The term graphene was coined using the prefix “graph” taken from graphite and the suffix “-ene” for the C=C bond, by Boehm et al. in 1986. The synthesis of graphene can be done using various methods. The synthesized graphene was further oxidized to graphene oxide (GO) using different methods, to enhance its multitude of applications. Graphene oxide (GO) is the oxidized analogy of graphene, familiar as the only intermediate or precursor for obtaining the latter at a large scale. Graphene oxide has recently obtained enormous popularity in the energy, environment, sensor, and biomedical fields and has been handsomely exploited for water purification membranes. GO is a unique class of mechanically robust, ultrathin, high flux, high-selectivity, and fouling-resistant separation membranes that provide opportunities to advance water desalination technologies. The facile synthesis of GO membranes opens the doors for ideal next-generation membranes as cost-effective and sustainable alternative to long existing thin-film composite membranes for water purification applications.
1. Introduction
Graphene is a purified form of graphite that recently gained enormous popularity in the energy
[1][2][3][1,2,3], environment
[4][5][6][7][8][4,5,6,7,8], membranes
[1][7][1,7], sensor
[9][10][11][12][9,10,11,12], and biomedical fields
[13][14][15][16][17][18][19][20][21][22][23][24][25][26][13,14,15,16,17,18,19,20,21,22,23,24,25,26]. It is a sp
2 hybridized, hexagonally arranged, chain of polycyclic aromatic hydrocarbon with a honeycomb crystal lattice
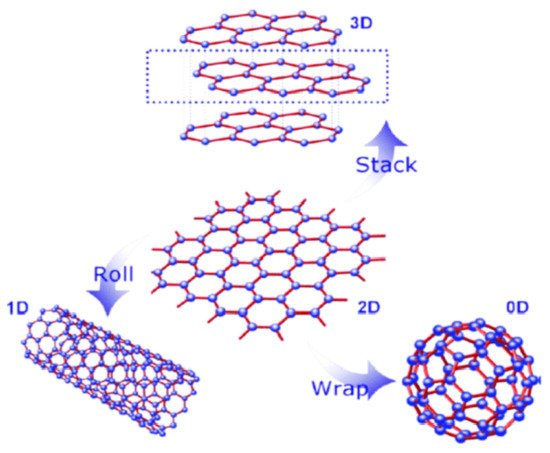
[27]. It is the most recent element of carbon allotropes and is actually the basic building block of other important carbon allotropes, including 3D graphite, 1D carbon nanotubes (CNTs), and 0D fullerene (C60), as shown in
Figure 1.
Figure 1. Structural representation of 2D graphene with different dimensions. [Reprinted with permission from ref.
[28], Wan, X., Huang, Y., & Chen, Y. (2012). Focusing on energy and optoelectronic applications: a journey for graphene and graphene oxide at large scale.
Accounts of chemical research,
45(4), 598–607. Copyright © American Chemical Society].
The name graphene was coined by Boehm in 1986
[1], taking the prefix “graph” from graphite and the suffix “-ene” for sp
2 hybridized carbon, and was finally accepted by the International Union for Pure and Applied Chemistry in 1997
[29][30][31][32][33][29,30,31,32,33]. Furthermore, it became famous worldwide in 2004 when Geim and Novoselov obtained a single sheet of graphene on solid support, for which they were honored with the Nobel Prize in Physics in 2010
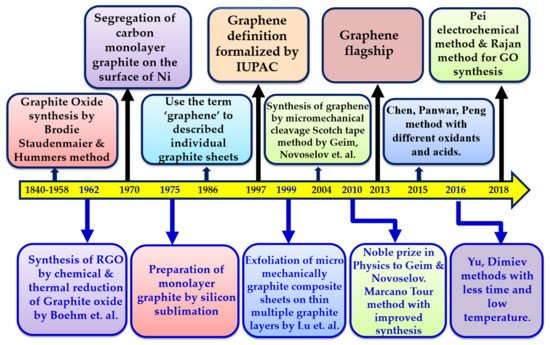
[34]. The main achievements of graphene in a timeline of history from 1840 to 2018 are shown in
Figure 2.
Figure 2.
Schematic representation of a graphene timeline.
2. General Methods of Graphene Synthesis
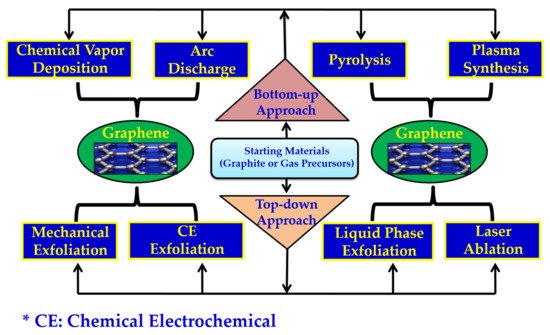
Generally, graphene can be synthesized using two different routes, viz, bottom-up and top-down
[33][35][36][33,35,36], as depicted in
Figure 3.
Figure 3.
Schematic representation of the general methods for graphene synthesis.
