Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Shizuka Uchida and Version 2 by Dean Liu.
Angiogenesis is the process of new blood vessel formation through the migration, growth, and differentiation of endothelial cells.
- cancer
- cardiovascular disease
- miRNA
1. Introduction
Cancer and cardiovascular disease are the leading causes of death across the globe accounting for one in six deaths [1] and 32% of all deaths worldwide [2], respectively, according to World Health Organization (WHO). Both cancer and cardiovascular disease are the umbrella terms commonly used to describe several disease etiologies. Each etiology of cancer and cardiovascular disease (e.g., lung cancer and ischemic heart disease, respectively) has its own distinct cause and progression pattern. However, recent research suggests that many aspects of cancer and cardiovascular disease are similar in terms of pathogenesis [3][4][5][3,4,5], leading to the development of specific field of study called cardio-oncology [6][7][6,7]. For example, both diseases involve dysregulated functionalities in vasculature, where abnormal vasculature (called, tumor vasculature [8]) occurs in cancer, while coronary artery disease is a type of cardiovascular disease caused by the narrowing or blockage of coronary arteries [9]. Another example is the involvement of immune responses, where prolonged or chronic inflammation is a hallmark of cancer [10][11][12][10,11,12] as well as cardiovascular disease [13][14][15][13,14,15]. The activation of immune responses often leads to the deposition of excessive extracellular matrices [16][17][16,17], which are another hallmark of cancer [17] and cardiac fibrosis as the end-stage of heart failure [18].
MicroRNAs (miRNAs) are evolutionary-conserved, regulatory short [~22 nucleotides (nt)] non-protein-coding RNAs that function as translational inhibitors by binding to the 3′-untranslated regions (3′-UTRs) of messenger RNAs (mRNAs) [19][20][19,20]. As one miRNA is predicted to bind hundreds of mRNAs due to its very short seed sequence (~6 nt) [21][22][23][21,22,23], it is speculated and experimentally shown for some miRNAs to regulate cascades of signaling pathways and their downstream targets. Due to their versatilities, dysregulation in miRNAs is linked to a variety of diseases, including cancer [24][25][24,25] and cardiovascular disease [26][27][28][26,27,28]. As the regulatory importance of miRNAs is experimentally proven, the therapeutic silencing of miRNAs is being explored [29][30][31][32][33][34][29,30,31,32,33,34]. However, due to their biodistributions (e.g., including their presence in the circulation [35][36][37][35,36,37]) and the presence of many target mRNAs for one miRNA, the precise mechanistic elucidation of each miRNA is urgently needed to advance into clinics. Since a specific miRNA is highly dependent on which target mRNAs are present in a specific biological context, it must be taken into consideration that the same miRNA can yield different biological outcomes depending on the specific cell or tissue [38]. This is especially important when considering miRNAs as potential therapeutic targets.
As cancer and cardiovascular disease share several aspects of disease causes and progressions, it is no surprise that many miRNAs are shown to be involved in pathogeneses of both cancer and cardiovascular disease. Because the heart is the least likely organ to harbor tumor growth [39], the communication between researchers working in miRNAs for either cancer or cardiovascular biology is scarce, although many miRNAs are found to be dysregulated in both diseases.
2. Angiogenesis: AngiomiRs
Angiogenesis is the process of new blood vessel formation through the migration, growth, and differentiation of endothelial cells [40][41][57,58]. In cancer, angiogenesis allows for a tumor to grow as new vessels provide nutrients and oxygen to malignant cells [42][43][59,60]. In cardiovascular disease, therapeutic angiogenesis aims to provide the blood flow to the ischemic heart tissue [44][45][61,62]. Thus, in both diseases, angiogenesis is an important therapeutic target, although the opposite effects are observed. During angiogenesis, several miRNAs are functionally involved, which has created a specific term to describe these angiogenesis-related miRNAs called, angiomiRs (Figure 12). AngiomiRs include miR-15/16, miR-17~92 cluster, miR-18a, miR-19, miR-21, miR-23b, miR-27a/b, miR-29b, miR-30, miR-34a, miR-57, miR-125b, miR-126, miR-128, miR-143, miR-145, miR-155, miR-192, miR-194, miR-199a, miR-200 family, miR-204, miR-210, miR-217, miR-296, miR-378, miR-484, miR-494, miR-497, miR-542-3p, miR-573, miR-642, and let-7b [46][47][63,64], which some are discussed below.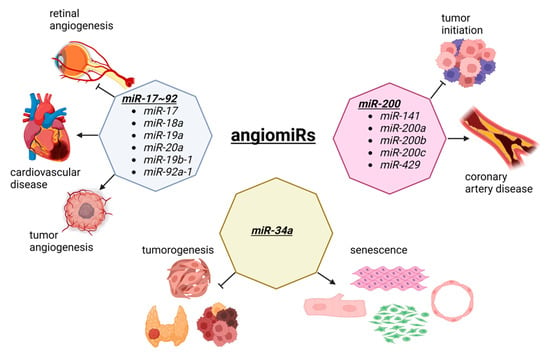
Figure 12. The dual role of angiomiRs in cancer and cardiac pathophysiology. The miR-17~92 cluster is involved in tumorigenesis and tumor vascularization. This cluster is also involved in retinal angiogenesis and the progression of cardiovascular disease. The members of the miR-200 family prevent the tumor initiation and malignant transformation, although they are upregulated in coronary artery disease. MiR-34a is a tumor suppressor involved in the development of thyroid cancer, head and neck squamous cell carcinoma, and cancer stem cells division. The overexpression of miR-34a suppress the proliferation and induces senescence in cardiomyocytes, fibroblasts, smooth muscle, and endothelial cells, by inhibiting sirtuin 1 (SIRT1). Figure created with BioRender.com, accessed on 24 October 2022.
