-200b-3p is a potential therapeutic target for atherosclerosis.
MiR-34a is a tumor suppressor and considered as a diagnostic and prognostic biomarker as well as a therapeutic target in various cancers, including head and neck squamous cell carcinoma, thyroid cancer, and cancer stem cells [59][60]. Interestingly, the expression of miR-34a is increased in senescent HUVECs and in the heart and spleen of older mice [61]. When overexpressed, miR-34a suppressed cell cycle and proliferation by inhibiting sirtuin 1 (SIRT1). Because ageing is a hot topic to be investigated, subsequent research shows the functional importance of miR-34a in cell types other than endothelial cells in the heart, including in cardiomyocytes [62][63], fibroblasts [64], and smooth muscle cells [65][66]. This is not an isolated case as many other angiomiRs (and other miRNAs) are expressed rather ubiquitously, suggesting that examining miRNAs as a common mechanism of action for cardio-oncology is not a big surprise.
References
- Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 3 October 2022).
- Available online: https://www.who.int/health-topics/cardiovascular-diseases#tab=tab_1 (accessed on 3 October 2022).
- Seton-Rogers, S. Cardiovascular disease and cancer communicate. Nat. Rev. Cancer 2020, 20, 552.
- Knisely, J.P.S.; Henry, S.A.; Saba, S.G.; Puckett, L.L. Cancer and cardiovascular disease. Lancet 2020, 395, 1904.
- De Boer, R.A.; Meijers, W.C.; van der Meer, P.; van Veldhuisen, D.J. Cancer and heart disease: Associations and relations. Eur. J. Heart Fail. 2019, 21, 1515–1525.
- Wang, Y.; Wang, Y.; Han, X.; Sun, J.; Li, C.; Adhikari, B.K.; Zhang, J.; Miao, X.; Chen, Z. Cardio-Oncology: A Myriad of Relationships Between Cardiovascular Disease and Cancer. Front. Cardiovasc. Med. 2022, 9, 727487.
- Koutsoukis, A.; Ntalianis, A.; Repasos, E.; Kastritis, E.; Dimopoulos, M.A.; Paraskevaidis, I. Cardio-oncology: A Focus on Cardiotoxicity. Eur. Cardiol. Rev. 2018, 13, 64–69.
- Ruoslahti, E. Specialization of tumour vasculature. Nat. Rev. Cancer 2002, 2, 83–90.
- Malakar, A.K.; Choudhury, D.; Halder, B.; Paul, P.; Uddin, A.; Chakraborty, S. A review on coronary artery disease, its risk factors, and therapeutics. J. Cell. Physiol. 2019, 234, 16812–16823.
- Hiam-Galvez, K.J.; Allen, B.M.; Spitzer, M.H. Systemic immunity in cancer. Nat. Rev. Cancer 2021, 21, 345–359.
- Gordon-Weeks, A.; Yuzhalin, A.E. Cancer Extracellular Matrix Proteins Regulate Tumour Immunity. Cancers 2020, 12, 3331.
- Gonzalez, H.; Hagerling, C.; Werb, Z. Roles of the immune system in cancer: From tumor initiation to metastatic progression. Genes Dev. 2018, 32, 1267–1284.
- Sorriento, D.; Iaccarino, G. Inflammation and Cardiovascular Diseases: The Most Recent Findings. Int. J. Mol. Sci. 2019, 20, 3879.
- Lopez-Candales, A.; Hernandez Burgos, P.M.; Hernandez-Suarez, D.F.; Harris, D. Linking Chronic Inflammation with Cardiovascular Disease: From Normal Aging to the Metabolic Syndrome. J. Nat. Sci. 2017, 3, e341.
- Mason, J.C.; Libby, P. Cardiovascular disease in patients with chronic inflammation: Mechanisms underlying premature cardiovascular events in rheumatologic conditions. Eur. Heart J. 2015, 36, 482–489.
- Boyd, D.F.; Thomas, P.G. Towards integrating extracellular matrix and immunological pathways. Cytokine 2017, 98, 79–86.
- Pickup, M.W.; Mouw, J.K.; Weaver, V.M. The extracellular matrix modulates the hallmarks of cancer. EMBO Rep. 2014, 15, 1243–1253.
