
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Rohit Goyat | -- | 1595 | 2022-11-17 05:13:50 | | | |
| 2 | Vivi Li | Meta information modification | 1595 | 2022-11-18 02:41:37 | | |
Video Upload Options
The term graphene was coined using the prefix “graph” taken from graphite and the suffix “-ene” for the C=C bond, by Boehm et al. in 1986. The synthesis of graphene can be done using various methods. The synthesized graphene was further oxidized to graphene oxide (GO) using different methods, to enhance its multitude of applications. Graphene oxide (GO) is the oxidized analogy of graphene, familiar as the only intermediate or precursor for obtaining the latter at a large scale. Graphene oxide has recently obtained enormous popularity in the energy, environment, sensor, and biomedical fields and has been handsomely exploited for water purification membranes. GO is a unique class of mechanically robust, ultrathin, high flux, high-selectivity, and fouling-resistant separation membranes that provide opportunities to advance water desalination technologies. The facile synthesis of GO membranes opens the doors for ideal next-generation membranes as cost-effective and sustainable alternative to long existing thin-film composite membranes for water purification applications.
1. Introduction
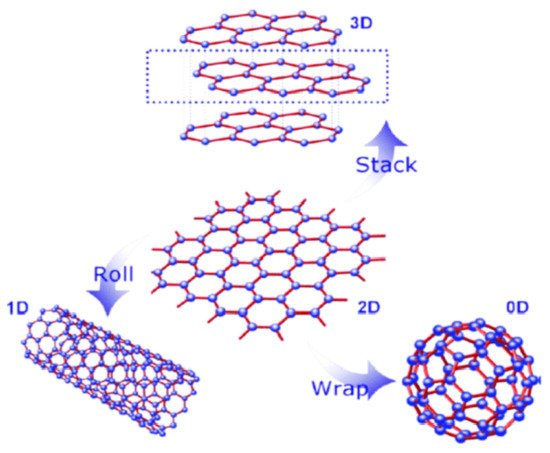
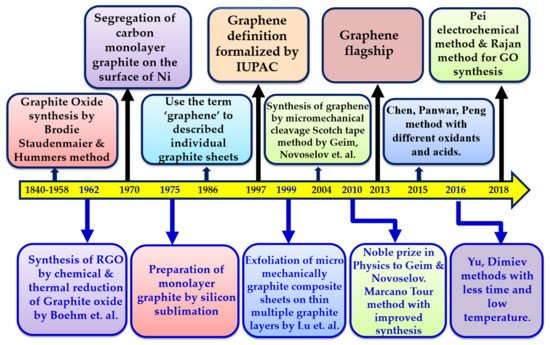
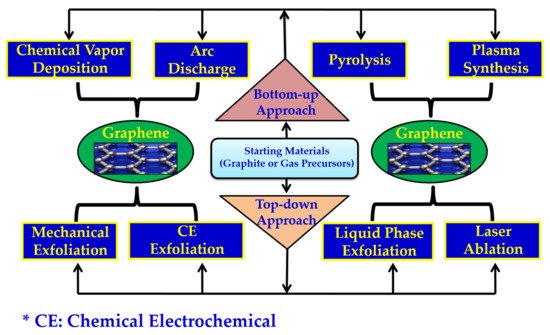
2. General Methods of Graphene Synthesis
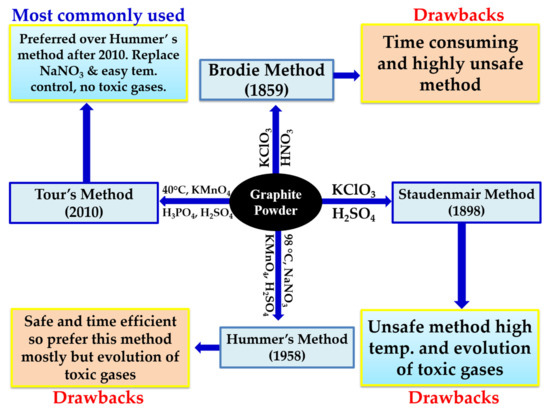
3. Graphene Oxide (GO)
3.1. Synthesis of GO
| Methods | Year | Starting Material | Different Oxidants Used | Reaction Time for GO Synthesis | Temperature °C | Features | References |
|---|---|---|---|---|---|---|---|
| Brodie | 1859 | Graphite | KclO3, HNO3 | 3–4 days | 60 | First attempt to synthesize GO | [45] |
| Staudenmaier | 1898 | Graphite | KclO3, H2SO4, HNO3 | 96 h | Room temperature | Improved efficiency | [46] |
| Hummers | 1958 | Graphite | KmnO4, H2SO4, NaNO3 | <2 h | <20–35–98 | Water-free, less than 2 h of reaction time | [47] |
| Fu | 2005 | Graphite | KmnO4, H2SO4, NaNO3 | <2 h | 35 | Validation of NaNO3 | [48] |
| Shen | 2009 | Graphite | Benzoyl peroxide | 10 min | 110 | Fast and non-acidic | [49] |
