Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Mohammad Abu Jafar Mazumder | -- | 1497 | 2022-11-08 11:27:43 | | | |
| 2 | Vivi Li | -37 word(s) | 1460 | 2022-11-09 03:47:56 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Goni, L.K.M.O.; Mazumder, M.A.J.; Tripathy, D.B.; Quraishi, M.A. Acridine. Encyclopedia. Available online: https://encyclopedia.pub/entry/33522 (accessed on 02 March 2026).
Goni LKMO, Mazumder MAJ, Tripathy DB, Quraishi MA. Acridine. Encyclopedia. Available at: https://encyclopedia.pub/entry/33522. Accessed March 02, 2026.
Goni, Lipiar K. M. O., Mohammad A. Jafar Mazumder, Divya B. Tripathy, Mumtaz A. Quraishi. "Acridine" Encyclopedia, https://encyclopedia.pub/entry/33522 (accessed March 02, 2026).
Goni, L.K.M.O., Mazumder, M.A.J., Tripathy, D.B., & Quraishi, M.A. (2022, November 08). Acridine. In Encyclopedia. https://encyclopedia.pub/entry/33522
Goni, Lipiar K. M. O., et al. "Acridine." Encyclopedia. Web. 08 November, 2022.
Copy Citation
Acridine, an alkaloid from anthracene, also known by the names of 10-azaanthracene, dibenzopyridine, 2,3,5,6-dibenzopyridine, and its derivatives or analogs, have a great potential to be an efficient class of excellent inhibitor. The phenomenon of corrosion threatens metallic components, human safety, and the economy. Despite being eco-friendly and promising as a corrosion inhibitor, acridine has not been explored to its full potential.
acridines
corrosion
metallic corrosion
corrosion inhibitors
adsorption
corrosion protection
1. Introduction
The assault of corrosion on metallic structures has become an extremely widespread and persistent dilemma across the globe. In general, corrosion has been defined as the deterioration of metallic structures by reaction with the surrounding environment [1][2]. However, in a more technical sense, corrosion entails the movement of mass and charge across a metal/solution interface [3]. The phenomenon of corrosion ensues in the presence of four things: an anode, a cathode, a metallic path, and an electrolyte. Upon contact with an appropriate electrolyte, the anode part loses free electrons that travel through the electrolyte to reach the cathode. Those free electrons are consumed to produce molecular hydrogen in acidic media and hydroxyl ions in basic/neutral conditions contaminated with oxygen. There are acids (H2SO4, HCl, and HNO3) and bases (NaOH, CaCO3, NaHCO3) that commonly attack metallic structures. Besides, there is moisture/water (H2O), salts (NaCl), gases (formaldehyde, ammonia, sulfur-containing gases, etc.), and aggressive metal polishes as well that have also been found to be aggravating to metallic structures [4]. Metals have tremendous implications in the construction industry for building pipework, structural components, cladding materials, etc., owing to their outstanding strength and durability. Because of the ubiquitous nature of metallic elements, it is not surprising that they are always in contact with different corrosive scenarios, making corrosion damage prevalent and demanding so much attention [5].
Corrosion profoundly impacts human health and safety, environment, materials’ life span, and the economy. It has been reported that any country in the world spends around 1–5% of its gross national product (GNP) on corrosion [6]. The global cost of corrosion is so huge that a study done by the National Association of Corrosion Engineers (NACE) revealed that the global cost of corrosion for the year 2013 was 3.4% (equivalent to USD 2.5 trillion) of the global gross domestic product (GDP) [7]. A more detailed account of how corrosion impacted the economy and claimed human lives in the past can be found in the previously published literature [8]. Corrosion inhibition [9][10][11], anodic protection [12], cathodic protection [13][14], alloying [15][16], and coatings [17][18] are some of the most widely used approaches to combat corrosion. However, corrosion inhibitors (CIs) have been found to be very popular and effective for protecting metallic units in petroleum refining, oil and gas production, exploration, the chemical industry, water treatment plants, etc. CIs are chemical substances added to corrosive environments to reduce or impede corrosion rates [5]. CIs form a protective layer on the surface of the metal to keep aggressive electrolytes from coming into contact with the metal surface. As inorganic inhibitors have already been denoted to be environmentally harmful, organic inhibitors have recently gained widespread popularity [4][19]. These inhibitors get adsorbed onto the metal surface by either an electrostatic attraction (physical adsorption or physisorption) or a coordinate covalent bond (chemical adsorption or chemisorption) when inhibitor molecules containing lone pairs of electrons donate electrons to the vacant d-orbitals of the metal. On the other hand, the metal can sometimes donate electrons (retro-donation) to the inhibitor molecules to form that protective layer. As a consequence, organic compounds containing heteroatoms (O, N, P, and S), polar functional groups (−COOH, −OH, −NH2, −CN, −C=O, −NO2), conjugated bonds, aromatic rings, π-systems, etc., are known to provide the best corrosion protection.