- Ichiki, T.; Schirger, J.A.; Huntley, B.K.; Brozovich, F.V.; Maleszewski, J.J.; Sandberg, S.M.; Sangaralingham, S.J.; Park, S.J.; Burnett, J.C., Jr. Cardiac fibrosis in end-stage human heart failure and the cardiac natriuretic peptide guanylyl cyclase system: Regulation and therapeutic implications. J. Mol. Cell. Cardiol. 2014, 75, 199–205.
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297.
- Gebert, L.F.R.; MacRae, I.J. Regulation of microRNA function in animals. Nat. Rev. Mol. Cell Biol. 2019, 20, 21–37.
- Chen, Y.; Wang, X. miRDB: An online database for prediction of functional microRNA targets. Nucleic Acids Res. 2020, 48, D127–D131.
- Agarwal, V.; Bell, G.W.; Nam, J.W.; Bartel, D.P. Predicting effective microRNA target sites in mammalian mRNAs. Elife 2015, 4, e05005.
- Paraskevopoulou, M.D.; Georgakilas, G.; Kostoulas, N.; Vlachos, I.S.; Vergoulis, T.; Reczko, M.; Filippidis, C.; Dalamagas, T.; Hatzigeorgiou, A.G. DIANA-microT web server v5.0: Service integration into miRNA functional analysis workflows. Nucleic Acids Res. 2013, 41, W169–W173.
- Chen, S.; Wang, Y.; Li, D.; Wang, H.; Zhao, X.; Yang, J.; Chen, L.; Guo, M.; Zhao, J.; Chen, C.; et al. Mechanisms Controlling MicroRNA Expression in Tumor. Cells 2022, 11, 2852.
- Ding, L.; Gu, H.; Xiong, X.; Ao, H.; Cao, J.; Lin, W.; Yu, M.; Lin, J.; Cui, Q. MicroRNAs Involved in Carcinogenesis, Prognosis, Therapeutic Resistance and Applications in Human Triple-Negative Breast Cancer. Cells 2019, 8, 1492.
- Kansakar, U.; Varzideh, F.; Mone, P.; Jankauskas, S.S.; Santulli, G. Functional Role of microRNAs in Regulating Cardiomyocyte Death. Cells 2022, 11, 983.
- Song, R.; Hu, X.Q.; Zhang, L. Mitochondrial MiRNA in Cardiovascular Function and Disease. Cells 2019, 8, 1475.
- Mirna, M.; Paar, V.; Rezar, R.; Topf, A.; Eber, M.; Hoppe, U.C.; Lichtenauer, M.; Jung, C. MicroRNAs in Inflammatory Heart Diseases and Sepsis-Induced Cardiac Dysfunction: A Potential Scope for the Future? Cells 2019, 8, 1352.
- Forterre, A.; Komuro, H.; Aminova, S.; Harada, M. A Comprehensive Review of Cancer MicroRNA Therapeutic Delivery Strategies. Cancers 2020, 12, 1852.
- Szczepanek, J.; Skorupa, M.; Tretyn, A. MicroRNA as a Potential Therapeutic Molecule in Cancer. Cells 2022, 11, 1008.
- Momin, M.Y.; Gaddam, R.R.; Kravitz, M.; Gupta, A.; Vikram, A. The Challenges and Opportunities in the Development of MicroRNA Therapeutics: A Multidisciplinary Viewpoint. Cells 2021, 10, 3097.
- O’Neill, C.P.; Dwyer, R.M. Nanoparticle-Based Delivery of Tumor Suppressor microRNA for Cancer Therapy. Cells 2020, 9, 521.
- Seo, H.A.; Moeng, S.; Sim, S.; Kuh, H.J.; Choi, S.Y.; Park, J.K. MicroRNA-Based Combinatorial Cancer Therapy: Effects of MicroRNAs on the Efficacy of Anti-Cancer Therapies. Cells 2019, 9, 29.
- Quemener, A.M.; Centomo, M.L.; Sax, S.L.; Panella, R. Small Drugs, Huge Impact: The Extraordinary Impact of Antisense Oligonucleotides in Research and Drug Development. Molecules 2022, 27, 536.
- Cui, M.; Wang, H.; Yao, X.; Zhang, D.; Xie, Y.; Cui, R.; Zhang, X. Circulating MicroRNAs in Cancer: Potential and Challenge. Front. Genet. 2019, 10, 626.
- Wu, Y.; Li, Q.; Zhang, R.; Dai, X.; Chen, W.; Xing, D. Circulating microRNAs: Biomarkers of disease. Clin. Chim. Acta 2021, 516, 46–54.