| Su | 2009 | Graphite | KmnO4, H2SO4 | 4 h | Room temperature | Large-size GO sheets formed | [50] |
| Marcano and Tour | 2010 & 2018 | Graphite | KmnO4, H3PO4, H2SO4 | 12 h | 50 | Eco-friendly resulting in a high yield | [51] |
| Sun | 2013 | Graphite | KmnO4, H2SO4 | 1.5 h | Room temperature-90 | High-yield and safe method | [52] |
| Eigler | 2013 | Graphite | KmnO4, NaNO3, H2SO4 | 16 h | 10 | High-quality GO produced | [53] |
| Chen | 2015 | Graphite | KmnO4, H2SO4 | <1 h | 40–95 | High-yield product | [54] |
| Panwar | 2015 | Graphite | H2SO4, H3PO4, KmnO4, HNO3 | 3 h | 50 | Three component acids and high-yield product | [55] |
| Peng | 2015 | Graphite | K2FeO4, H2SO4 | 1 h | Room temperature | Results in a high-yield and eco-friendly method | [56] |
| Rosillo-Lopez | 2016 | Graphite | HNO3 | 20 h | Room temperature | Nano-sized GO obtained | [57] |
| Yu | 2016 | Graphite | K2FeO4, KmnO4 H2SO4, H3BO3 (NH4)2S2O8 | 5 h | <5–35–95 | Low manganite impurities and high yield obtained | [58] |
| Dimiev | 2016 | Graphite | 98% H2SO4, fuming H2SO4 | 3–4 h | Room temperature | 25 nm thick and ~100%conversion rate | [59] |
| Pei | 2018 | Graphite foil | H2SO4 | <5 min | Room temperature | High efficiency | [60] |
| Ranjan | 2018 | Graphite | H2SO4, H3PO4, KmnO4 | <24 h | <RT-35–95 | Cooled exothermal reaction to make the process safe |
[61] |
| S. No. | Source of Carbon | H2SO4 (in mL) |
Other Ingredients | Temp. (in °C) |
Time (in h) |
C:O | Colour of GO Obtained |
|---|---|---|---|---|---|---|---|
| 1 | Graphite | 15.0 | 1.0 g Na2Cr2O7 | 30 | 72 | 16:1 | Black |
| 2 | Graphite | 15.0 | 4.0 g Na2Cr2O7 | 30 | 72 | 3.4:1 | Black |
| 3 | Graphite | 15.0 | 15.0 mL 70% HNO3 3.0 g KmnO4, |
30 | 24 | -- | Black |
| 4 | Graphite | 20.0 | 11.0 g KclO3, 10.0 mL 70% HNO3 |
0–60 | 33 | 3.1:1 | Midnight green |
| 5 | Graphite | 30.0 | 3.0 g KmnO4,1.0 g NaNO3 |
30 | 2 | 3.0:1 | Bluish green |
| 6 | Graphite | 30.0 | 3.0 g KmnO4,1.0 g NaNO3 |
45 | 1 | -- | Green |
| 7 | Graphite | 22.5 | 3.0 g KmnO4,1.0 g NaNO3 |
45 | 1 | -- | Brittle yellow |
| 8 | Graphite | 22.5 | 3.0 g KmnO4,0.5 g NaNO3 |
45 | 1 | -- | Yellow |
| 9 | Graphite | 22.5 | 3.0 g KmnO4,0.5 g NaNO3 |
