
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sudhagar Mani | -- | 1988 | 2022-10-11 04:38:08 | | | |
| 2 | Amina Yu | Meta information modification | 1988 | 2022-10-31 02:14:15 | | |
Video Upload Options
Repeated and arranged polymer molecular units are present in some animal and plant biomass. They behave as fossil-based polymeric materials and are called natural polymers. The natural polymers that were extracted from different biomass resources are classified based on the resources used. They are present in animals and plants as protein macromolecules of amino acids bonded by peptides or as polysaccharide macromolecules of monosaccharides bonded by glycosidic bonds or as lipid–long chain hydrocarbon molecules containing a carboxylic acid moiety.
1. Introduction
Natural polymers are abundantly available in many renewable resources. At present, biomass resources are mainly utilized for the production of various food products, oil, feed grains, bioenergy, and cosmetic products. The production and utilization details of various resources used for the manufacture of natural polymers in the U.S.A. are presented in Table 1.
| Renewable Resources |
Natural Polymer Type and Compositions | Production Volume (Million Metric Tons) |
Current Use | Reference |
|---|---|---|---|---|
| Milk | Contains 33 g of protein/L. 80% casein and 20% whey protein | 97.76 | Used as a fat substitute. Butter, dry skim milk, cheese, whey, whey protein concentrate, and lactose are produced from milk. | [1][2] |
| Pork & Beef | More than 29% gelatin is available in pig skin. In beef meat, 10.6~21.9% of gelatin protein available in rib and shank | 11.91 | Used as meat. By-products such as skin, bones, and connective tissues are used to produce gelatin | [2][3] |
| Wheat | Contains 76.5% starch | 47.38 | Used for the production of food products | [2][4] |
| Soybeans | Contains 31.7 to 58.9% protein | 120.07 | Source for animal protein and vegetable oil | [2][5] |
| Corn grain | Contains about 70–72% starch | 371.10 | Source for corn meal, starch, oil, bioethanol, syrup, sugar, and feed grain | [2][6] |
| Potato | Contains 20% of potato dry matter with 60–80% of starch | 22.91 | Source for food products and starch | [2][7] |
| Crustaceans (Shrimp and Crab) | Crab shell contains 9.6% chitin and shrimp shell contains 4% chitin | 0.32 | Source for seafood and compost | [8][9] |
| Forestry biomass resources | 40~50% cellulose | 139.71 | Biofuels, wood products such as timber, lumber, etc. | [10][11] |
| Agricultural biomass resources | 25~40% cellulose | 130.64 | Source for bioenergy, biofuels, and bioproducts | [10][11] |
| Waste (Agricultural wastes, forestry wastes) | 25~50% cellulose | 61.69 | Source for compost, bioenergy | [10][11] |
2. Protein-Based Natural Polymers
2.1. Proteins-Based Natural Polymers from an Animal Resource
| Natural Polymer | Plasticizer/ Crosslinker |
TS a (MPa) |
YM b (MPa) |
Elongation at Break (%) |
Tensile Test Conditions | WVP c × 1020 (g m h−1 kPa−1 m−2) |
Reference |
|---|---|---|---|---|---|---|---|
| αs1—Casein Films | -- | 0.004 | -- | 38 | Film size 20 × 50 mm. Loading Rate—50 mm/min | -- | [28] |
| αs1—Casein Films | Transglutaminase (Enzyme) | 0.01 | -- | 75 | -- | ||
| α, β and κ—Casein Films f | -- | 52 ± 0.20 | 1107 ± 11 | 8 ± 2 | Loading rate—20 mm/min d | -- | [29] |
| α, β and κ—Casein Films g | -- | 49 ± 3 | 1391 ± 48 | 6 ± 2 | -- | ||
| α, β and κ—Casein Films h | 10% Glycerol | 36 ± 0.40 | 693 ± 38 | 25 ± 9 | -- | ||
| α, β and κ—Casein Films i | 10% Glycerol | 21 ± 0.30 | 497 ± 40 | 17 ± 1 | -- | ||
| α, β and κ—Casein Films j | 10% Glycerol | 33 ± 2 | 765 ± 109 | 15 ± 7 | -- | ||
| α, β and κ—Casein Films k | 10% Glycerol | 48 ± 2 | 1004 ± 40 | 8 ± 3 | -- | ||
| Whey Protein Films | 40% Sorbitol | 18 | 650 | 5 | Loading rate—100 mm/min d | -- | [30] |
| Whey Protein Films | 15% Glycerol | 29 | 1100 | 4 | -- | ||
| Fish Gelatin Films l | 10 wt.% glycerol | 36.52 ± 2.98 | 1.79 ± 0.54 | Sample size 4.75 × 22.25 mm e | 5.33 ± 0.16 | [22] | |
| Fish Gelatin Films m | 10 wt.% glycerol | 43.02 ± 0.52 | 2.31 ± 0.33 | 5.47 ± 0.10 | |||
| Fish Gelatin Films n | 10 wt.% glycerol | 52.36 ± 3.16 | 2.88 ± 0.68 | 6.52 ± 0.16 |
a TS—Tensile strength. b YM—Young’s modulus. c ASTM test for water vapor permeability—E96-95. d ASTM test for tensile strength—D882. e ASTM test for tensile strength—D1708-93. f Casein in NaOH/H2O solution and Heat treated at 130 °C/18 h. g Casein in 3-aminopropyl triethoxy silane solution—Heat treated at 130 °C/18 h. h Casein in NaOH/H2O solution- Air dried. i Casein in NaOH/H2O solution—Heat treated at 130 °C/18 h. j Casein in 3-aminopropyl triethoxy silane solution- Heat treated at 130 °C/18 h. k Casein in 3-aminopropyl triethoxy silane solution- Air dried. l Gelatin solution without pH modification. m Gelatin solution with HCl acid modified pH (2.0). n Gelatin solution with NaOH base modified pH (10.0).