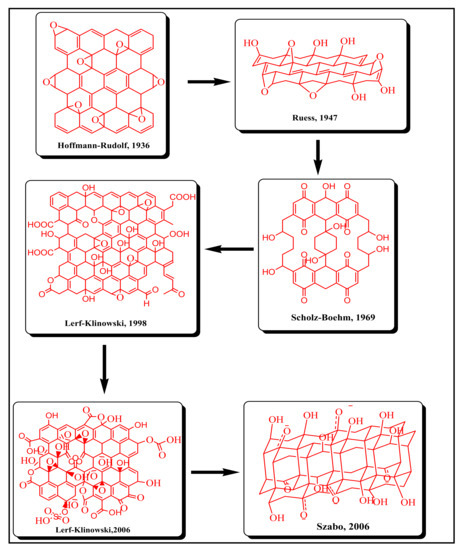
3. Graphene Oxide (GO)
In comparison to graphene, graphene oxide is considered a more versatile and advanced material. GO has a broad range of oxygen containing functional groups such as carboxyl, hydroxyl, epoxy, carbonyl, and keto groups on its surface.
GO has shown great potential in a variety of fields by virtue of its high surface area
[37][88], unique mechanical strength
[38][89], and excellent optical and magnetic properties
[39][90]. In comparison to other carbon-based nanomaterials, GO is considered a green oxidant, as it is enriched with oxygen-containing functional groups
[40][41][91,92]. Further, GO has an aromatic scaffold, which acts as a template to anchor active species behaving as an organo-catalyst
[42][43][93,94].
3.1. Synthesis of GO
In 1840, German scientist Schafhacutl was given the first report on the synthesis of graphene oxide and graphite intercalated compounds
[44][95]. For the very first time, he attempted to exfoliate graphite and tried to purify impure graphite “kish” from iron smelters
[27]. To date, several methods, as shown in
Table 12, have been proposed.
Table 12.
List of different methods used to synthesize graphene oxide.
| Methods |
Year |
Starting Material |
Different Oxidants Used |
Reaction Time for GO Synthesis |
Temperature °C |
Features |
References |
| Brodie |
1859 |
Graphite |
KclO3, HNO3 |
3–4 days |
60 |
First attempt to synthesize GO |
[45][96] |
2, N
2O
4 or ClO
2, along with easy temperature control and better yield
[51][102]. In addition to this, the GO suspension obtained was treated with hydrogen peroxide (H
2O
2) to eliminate all impurities due to permanganate and manganese dioxide.
Furthermore, the final color of the product GO varies from army green to light yellow, depending on the carbon-to-oxygen ratios
[62][113], as depicted in
Table 23.
Table 23. Effect of acid concentration, reaction temperature, reaction time, and the quantity of the oxidizing agent on the oxidation of graphene [62]. Effect of acid concentration, reaction temperature, reaction time, and the quantity of the oxidizing agent on the oxidation of graphene [113].
| S. No. |
Source of Carbon |
H2SO4
(in mL) |
Other Ingredients |
Temp.
(in °C) |
Time
(in h) |
C:O |
Colour of GO Obtained |
| 1 |
Graphite |
15.0 |
1.0 g Na2Cr2O7 |
30 |
72 |
16:1 |
Black |
| Staudenmaier |
1898 |
Graphite |
KclO3, H2SO4 |
| 2 |
Graphite | , HNO |
15.03 |
4.0 g Na296 h |
Cr2ORoom temperature |
7Improved efficiency |
[46][97] |
| 30 |
72 |
3.4:1 |
Black |
Hummers |
1958 |
Graphite |
KmnO4, H2SO4, NaNO3 |
<2 h |
<20–35–98 |
| 3 |
Graphite |
15.0 |
15.0 mL 70% HNO3
3.0 g KmnO4, |
30 |
24 | Water-free, less than 2 h of reaction time |
[47][98] |
| -- |
Black |
Fu |
2005 |
Graphite |
KmnO4, H2SO4, NaNO3 |
<2 h |
| 4 |
Graphite |
20.0 | 35 |
11.0 g KclO3,
10.0 mL 70% HNO3 | Validation of NaNO | 3 |
0–60[ |
3348 |
3.1:1][99] |
| Midnight green |
Shen |
2009 |
Graphite |
Benzoyl peroxide |
10 min |
110 |
| 5 |
Graphite |
30.0 |
3.0 g KmnO4,1.0 g
NaNO3 |
30 |
2 | Fast and non-acidic |
[49][100] |
| 3.0:1 |
Bluish green |
Su |
2009 |
Graphite |
KmnO4, H2SO |
| 6 | 4 |
Graphite |
30.0 |
3.0 g KmnO4,1.0 g4 h |
Room temperature |
Large-size GO sheets formed |
NaNO3[50] |
45[ |
1101 |
--] |
| Green |
Marcano and Tour |
2010 & 2018 |
Graphite |
KmnO4, H3PO4, H2SO4 |