Acridine (Figure 1), an alkaloid from anthracene, also known by the names of 10-azaanthracene, dibenzopyridine, 2,3,5,6-dibenzopyridine, and its derivatives or analogs, have a great potential to be an efficient class of excellent inhibitor. Aromatic rings, π-systems, and a nitrogen atom with an available lone pair of electrons already present in the chemical structure of acridine hint at the possibility of it acting as a suitable inhibitor. Additionally, incorporating different functional groups that can undergo chemisorption or physisorption with the vacant d-orbitals of the metal in the acridine can make it even better as a CI. Furthermore, acridine and its analogs have been reported to have shown loads of biological activities, rendering it environmentally safe as well.
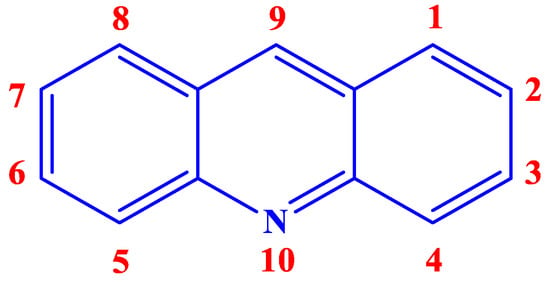
Figure 1. Chemical structure and numeration of acridine.
2. Biological Properties of Acridines
Inorganic salts and salts of heavy metals had been used historically as CIs, including molybdates, nitrates, phosphates, polyphosphates, phosphonates, sodium chromates, silicates, hydroxides, etc. [20]. However, even after showing practical efficiencies, these types of inhibitors have been restricted in their use, having been identified as toxic to human life and the environment. As a consequence, several tough regulations, including the US Occupational Safety and Health Administration (OSHA) in 1993, the Emergency Planning and Community Right to Know Act of 1986, the Chemical Hazard Assessment and Risk Management (CHARM) model adopted in the UK and other European countries, caused researchers to look for non-toxic yet efficient corrosion inhibitors. Acridine and its derivatives are found in natural plants and marine organisms, forming an important class of N-containing heterocycles. Due to their unique chemical and physical properties and biological activities, acridine derivatives are used across different industries. Pharmaceutically, acridine derivatives have been found to show bio-activities, such as anticancer, antitubercular, antiviral, antimalarial, antimicrobial, anti-inflammatory, antiparasitic, and fungicidal activities [21]. Some acridine derivatives, their structures, and relevant bio-activity have been included in Table 1 to show that acridine and its derivatives are being explored as environmentally friendly and efficient CIs.
Table 1. Several pharmaceutically important acridine derivatives and their chemical structures.