45 | 0.5 | 2.3:1 | Yellow |
| 10 | Graphite | 22.5 | 3.0 g KmnO4,0.5 g NaNO3 |
35 | 0.5 | 2.05:1 | Bright yellow |
| 11 | Graphite | 22.5 | 3.0 g KmnO4, 1.0 g fuming HNO3 |
35 | 1 | -- | Bright yellow |
| 12 | Graphite | 22.5 | 3.0 g KmnO4, 1.0 g BaNO3 |
45 | 2 | -- | Light green |
3.2. Structural Aspects of GO
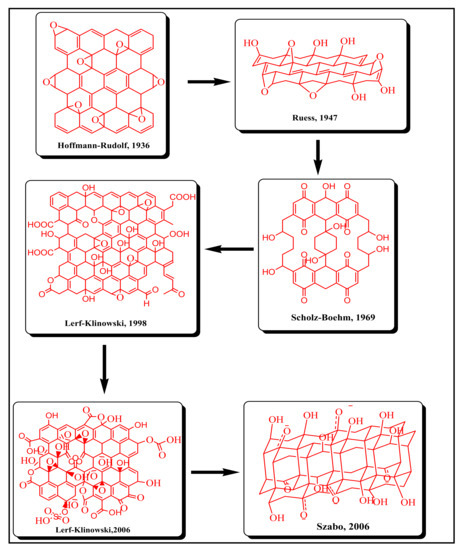
3.3. Characterization of GO
References
- Xu, S.; Che, J.; An, Y.; Zhu, T.; Pang, L.; Liu, X.; Li, X.; Lv, M. Research and Preparation of Graphene Foam Based on Passive Ultra-Low Energy Consumption Building New Ultra-Thin External Wall Insulation Material. J. Nanoelectron. Optoelectron. 2021, 16, 1467–1474.
- Banciu, C.A.; Nastase, F.; Istrate, A.-I.; Veca, L.M. 3D Graphene Foam by Chemical Vapor Deposition: Synthesis, Properties, and Energy-Related Applications. Molecules 2022, 27, 3634.
- Umar, A.; Kumar, S.A.; Inbanathan, S.S.R.; Modarres, M.; Kumar, R.; Algadi, H.; Baskoutas, S. Enhanced sunlight-driven photocatalytic, supercapacitor and antibacterial applications based on graphene oxide and magnetite-graphene oxide nanocomposites. Ceram. Int. 2022, 48, 29349–29358.
- Isaeva, V.I.; Vedenyapina, M.D.; Kurmysheva, A.Y.; Weichgrebe, D.; Nair, R.R.; Nguyen, N.P.T.; Kustov, L.M. Modern Carbon–Based Materials for Adsorptive Removal of Organic and Inorganic Pollutants from Water and Wastewater. Molecules 2021, 26, 6628.
- Pelosato, R.; Bolognino, I.; Fontana, F.; Sora, I.N. Applications of Heterogeneous Photocatalysis to the Degradation of Oxytetracycline in Water: A Review. Molecules 2022, 27, 2743.
- Huang, H.-H.; Joshi, R.K.; de Silva, K.K.H.; Badam, R.; Yoshimura, M.J. Fabrication of reduced graphene oxide membranes for water desalination. Membr. Sci. 2019, 572, 12.
- Franco, P.; Cardea, S.; Tabernero, A.; De Marco, I. Porous Aerogels and Adsorption of Pollutants from Water and Air: A Review. Molecules 2021, 26, 4440.
- Santoso, E.; Ediati, R.; Kusumawati, Y.; Bahruji, H.; Sulistiono, D.O.; Prasetyoko, D. Mater. Today Chem. 2020, 16, 100233.
- Xiao, C.; Li, C.; Hu, J.; Zhu, L. The Application of Carbon Nanomaterials in Sensing, Imaging, Drug Delivery and Therapy for Gynecologic Cancers: An Overview. Molecules 2022, 27, 4465.