12 h |
50 |
Eco-friendly resulting in a high yield |
[51][102] |
| Sun |
2013 |
Graphite |
KmnO4, H2SO4 |
1.5 h |
Room temperature-90 |
High-yield and safe method |
[52 |
| 7 |
Graphite |
22.5 |
3.0 g KmnO4,1.0 g
NaNO3 |
45 |
1 |
-- |
Brittle yellow |
][103] |
| Eigler |
2013 |
Graphite |
KmnO4, NaNO3, H2SO4 |
16 h |
10 |
High-quality GO produced |
[53][104] |
| [ | 54 | ] | [ | 105 | ] |
| Panwar |
2015 |
Graphite |
H2SO4, H3PO4, KmnO4, HNO3 |
3 h |
50 |
Three component acids and high-yield product |
[55][106] |
| Peng |
2015 |
Graphite |
K2FeO4, H2SO4 |
1 h |
Room temperature |
Results in a high-yield and eco-friendly method |
[56][107] |
| Rosillo-Lopez |
2016 |
Graphite |
HNO3 |
20 h |
| 8 |
Graphite |
22.5 |
3.0 g KmnO4,0.5 g
NaNO3 |
45 |
1 |
-- |
Yellow |
| 9 |
Graphite |
22.5 |
3.0 g KmnO4,0.5 g
NaNO3 |
45 |
0.5 |
2.3:1 |
Yellow |
Chen |
2015 |
Graphite |
KmnO4, H2SO4 |
<1 h |
40–95 |
High-yield product |
| 10 |
Graphite |
22.5 |
3.0 g KmnO4,0.5 g
NaNO3 |
35 |
0.5 |
2.05:1 |
Bright yellow |
| 11 |
Graphite |
22.5 |
3.0 g KmnO4, 1.0 g
fuming HNO3 |
35 |
1 |
-- |
Bright yellow |
| 12 |
Graphite |
22.5 |
3.0 g KmnO4, 1.0 g
BaNO3 |
45 |
2 |
-- |
Light green |
Room temperature |
Nano-sized GO obtained |
[57][108] |
| Yu |
2016 |
Graphite |
K2FeO4, KmnO4 H2SO4, H3BO3 (NH4)2S2O8 |
5 h |
<5–35–95 |
Low manganite impurities and high yield obtained |
[58][109] |
| Dimiev |
2016 |
Graphite |
98% H2SO4, fuming H2SO4 |
3–4 h |
Room temperature |
25 nm thick and ~100%conversion rate |
[59][110] |
| Pei |
2018 |
Graphite foil |
H2SO4 |
<5 min |
Room temperature |
High efficiency |
[60][111] |
| Ranjan |
2018 |
Graphite |
H2SO4, H3PO4, KmnO4 |
<24 h |
<RT-35–95 |
Cooled exothermal reaction to make the process
safe |
[61][112] |
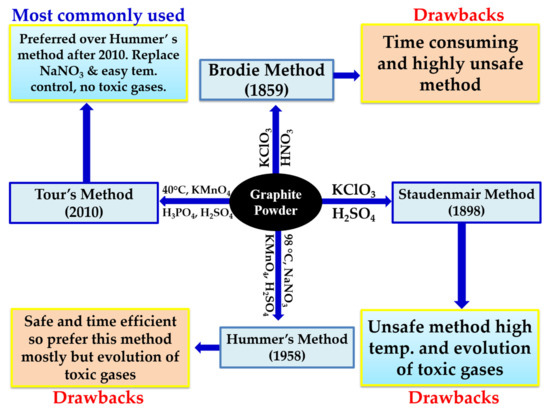
The most preferred methods are Brodie
[45][96], Staudenmaier
[46][97], and Hummers
[47][98], as shown in
Figure 411. From these familiar methods, a number of variations have been derived to improve the overall yield and quality of the GO. In 1859, Brodie used graphite as the starting material for the synthesis of graphene oxide (GO). In his experimental work, he used KclO
4 (strong oxidizing agent) along with nitric acid and heated the content at 60 °C for 3–4 days
[45][96]. The GO obtained was soluble in pure or basic water. The chemical composition showed mainly carbon, oxygen, and hydrogen with the general formula C
11H
4O
5. After nearly four decades, in 1898, Staudenmaier and Hoffmann modified Brodie’s method and trimmed down the reaction time of graphene oxide synthesis from 4 days to 2 days
[46][97]. The nitric acid used in Brodie method was also replaced with sulfuric acid, which further reduced the liberation of toxic gases such as NO
2 or N
2O
4.
Figure 411.
Schematic representation of the synthesis of graphene oxide with different methods.
In 1958, Hummer reduced the reaction time from 2 days to 12 h by using KmnO
4 as the oxidizing agent instead of KclO
4, followed by the addition of sodium nitrate, but the problem of toxic gases still remains a challenge
[47][98]. Further, in 2010, at Rice University, Tour’s group
[51][102] replaced sodium nitrate with phosphoric acid and increased the amount of KmnO
4. This improvement made the process eco-friendly, as it completely stops the release of toxic gases such as NO