| Bioactivity | Acridine Derivative | Remarks | References |
|---|---|---|---|
| Antimalarial | 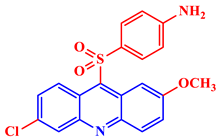 2-Methoxy-6-chloro-9-(4′-amino-phenylsulfonyl)-acridine |
IC50 values of 14.3 and 20 μM are shown against 3D7 and W2 strains, respectively, of Plasmodium falciparum. | [22] |
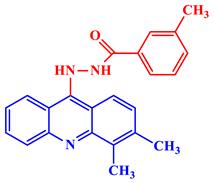 N′-(3,4-dimethyl-9-acridinyl)-2-hydroxybenzohydrazide |
IC50 values of 0.5 ± 0.05 and 0.13 ± 0.03 nM are shown against human cathepsin D and Plasmodium falciparum plasmepsin-II, respectively. | [23] | |
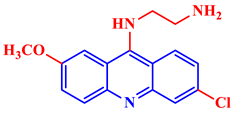 9-(N-ethylamino)-6-chloro-2-methoxyacridine |
IC50 values of 42 ± 19 and 280 ± 40 nM are shown against chloroquinone-susceptible and chloroquinone-resistant Plasmodium falciparum strains, respectively. | [24] | |
| Antiviral | 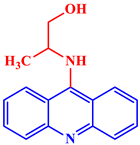 9-(N-2-methyl hydroxyethyl)acridine |
An MIC50 of 5 μg/mL was enough to reduce the infection viral titer of HSV-L2 in an experiment by order of 6.5 to that of the control. | [25] |
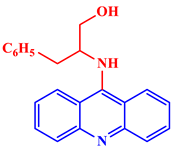 9-(N-2-benzyl hydroxyethyl)acridine |
An MIC50 of 5 μg/mL was enough to reduce the infection viral titer of HSV-L2 in an experiment by order of 1.3 to that of the control. | [25] | |
| Antibacterial | 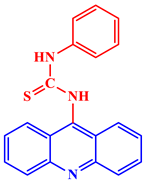 1-(9-AcridinyI)-3-phenyl-2-thiourea |
At 400 μg/mL levels, the compound effectively inhibited strains of several bacterial cell lines, including Salmonella pullorum and Escherichia coli. | [26] |
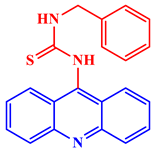 1-(9-AcridinyI)-3-benzyl-2-thiourea |
At 400 μg/mL levels, the compound effectively inhibited strains of several additional bacterial cell lines. | [26] | |
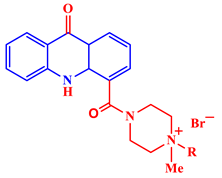 1-Methyl-1-[(5-nitrofuran-2-yl)methyl]-4-(9-oxo-9,10-dihydroacridine-4-carbonyl)piperazin-1-ium bromide |
The compound showed commendable antibacterial activity against multiple test strains, including S. aureus and E. coli. | [27] | |
| Antitumor | 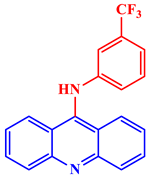 9-[(3′-Trifluoromethyl)phenylamino]acridine hydrochloride |
CTC50 values of 31.25 and 36.25 μg/mL were shown against cervical cancer cell (HeLa) and lung cancer cell (A-549) lines, respectively. | [28] |
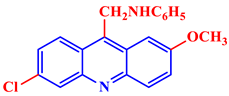 6-Chloro-2-methoxy-N-benzylacridin-9-amine |
IC50 values of 0.65 and 1.40 μM were shown against K562 leukemia and hepatoma HepG-2 cell lines, respectively. | [29] | |
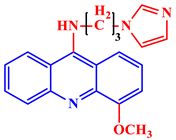 N-(3-(1H-imidazol-1-yl)propyl)-4-methoxyacridin-9-amine |
Excellent anticancer activity was shown against multiple cell lines, including liver (HEP-2), breast (MCF-7), and lung (A-549) cell lines. | [30] |
3. Synthesis of Acridines
Acridine was isolated for the first time, from a high boiling fraction of coal tar, by Grabe and Heinrich in Germany in 1870. Due to the scarcity of quinine during World War II, an acridine-based antimalarial drug called mepacrine gained widespread popularity. However, current antibacterial therapy like penicillin and sulfonamide suppress mepacrine. Recently, an across-the-board increase in drug resistance has pushed scientists and researchers to look for alternatives, giving acridine research a boost once again, possibly making acridines suitable for use across other platforms, such as corrosion protection [31]. Although several methods are available for synthesizing acridines, some important, popular, simple, and named synthesis processes of acridines are outlined below.
Ullmann synthesis [21], a prevalent method to produce acridines, involves condensing a primary amine with an aromatic aldehyde/carboxylic acid in the presence of a strong mineral acid (H2SO4/HCl), followed by a cyclization step to produce acridone. After that, two additional steps of reduction and dehydrogenation afford acridine (Scheme 1a). 9-substituted acridines can be produced by exploiting Bernthsen synthesis [32], which involves reacting diphenylamine with a carboxylic acid in the presence of zinc chloride (Scheme 1b). On the other hand, 9-methyl acridine can be afforded via Friedlander synthesis [33] when the salt of anthranilic acid is treated with 2-cyclohexenone at 120 °C (Scheme 1c). C-Acylated diphenylamine, a little variation of the Bernthsen synthesis reactant, can produce 9-phenyl acridines when treated with I2/HI (Scheme 1d).