- Guo, W.; Umar, A.; Alsaiari, M.A.; Wang, L.; Pei, M. Ultrasensitive and selective label-free aptasensor for the detection of penicillin based on nanoporousPtTi/graphene oxide-Fe3O4/MWCNT-Fe3O4 nanocomposite. Microchem. J. 2020, 158, 105270.
- Pandey, R.R.; Chusuei, C.C. Carbon Nanotubes, Graphene, and Carbon Dots as Electrochemical Biosensing Composites. Molecules 2021, 26, 6674.
- Hu, H.; Liang, H.; Fan, J.; Guo, L.; Li, H.; de Rooij, N.F.; Umar, A.; Algarni, H.; Wang, Y.; Zhou, G. Assembling Hollow Cactus-Like ZnO Nanorods with Dipole-Modified Graphene Nanosheets for Practical Room-Temperature Formaldehyde Sensing. ACS Appl. Mater. Interfaces 2022, 14, 13186–13195.
- Al Fatease, A.; Guo, W.; Umar, A.; Zhao, C.; Alhamhoom, Y.; Muhsinah, A.B.; Mahnashi, M.H.; Ansari, Z.A. A dual-mode electrochemical aptasensor for the detection of Mucin-1 based on AuNPs-magnetic graphene composite. Microchem. J. 2022, 180, 107559.
- Treerattrakoon, K.; Jiemsakul, T.; Tansarawiput, C.; Pinpradup, P.; Iempridee, T.; Luksirikul, P.; Khoothiam, K.; Dharakul, T.; Japrung, D. Rolling circle amplification and graphene-based sensor-on-a-chip for sensitive detection of serum circulating miRNAs. Anal. Biochem. 2019, 577, 89.
- Cosma, D.; Urda, A.; Radu, T.; Rosu, M.C.; Mihet, M.; Socaci, C. Evaluation of the Photocatalytic Properties of Copper Oxides/Graphene/TiO2 Nanoparticles Composites. Molecules 2022, 27, 5803.
- Wang, H.-L.; Wang, Z.-G.; Liu, S.-L. Lipid Nanoparticles for mRNA Delivery to Enhance Cancer Immunotherapy. Molecules 2022, 27, 5607.
- Gong, Y.; Li, H.; Pei, W.; Fan, J.; Umar, A.; Al-Assiri, M.S.; Wang, Y.; de Rooij, N.; Zhou, G. Assembly with copper(II) ions and D–π–A molecules on a graphene surface for ultra-fast acetic acid sensing at room temperature. RSC Adv. 2019, 9, 30432–30438.
- Gong, P.; Zhang, L.; Yuan, X.; Liu, X.; Diao, X.; Zhao, Q.; Tian, Z.; Sun, J.; Liu, Z.; You, J. Multifunctional fluorescent PEGylated fluorinated graphene for targeted drug delivery: An experiment and DFT study. Dye. Pigment. 2019, 162, 573.
- Xu, L.; Gai, L.; Yang, F.; Wu, S. Preparation of Nanographene and Its Effect on the Correlation Between N-Terminal Pro Brain-Type Natriuretic Peptide, Peripheral Blood Copeptin Level and Cardiac Function Grading of Patients with Heart Failure. Sci. Adv. Mater. 2021, 13, 1937–1944.
- Patil, T.V.; Patel, D.K.; Dutta, S.D.; Ganguly, K.; Lim, K.-T. Graphene Oxide-Based Stimuli-Responsive Platforms for Biomedical Applications. Molecules 2021, 26, 2797.
- Cirillo, G.; Pantuso, E.; Curcio, M.; Vittorio, O.; Leggio, A.; Iemma, F.; De Filpo, G.; Nicoletta, F.P. Alginate Bioconjugate and Graphene Oxide in Multifunctional Hydrogels for Versatile Biomedical Applications. Molecules 2021, 26, 1355.
- Bahrami, S.; Solouk, A.; Mirzadeh, H.; Seifalian, A.M. Electroconductive polyurethane/graphene nanocomposite for biomedical applications. Compos. Part B Eng. 2019, 168, 421–431.