2.2. Protein-Based Natural Polymers from Plant Resources
| Natural Polymer | Plasticizer/Crosslinker | TS a (MPa) |
YM b (MPa) |
Elongation at Break (%) |
Tensile Test Conditions c | WVP d (g m h−1 kPa−1 m−2) |
Reference |
|---|---|---|---|---|---|---|---|
| Glutenin-rich Wheat Gluten Film | 20% Glycerol | 5 | -- | 100 | Sample Size: 2.54 × 10 cm. Loading rate: 508 mm/min | 6.94 × 104 | [39] |
| Gliadin-rich Wheat Gluten Film | 20% Glycerol | 15 | -- | 350 | -- | 1.11 × 105 | |
| SPI Film | 50% Glycerin | 2.80 ± 0.30 | -- | 165.70 ± 15 | Film size: 2.54 × 15 cm | 9.66 × 10−9 | [40] |
| Zein Film | -- | 6.70 ± 0.37 | 409.86 ± 7.62 | 1.96 ± 0.18 | Film size: 40 × 10 × 0.47 ± 0.12 mm | 1.69 × 10−4 | [41] |
| Zein Film | 10% Tributyl Citrate | 17.80 ± 4.26 | 556.29 ± 29.42 | 4.53 ± 0.54 | 1.64 × 10−4 |
a TS—Tensile strength. b YM—Young’s Modulus. c Films conditioned in a chamber at 23 °C and 50% RH for at least 48 h. d ASTM test method for Water vapor permeability—E96-95.
3. Polysaccharide-Based Natural Polymers
3.1. Polysaccharide-Based Natural Polymers from an Animal Resource
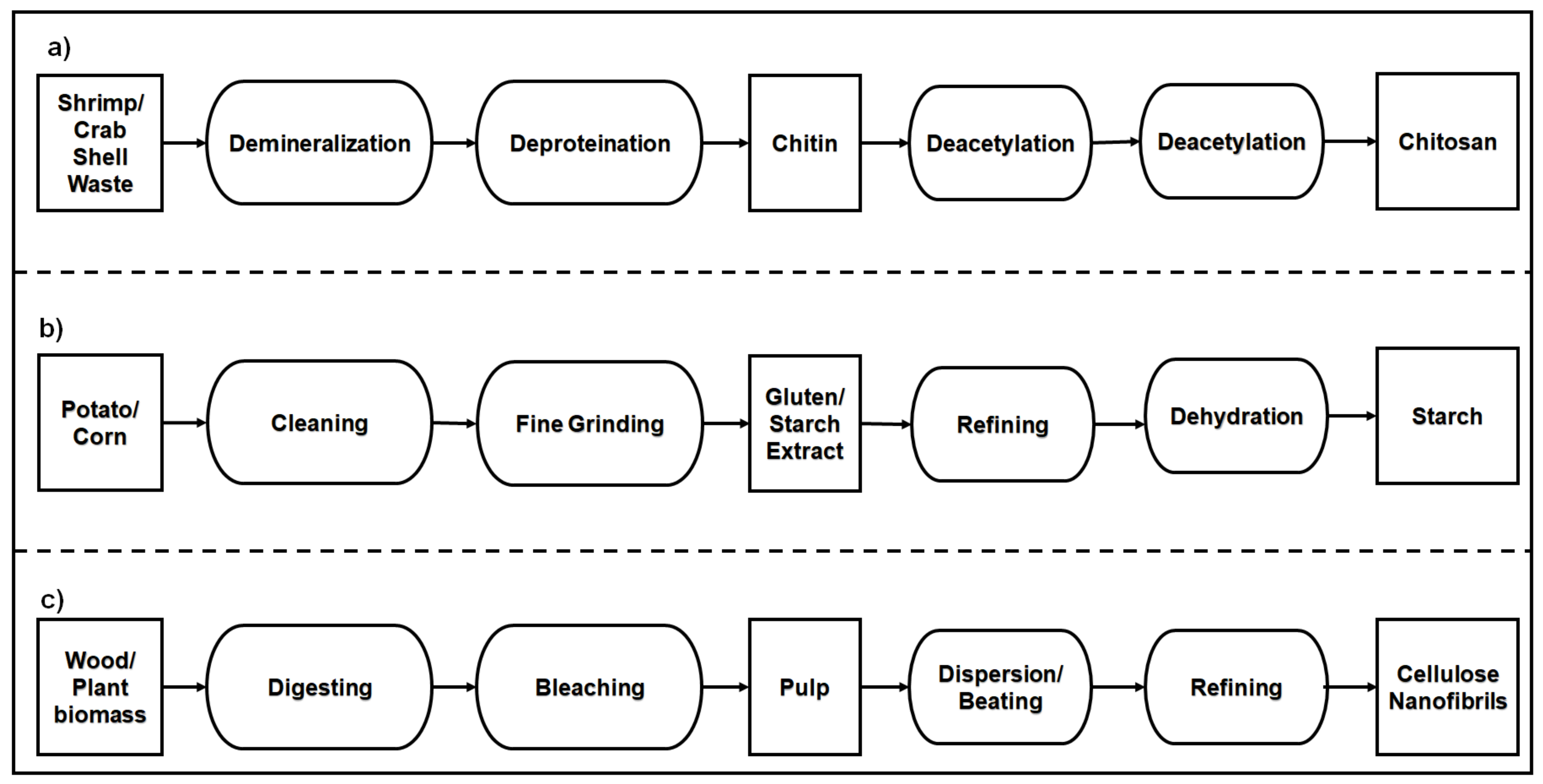
3.2. Polysaccharide-Based Natural Polymers from Plant Resource
4. Lipid-Based Natural Polymers
References
- Khwaldia, K.; Perez, C.; Banon, S.; Desobry, S.; Hardy, J. Milk proteins for edible films and coatings. Crit. Rev. Food Sci. Nutr. 2004, 44, 239–251.
- U.S. Department of Agriculture, E.R.S. Available online: https://www.ers.usda.gov/data-products/ (accessed on 18 September 2022).
- Mitchell, H.H.; Zimmerman, R.L.; Hamilton, T.S. Determination of the amount of connective tissue in meat. J. Anim. Sci. 1927, 1927, 257.
- Domenek, S.; Feuilloley, P.; Gratraud, J.; Morel, M.H.; Guilbert, S. Biodegradability of wheat gluten based bioplastics. Chemosphere 2004, 54, 551–559.