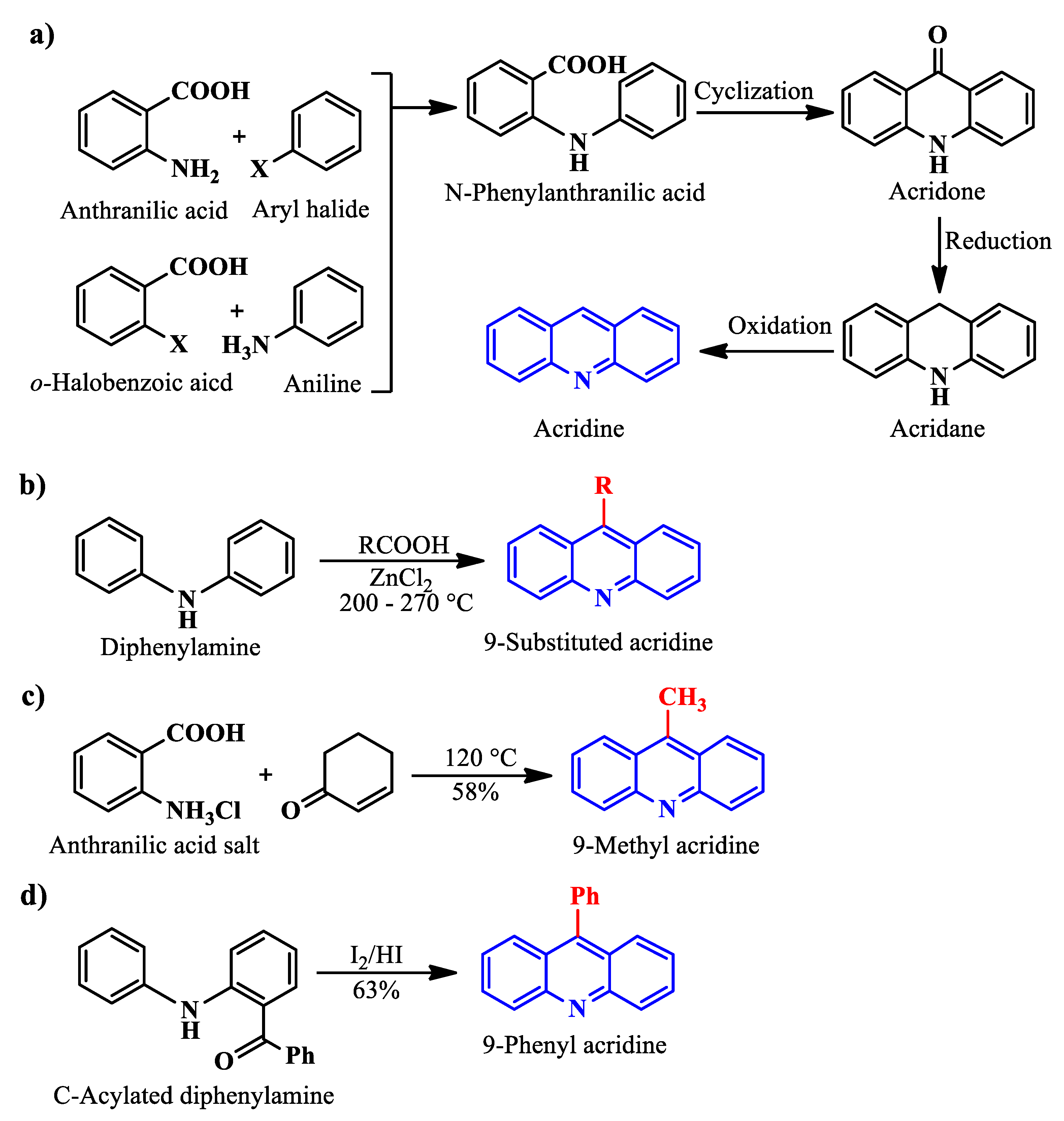
Scheme 1. Acridine and substituted acridine synthesis using Ullman (a), Bernthsen (b), Friedlander (c) synthesis, and from C-acylated diphenylamine (d).