- Wang, W.; Junior, J.R.P.; Nalesso, P.R.L.; Musson, D.; Cornish, J.; Mendonça, F.; Caetano, G.F.; Bártolo, P. Engineered 3D printed poly (ɛ-caprolactone)/graphene scaffolds for bone tissue engineering. Mater. Sci. Eng. C 2019, 100, 759.
- Zhang, S.; Zhuang, Y.; Jin, S.; Wang, K. Preparation of Nanoscale Graphene Oxide and Its Application on β-Catenin Expression in Pediatric Osteosarcoma Cells Combine with Probiotics. Sci. Adv. Mater. 2021, 13, 2131–2137.
- Bai, H.; Guo, H.; Wang, J.; Dong, Y.; Liu, B.; Xie, Z.; Guo, F.; Chen, D.; Zhang, R.; Zheng, Y. A room-temperature NO2 gas sensor based on CuO nanoflakes modified with rGO nanosheets. Sens. Actuators B Chem. 2021, 337, 129783.
- Hussein-Al-Ali, S.H.; Hussein Alali, S.H.; Al-Ani, R.; Alkrad, J.A.; Abudoleh, S.M.; Abdallah Abualassal, Q.I.; Ayoub, R.; Hussein, M.Z.; Bullo, S.; Palanisamy, A. Betulinic Acid-Graphene Oxide Nanocomposites for Cancer Treatment. Sci. Adv. Mater. 2021, 13, 2138–2148.
- Singh, J.; Goyat, R. Graphene oxide: Synthesis, characterization and its applications. Res. J. Chem. Environ. 2022, 26, 150–156.
- Wan, X.; Huang, Y.; Chen, Y. Focusing on energy and optoelectronic applications: A journey for graphene and graphene oxide at large scale. Acc. Chem. Res. 2012, 45, 598–607.
- Ramu, A.G.; Umar, A.; Ibrahim, A.A.; Algadi, H.; Ibrahim, Y.S.A.; Choi, Y.W.D. Synthesis of porous 2D layered nickel oxide-reduced graphene oxide (NiO-rGO) hybrid composite for the efficient electrochemical detection of epinephrine in biological fluid. Environ. Res. 2021, 200, 111366.
- Santosh, K.; Sahoo, S.; Wang, N.; Huczko, A. Graphene research and their outputs: Status and prospect. J. Sci. Adv. Mater. Devices 2020, 5, 10–29, ISSN 2468-2179.
- Aliofkhazraei, M.; Ali, N.; Milne, W.I.; Ozkan, C.S.; Mitura, S.; Gervasoni, J.L. Graphene Science Handbook: Fabrication Methods; CRC Press: Boca Raton, FL, USA, 2016; p. 587.
- Dasari, B.L.; Nouri, J.M.; Brabazon, D.; Naher, S. Graphene and derivatives–Synthesis techniques, properties and their energy applications. Energy 2017, 140, 766.
- Toh, S.Y.; Loh, K.S.; Kamarudin, S.K.; Daud, W.R.W. Graphene production via electrochemical reduction of graphene oxide: Synthesis and characterization. Chem. Eng. J. 2014, 251, 422.
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666.
- Ma, J.; Syed, J.A.; Su, D. Hybrid Supercapacitors Based on Self-Assembled Electrochemical Deposition of Reduced Graphene Oxide/Polypyrrole Composite Electrodes. J. Nanoelectron. Optoelectron. 2021, 16, 949–956.
- Taghioskoui, M. Trends in graphene research. Mater. Today 2009, 12, 34–37.
- Chen, Z.; Wang, J.; Cao, N.; Wang, Y.; Li, H.; de Rooij, N.F.; Umar, A.; Feng, Y.; French, P.J.; Zhou, G. Three-Dimensional Graphene-Based Foams with “Greater Electron Transferring Areas” Deriving High Gas Sensitivity. ACS Appl. Nano Mater. 2021, 4, 13234–13245.