- Creemers, E.E.; Tijsen, A.J.; Pinto, Y.M. Circulating microRNAs: Novel biomarkers and extracellular communicators in cardiovascular disease? Circ. Res. 2012, 110, 483–495.
- Murakami, Y.; Yasuda, T.; Saigo, K.; Urashima, T.; Toyoda, H.; Okanoue, T.; Shimotohno, K. Comprehensive analysis of microRNA expression patterns in hepatocellular carcinoma and non-tumorous tissues. Oncogene 2006, 25, 2537–2545.
- Tyebally, S.; Chen, D.; Bhattacharyya, S.; Mughrabi, A.; Hussain, Z.; Manisty, C.; Westwood, M.; Ghosh, A.K.; Guha, A. Cardiac Tumors: JACC CardioOncology State-of-the-Art Review. Cardio Oncol. 2020, 2, 293–311.
- Potente, M.; Carmeliet, P. The Link Between Angiogenesis and Endothelial Metabolism. Annu. Rev. Physiol. 2017, 79, 43–66.
- Chung, A.S.; Ferrara, N. Developmental and pathological angiogenesis. Annu. Rev. Cell. Dev. Biol. 2011, 27, 563–584.
- Lugano, R.; Ramachandran, M.; Dimberg, A. Tumor angiogenesis: Causes, consequences, challenges and opportunities. Cell. Mol. Life Sci. 2020, 77, 1745–1770.
- Eelen, G.; Treps, L.; Li, X.; Carmeliet, P. Basic and Therapeutic Aspects of Angiogenesis Updated. Circ. Res. 2020, 127, 310–329.
- Yla-Herttuala, S.; Bridges, C.; Katz, M.G.; Korpisalo, P. Angiogenic gene therapy in cardiovascular diseases: Dream or vision? Eur. Heart J. 2017, 38, 1365–1371.
- Korpela, H.; Jarvelainen, N.; Siimes, S.; Lampela, J.; Airaksinen, J.; Valli, K.; Turunen, M.; Pajula, J.; Nurro, J.; Yla-Herttuala, S. Gene therapy for ischaemic heart disease and heart failure. J. Intern. Med. 2021, 290, 567–582.
- Salinas-Vera, Y.M.; Marchat, L.A.; Gallardo-Rincon, D.; Ruiz-Garcia, E.; Astudillo-De La Vega, H.; Echavarria-Zepeda, R.; Lopez-Camarillo, C. AngiomiRs: MicroRNAs driving angiogenesis in cancer (Review). Int. J. Mol. Med. 2019, 43, 657–670.
- Wang, S.; Olson, E.N. AngiomiRs--key regulators of angiogenesis. Curr. Opin. Genet. Dev. 2009, 19, 205–211.
- Ota, A.; Tagawa, H.; Karnan, S.; Tsuzuki, S.; Karpas, A.; Kira, S.; Yoshida, Y.; Seto, M. Identification and characterization of a novel gene, C13orf25, as a target for 13q31-q32 amplification in malignant lymphoma. Cancer Res. 2004, 64, 3087–3095.
- Mogilyansky, E.; Rigoutsos, I. The miR-17/92 cluster: A comprehensive update on its genomics, genetics, functions and increasingly important and numerous roles in health and disease. Cell Death Differ. 2013, 20, 1603–1614.
- Mendell, J.T. miRiad roles for the miR-17-92 cluster in development and disease. Cell 2008, 133, 217–222.
- Chamorro-Jorganes, A.; Lee, M.Y.; Araldi, E.; Landskroner-Eiger, S.; Fernandez-Fuertes, M.; Sahraei, M.; Quiles Del Rey, M.; van Solingen, C.; Yu, J.; Fernandez-Hernando, C.; et al. VEGF-Induced Expression of miR-17-92 Cluster in Endothelial Cells Is Mediated by ERK/ELK1 Activation and Regulates Angiogenesis. Circ. Res. 2016, 118, 38–47.
- Gu, H.; Liu, Z.; Zhou, L. Roles of miR-17-92 Cluster in Cardiovascular Development and Common Diseases. Biomed. Res. Int. 2017, 2017, 9102909.
- Danielson, L.S.; Park, D.S.; Rotllan, N.; Chamorro-Jorganes, A.; Guijarro, M.V.; Fernandez-Hernando, C.; Fishman, G.I.; Phoon, C.K.; Hernando, E. Cardiovascular dysregulation of miR-17-92 causes a lethal hypertrophic cardiomyopathy and arrhythmogenesis. FASEB J. 2013, 27, 1460–1467.