References
- Roberge, P.R. Corrosion Engineering Principles and Practice, 1st ed.; McGraw-Hill: New York, NY, USA, 2008.
- Revie, R.W.; Ulhig, H.H. Corrosion and Corrosion Control, 4th ed.; Wiley-Interscience: Hoboken, NJ, USA, 2008.
- McCafferty, E. Introduction to Corrosion Science, 1st ed.; Springer: New York, NY, USA, 2010.
- Goni, L.K.M.O.; Mazumder, M.A.J. Green Corrosion Inhibitors. In Corrosion Inhibitors; Singh, A., Ed.; IntechOpen: London, UK, 2019; pp. 77–94.
- Goni, L.K.M.O.; Jafar Mazumder, M.A.; Quraishi, M.A.; Mizanur Rahman, M. Bioinspired Heterocyclic Compounds as Corrosion Inhibitors: A Comprehensive Review. Chem. Asian J. 2021, 16, 1324–1364.
- Javaherdashti, R. How Corrosion Affects Industry and Life. Anti-Corros. Methods Mater. 2000, 47, 30–34.
- Koch, G.; Varney, J.; Thompson, N.; Moghissi, O.; Gould, M.; Payer, J. International Measures of Prevention, Application, and Economics of Corrosion Technologies Study; NACE International: Houston, TX, USA, 2016.
- Jafar Mazumder, M.A. Global Impact of Corrosion: Occurrence, Cost and Mitigation. Glob. J. Eng. Sci. 2020, 5, 1–5.
- Shinato, K.W.; Zewde, A.A.; Jin, Y. Corrosion Protection of Copper and Copper Alloys in Different Corrosive Medium Using Environmentally Friendly Corrosion Inhibitors. Corros. Rev. 2020, 38, 101–109.
- Farag, A.A. Applications of Nanomaterials in Corrosion Protection Coatings and Inhibitors. Corros. Rev. 2020, 38, 1–20.
- Obot, I.B.; Onyeachu, I.B.; Umoren, S.A. Pyrazines as Potential Corrosion Inhibitors for Industrial Metals and Alloys: A Review. J. Bio-Tribo-Corros. 2018, 4, 18–31.
- Fernández, A.G.; Cabeza, L.F. Anodic Protection Assessment Using Alumina-Forming Alloys in Chloride Molten Salt for CSP Plants. Coatings 2020, 10, 138.
- Christodoulou, C.; Glass, G.; Webb, J.; Austin, S.; Goodier, C. Assessing the Long-Term Benefits of Impressed Current Cathodic Protection. Corros. Sci. 2010, 52, 2671–2679.
- Broomfield, J.P. An Overview of Cathodic Protection Criteria for Steel in Atmospherically Exposed Concrete. Corros. Eng. Sci. Technol. 2020, 55, 303–310.
- Li, X.; Xia, W.; Yan, H.; Chen, J.; Li, X. Improving Strength and Corrosion Resistance of High Mg Alloyed Al–Mg–Mn Alloys through Ce Addition. Corros. Eng. Sci. Technol. 2020, 55, 381–391.
- Nowak, P.; Mosialek, M.; Kharitonov, D.S.; Adamiec, J.; Turowska, A. Effect of TIG Welding and Rare Earth Elements Alloying on Corrosion Resistance of Magnesium Alloys. J. Electrochem. Soc. 2020, 167, 131504.
- Zhang, K.; Yang, W.; Ge, F.; Xu, B.; Chen, Y.; Yin, X.; Liu, Y.; Zuo, H. A Self-Curing Konjac Glucomannan/CaCO3 Coating for Corrosion Protection of AA5052 Aluminum Alloy in NaCl Solution. Int. J. Biol. Macromol. 2020, 151, 691–701.
- Rodič, P.; Lekka, M.; Andreatta, F.; Fedrizzi, L.; Milošev, I. The Effect of Copolymerisation on the Performance of Acrylate-Based Hybrid Sol-Gel Coating for Corrosion Protection of AA2024-T3. Prog. Org. Coat. 2020, 147, 105701.