- Wang, C.; Peng, Q.; Wu, J.; He, X.; Tong, L.; Luo, Q.; Li, J.; Moody, S.; Liu, H.; Wang, R.; et al. Mechanical characteristics of individual multi-layer graphene-oxide sheets under direct tensile loading. Carbon 2014, 80, 279–289.
- Pei, W.; Zhang, T.; Wang, Y.; Chen, Z.; Umar, A.; Li, H.; Guo, W. Enhancement of Charge Transfer between Graphene and Donor-π-Acceptor Molecule for Ultrahigh Sensing Performance. Nanoscale 2017, 9, 16273–16280.
- Zhang, J.; Li, P.; Zhang, Z.; Wang, X.; Tang, J.; Liu, H.; Shao, Q.; Ding, T.; Umar, A.; Guo, Z. Solvent-free graphene liquids: Promising candidates for lubricants without the base oil. J. Colloid Interface Sci. 2019, 542, 159–167.
- Chen, Z.; Wang, Y.; Shang, Y.; Umar, A.; Xie, P.; Qi, Q.; Zhou, G. One-Step Fabrication of Pyranine Modified- Reduced Graphene Oxide with Ultrafast and Ultrahigh Humidity Response. Sci. Rep. 2017, 7, 2713.
- Erickson, K.; Erni, R.; Lee, Z.; Alem, N.; Gannett, W.; Zettl, A. Determination of the local chemical structure of graphene oxide and reduced graphene oxide. Adv. Mater. 2010, 22, 4467–4472.
- Su, C.; Acik, M.; Takai, K.; Lu, J.; Hao, S.-J.; Zheng, Y.; Wu, P.; Bao, Q.; Enoki, T.; Chabal, Y.J. Probing the catalytic activity of porous graphene oxide and the origin of this behaviour. Nat. Comm. 2012, 3, 1298.
- Schafhaeutl, C. Ueber die Verbindungen des Kohlenstoffes Mit Silicium, Eisen und Andern Metallen, Welche die Verschiedenen Arten von Gusseisen, Stahl und Schmiedeeisen Bilden. J. Prakt. Chem. 1840, 19, 159–174.
- Brodie, B.C. On the atomic weight of graphite. Philos. Trans. R. Soc. London 1859, 149, 249–259.
- Staudenmaier, L. Verfahrenzurdarstellung der graphits äure. Ber. Dtsch. Chem. Ges. 1898, 31, 1481–1487.
- Hummers Jr, W.S.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339.
- Fu, L.; Liu, H.B.; Zou, Y.; Li, B. Technology research on oxidative degree of graphite oxide prepared by Hummers method. Carbon 2005, 124, 10–14. (In Chinese)
- Jeong, T.K.; Choi, M.K.; Sim, Y.; Lim, J.T.; Kim, G.S.; Seong, M.J.; Hyung, J.H.; Kim, K.S.; Umar, A.; Lee, S.K. Effect of graphene oxide ratio on the cell adhesion and growth behavior on a graphene oxide-coated silicon substrate. Sci. Rep. 2016, 6, 33835.
- Su, C.Y.; Xu, Y.; Zhang, W.; Zhao, J.; Tang, X.; Tsai, C.H.; Li, L.J. Electrical and spectroscopic characterizations of ultra-large reduced graphene oxide monolayers. Chem. Mater. 2009, 21, 5674–5680.
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved synthesis of graphene oxide. ACS Nano 2010, 4, 4806–4814.
- Sun, L.; Fugetsu, B. Mass production of graphene oxide from expanded graphite. Mater. Lett. 2013, 109, 207–210.
- Eigler, S.; Enzelberger-Heim, M.; Grimm, S.; Hofmann, P.; Kroener, W.; Geworski, A.; Dotzer, C.; Röckert, M.; Xiao, J.; Papp, C.; et al. Wet chemical synthesis of graphene. Adv. Mater. 2013, 25, 3583–3587.
- Chen, J.; Li, Y.; Huang, L.; Li, C.; Shi, G. High-yield preparation of graphene oxide from small graphite flakes via an improved Hummers method with a simple purification process. Carbon 2015, 81, 826–834.