- Li, M.; Guan, X.; Sun, Y.; Mi, J.; Shu, X.; Liu, F.; Li, C. miR-92a family and their target genes in tumorigenesis and metastasis. Exp. Cell Res. 2014, 323, 1–6.
- Bonauer, A.; Carmona, G.; Iwasaki, M.; Mione, M.; Koyanagi, M.; Fischer, A.; Burchfield, J.; Fox, H.; Doebele, C.; Ohtani, K.; et al. MicroRNA-92a controls angiogenesis and functional recovery of ischemic tissues in mice. Science 2009, 324, 1710–1713.
- Cavallari, I.; Ciccarese, F.; Sharova, E.; Urso, L.; Raimondi, V.; Silic-Benussi, M.; D’Agostino, D.M.; Ciminale, V. The miR-200 Family of microRNAs: Fine Tuners of Epithelial-Mesenchymal Transition and Circulating Cancer Biomarkers. Cancers 2021, 13, 5874.
- Humphries, B.; Yang, C. The microRNA-200 family: Small molecules with novel roles in cancer development, progression and therapy. Oncotarget 2015, 6, 6472–6498.
- Zhang, F.; Cheng, N.; Du, J.; Zhang, H.; Zhang, C. MicroRNA-200b-3p promotes endothelial cell apoptosis by targeting HDAC4 in atherosclerosis. BMC Cardiovasc. Disord. 2021, 21, 172.
- Li, W.J.; Wang, Y.; Liu, R.; Kasinski, A.L.; Shen, H.; Slack, F.J.; Tang, D.G. MicroRNA-34a: Potent Tumor Suppressor, Cancer Stem Cell Inhibitor, and Potential Anticancer Therapeutic. Front. Cell Dev. Biol. 2021, 9, 640587.
- Kalfert, D.; Ludvikova, M.; Pesta, M.; Ludvik, J.; Dostalova, L.; Kholova, I. Multifunctional Roles of miR-34a in Cancer: A Review with the Emphasis on Head and Neck Squamous Cell Carcinoma and Thyroid Cancer with Clinical Implications. Diagnostics 2020, 10, 563.
- Ito, T.; Yagi, S.; Yamakuchi, M. MicroRNA-34a regulation of endothelial senescence. Biochem. Biophys. Res. Commun. 2010, 398, 735–740.
- Yang, Y.; Cheng, H.W.; Qiu, Y.; Dupee, D.; Noonan, M.; Lin, Y.D.; Fisch, S.; Unno, K.; Sereti, K.I.; Liao, R. MicroRNA-34a Plays a Key Role in Cardiac Repair and Regeneration Following Myocardial Infarction. Circ. Res. 2015, 117, 450–459.
- Boon, R.A.; Iekushi, K.; Lechner, S.; Seeger, T.; Fischer, A.; Heydt, S.; Kaluza, D.; Treguer, K.; Carmona, G.; Bonauer, A.; et al. MicroRNA-34a regulates cardiac ageing and function. Nature 2013, 495, 107–110.
- Huang, Y.; Qi, Y.; Du, J.Q.; Zhang, D.F. MicroRNA-34a regulates cardiac fibrosis after myocardial infarction by targeting Smad4. Expert Opin. Ther. Targets 2014, 18, 1355–1365.
- Chen, Q.; Yang, F.; Guo, M.; Wen, G.; Zhang, C.; Zhu, J.; Xiao, Q.; Zhang, L. miRNA-34a reduces neointima formation through inhibiting smooth muscle cell proliferation and migration. J. Mol. Cell. Cardiol. 2015, 89, 75–86.
- Badi, I.; Mancinelli, L.; Polizzotto, A.; Ferri, D.; Zeni, F.; Burba, I.; Milano, G.; Brambilla, F.; Saccu, C.; Bianchi, M.E.; et al. miR-34a Promotes Vascular Smooth Muscle Cell Calcification by Downregulating SIRT1 (Sirtuin 1) and Axl (AXL Receptor Tyrosine Kinase). Arterioscler. Thromb. Vasc. Biol. 2018, 38, 2079–2090.
More
Information
Subjects:
Cardiac & Cardiovascular Systems; Oncology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
681
Revisions:
2 times
(View History)
Update Date:
21 Nov 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