- Aljeaban, N.A.; Goni, L.K.M.O.; Alharbi, B.G.; Mazumder, M.A.J.; Ali, S.A.; Chen, T.; Quraishi, M.A.; Al-Muallem, H.A. Polymers Decorated with Functional Motifs for Mitigation of Steel Corrosion: An Overview. Int. J. Polym. Sci. 2020, 2020, 9512680.
- Dariva, C.G.; Galio, A.F. Corrosion Inhibitors: Principles, Mechanisms and Applications. In Developments in Corrosion Protection; Aliofkhazraei, M., Ed.; IntechOpen: London, UK, 2014; pp. 365–379.
- Gensicka-Kowalewska, M.; Cholewiński, G.; Dzierzbicka, K. Recent Developments in the Synthesis and Biological Activity of Acridine/Acridone Analogues. RSC Adv. 2017, 7, 15776–15804.
- Santelli-Rouvier, C.; Pradines, B.; Berthelot, M.; Parzy, D.; Barbe, J. Arylsulfonyl Acridinyl Derivatives Acting on Plasmodium Falciparum. Eur. J. Med. Chem. 2004, 39, 735–744.
- Azim, M.K.; Ahmed, W.; Khan, I.A.; Rao, N.A.; Khan, K.M. Identification of Acridinyl Hydrazides as Potent Aspartic Protease Inhibitors. Bioorg. Med. Chem. Lett. 2008, 18, 3011–3015.
- Yu, X.M.; Ramiandrasoa, F.; Guetzoyan, L.; Pradines, B.; Quintino, E.; Gadelle, D.; Forterre, P.; Cresteil, T.; Mahy, J.P.; Pethe, S. Synthesis and Biological Evaluation of Acridine Derivatives as Antimalarial Agents. ChemMedChem 2012, 7, 587–605.
- Suveyzdis, Y.I.; Lyakhov, S.A.; Litvinova, L.A.; Rybalko, S.L.; Dyadyun, S.T. Antiviral Activity of Acridinylaminoalcohols and Acridinylaminoacid Esters. Pharm. Chem. J. 2000, 34, 528–529.
- Stewart, J.T. Synthesis and Biological Activity of 9-Substituted Acridines Isolation of P-Amyrin and Ellagic Acid from Couroupita Amazonica. J. Pharm. Sci. 1973, 62, 1357–1358.
- Kudryavtseva, T.N.; Lamanov, A.Y.; Klimova, L.G.; Nazarov, G.V. Synthesis and Antimicrobial Activity of Acridine Carboxylic Acid Derivatives Containing a Piperazine Moiety. Russ. Chem. Bull. 2017, 66, 123–128.
- Kumar, P.; Kumar, R.; Prasad, D.N. Synthesis and Anticancer Study of 9-Aminoacridine Derivatives. Arab. J. Chem. 2013, 6, 79–85.
- Lang, X.; Li, L.; Chen, Y.; Sun, Q.; Wu, Q.; Liu, F.; Tan, C.; Liu, H.; Gao, C.; Jiang, Y. Novel Synthetic Acridine Derivatives as Potent DNA-Binding and Apoptosis-Inducing Antitumor Agents. Bioorg. Med. Chem. 2013, 21, 4170–4177.
- Sondhi, S.M.; Singh, J.; Rani, R.; Gupta, P.P.; Agrawal, S.K.; Saxena, A.K. Synthesis, Anti-Inflammatory and Anticancer Activity Evaluation of Some Novel Acridine Derivatives. Eur. J. Med. Chem. 2010, 45, 555–563.
- Kumar, R.; Kaur, M.; Kumari, M. Acridine: A Versatile Heterocyclic Nucleus. Acta Pol. Pharm.-Drug Res. 2012, 69, 3–9.
- Patel, M.M.; Mali, M.D.; Patel, S.K. Bernthsen Synthesis, Antimicrobial Activities and Cytotoxicity of Acridine Derivatives. Bioorg. Med. Chem. Lett. 2010, 20, 6324–6326.
- Muscia, G.C.; Buldain, G.Y.; Asís, S.E. Design, Synthesis and Evaluation of Acridine and Fused-Quinoline Derivatives as Potential Anti-Tuberculosis Agents. Eur. J. Med. Chem. 2014, 73, 243–249.
More
Information
Subjects:
Chemistry, Organic
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.6K
Entry Collection:
Organic Synthesis
Revisions:
2 times
(View History)
Update Date:
09 Nov 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