- Panwar, V.; Chattree, A.; Pal, K. A new facile route for synthesizing of graphene oxide using mixture of sulfuric–nitric–phosphoric acids as intercalating agent. Phys. E Low-Dimens. Syst. Nanostruct. 2015, 73, 235–241.
- Peng, L.; Xu, Z.; Liu, Z.; Wei, Y.; Sun, H.; Li, Z.; Zhao, X.; Gao, C. An iron-based green approach to 1-hproduction of single-layer graphene oxide. Nat. Commun. 2015, 6, 5716.
- Rosillo-Lopez, M.; Salzmann, C.G. A simple and mild chemical oxidation route to high-purity nano-graphene oxide. Carbon 2016, 106, 56–63.
- Yu, H.; Zhang, B.; Bulin, C.; Li, R.; Xing, R. High-efficient synthesis of graphene oxide based on improved Hummers method. Sci. Rep. 2016, 6, 36143.
- Dimiev, A.M.; Ceriotti, G.; Metzger, A.; Kim, N.D.; Tour, J.M. Chemical mass production of graphene nanoplatelets in ~100% yield. ACS Nano 2016, 10, 274–279.
- Pei, S.; Wei, Q.; Huang, K.; Cheng, H.M.; Ren, W. Green synthesis of graphene oxide by seconds timescale water electrolytic oxidation. Nat. Commun. 2018, 9, 145.
- Ranjan, P.; Agrawal, S.; Sinha, A.; Rajagopala Rao, T.; Balakrishnan, J.; Thakur, A.D. A low-costnon-explosive synthesis of graphene oxide for scalable applications. Sci. Rep. 2018, 8, 12007.
- Hummers, J.W.S. Preparation of Graphitic Acid. U.S. Patent 2,798,878, 9 July 1957.
- Hofmann, U.; Holst, R. Über die Säurenatur und die Methylierung von Graphitoxyd. Ber. Dtsch. Chem. Ges. A B 1939, 72, 754–771.
- Ruess, G. Ber das Graphitoxyhydroxyd (Graphitoxyd). Mon. Fr. Chem. 1947, 76, 381–417.
- Scholz, W.; Boehm, H.P. Untersuchungen am Graphitoxid. VI. Betrachtungen zur Struktur des Graphitoxids. Z. Anorg. Allg. Chem. 1969, 369, 327–340.
- Nakajima, T.; Matsuo, Y. Formation process and structure of graphite oxide. Carbon 1994, 32, 469–475.
- Lerf, A.; He, H.; Forster, M.; Klinowski, J. Structure of graphite oxide revisited II. J. Phys. Chem. B 1998, 102, 4477–4482.
- Savazzi, F.; Risplendi, F.; Mallia, G.; Harrison, N.M.; Cicero, G. Unravelling some of the structure–property relationships in graphene oxide at low degree of oxidation. J. Phys. Chem. Lett. 2018, 9, 1746–1749.
- Szabó, T.; Berkesi, O.; Forgó, P.; Josepovits, K.; Sanakis, Y.; Petridis, D.; Dékány, I. Evolution of surface functional groups in a series of progressively oxidized graphite oxides. Chem. Mater. 2006, 18, 2740–2749.
- Liu, Z.; Nørgaard, K.; Overgaard, M.H.; Ceccato, M.; Mackenzie, D.M.A.; Stenger, N.; Stipp, S.L.S.; Hassenkam, T. Direct observation of oxygen configuration on individual graphene oxide sheets. Carbon 2018, 127, 141–148.
- Chen, L.; Yu, H.; Zhong, J.; Song, L.; Wu, J.; Su, W. Graphene field emitters: A review of fabrication, characterization and properties. Mater. Sci. Eng. B 2017, 220, 44–58.
- Chen, X.; Fan, K.; Liu, Y.; Li, Y.; Liu, X.; Feng, W.; Wang, X. Recent advances in fluorinated graphene from synthesis to applications: Critical review on functional chemistry and structure engineering. Adv. Mater. 2022, 34, 2101665.
- Yin, P.T.; Shah, S.; Chhowalla, M.; Lee, K.B. Design, synthesis, and characterization of graphene–nanoparticle hybrid materials for bioapplications. Chem. Rev. 2015, 115, 2483–2531.
- Steinert, B.W.; Dean, D.R. Magnetic field alignment and electrical properties of solution cast PET–carbon nanotube composite films. Polymer 2009, 50, 898–904.
- Jia, R.; Xie, P.; Feng, Y.; Chen, Z.; Umar, A.; Wang, Y. Dipole-Modified Graphene with Ultrahigh Gas Sensibility. Appl. Surface Sci. 2018, 440, 409–414.
- Sun, Z.; Yan, Z.; Yao, J.; Beitler, E.; Zhu, Y.; Tour, J.M. Growth of graphene from solid carbon sources. Nature 2010, 468, 549–552.
- Shen, J.; Hu, Y.; Shi, M.; Lu, X.; Qin, C.; Li, C.; Ye, M. Fast and Facile Preparation of Graphene Oxide and Reduced Graphene Oxide Nanoplatelets. Chem. Mater. 2009, 21, 3514–3520.
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.B.T.; Ruoff, R.S. Synthesis of Graphene-Based Nanosheets via Chemical Reduction of Exfoliated Graphite Oxide. Carbon 2007, 45, 1558–1565.
- Zhao, J.; Liu, L.; Li, F. Structural Characterization. In Graphene Oxide: Physics and Applications; Springer: Berlin/Heidelberg, Germany, 2015; pp. 15–29, Chapter 2.
- Ganguly, A.; Sharma, S.; Papakonstantinou, P.; Hamilton, J. Probing the Thermal Deoxygenation of Graphene Oxide using High-Resolution in Situ X-ray-based Spectroscopies. J. Phys. Chem. C 2011, 115, 17009–17019.
- Hintermueller, D.; Prakash, R. Comprehensive Characterization of Solution-Cast Pristine and Reduced Graphene Oxide Composite Polyvinylidene Fluoride Films for Sensory Applications. Polymers 2022, 14, 2546.
- Ahmed, A.; Ibrahim, A.A.; Algadi, H.; Albargi, H.; Alsairi, M.A.; Wang, Y.; Akbar, S. Supramolecularly assembled isonicotinamide/ reduced graphene oxide nanocomposite for room-temperature NO2 gas sensor. Environ. Technol. Innov. 2022, 25, 102066.
- Bagri, A.; Mattevi, C.; Acik, M.; Chabal, Y.J.; Chhowalla, M.; Shenoy, V.B. Structural Evolution during the Reduction of Chemically Derived Graphene Oxide. Nat. Chem. 2010, 2, 581–587.
- Schniepp, H.C.; Li, J.-L.; McAllister, M.J.; Sai, H.; Herrera-Alonso, M.; Adamson, D.H.; Prud’homme, R.K.; Car, R.; Saville, D.A.; Aksay, I.A. Functionalized Single Graphene Sheets derived from Splitting Graphite Oxide. J. Phys. Chem. B 2006, 110, 8535–8539.
- Shahriary, L.; Athawale, A.A. Graphene Oxide Synthesized by using Modified Hummers Approach. Int. J. Renew. Energy Environ. Eng. 2014, 2, 58–63.
- Wei, N.; Peng, X.; Xu, Z. Understanding Water Permeation in Graphene Oxide Membranes. ACS Appl. Mater. Interfaces 2014, 6, 5877–5883.
- Zhang, H.-B.; Zheng, W.G.; Yan, Q.; Yang, Y.; Wang, J.W.; Lu, Z.H.; Ji, G.Y.; Yu, Z.Z. Electrically conductive polyethylene terephthalate/graphene nanocomposites prepared by melt compounding. Polymer 2010, 51, 1191–1196.
- Seekaew, Y.; Lokavee, S.; Phokharatkul, D.; Wisitsoraat, A.; Kerdcharoen, T.; Wongchoosuk, C. Low-cost and flexible printed graphene–PEDOT: PSS gas sensor for ammonia detection. Org. Electron. 2014, 15, 2971–2981.