- Wang, J.; Chen, P.; Wang, D.; Shannon, G.; Zeng, A.; Orazaly, M.; Wu, C. Identification and mapping of stable QTL for protein content in soybean seeds. Mol. Breed. 2015, 35, 1–10.
- Rouf Shah, T.; Prasad, K.; Kumar, P. Maize—A potential source of human nutrition and health: A review. Cogent Food Agric. 2016, 2, 1166995.
- Robertson, T.M.; Alzaabi, A.Z.; Robertson, M.D.; Fielding, B.A. Starchy carbohydrates in a healthy diet: The role of the humble potato. Nutrients 2018, 10, 1764.
- Muñoz, I.; Rodríguez, C.; Gillet, D.; Moerschbacher, B.M. Life cycle assessment of chitosan production in India and Europe. Int. J. Life Cycle Assess. 2018, 23, 1151–1160.
- Lowther, A.; Liddel, M.; Yencho, M. Fisheries of the United States 2018: Current Fishery Statistics no. 2018; National Oceanic and Atmospheric Administration: Silver Spring, MD, USA, 2020.
- Wang, S.; Dai, G.; Yang, H.; Luo, Z. Lignocellulosic biomass pyrolysis mechanism: A state-of-the-art review. Prog. Energy Combust. Sci. 2017, 62, 33–86.
- Langholtz, M.; Stokes, B.; Eaton, L. 2016 Billion-ton report: Advancing domestic resources for a thriving bioeconomy (executive summary). Ind. Biotechnol. 2016, 12, 282–289.
- Chen, H. Functional properties and applications of edible films made of milk proteins. J. Dairy Sci. 1995, 78, 2563–2583.
- Dalgleish, D. Milk proteins. Chemistry and physics. In Food Proteins; Fox, P.F., Condon, J.J., Eds.; Applied Science Publishers: London, UK, 1982; pp. 155–178.
- Metzger, W. Method of Producing Casein Film. U.S. Patent No. 5,681,517, 28 October 1997.
- Patel, S. Emerging trends in nutraceutical applications of whey protein and its derivatives. J. Food Sci. Technol. 2015, 52, 6847–6858.
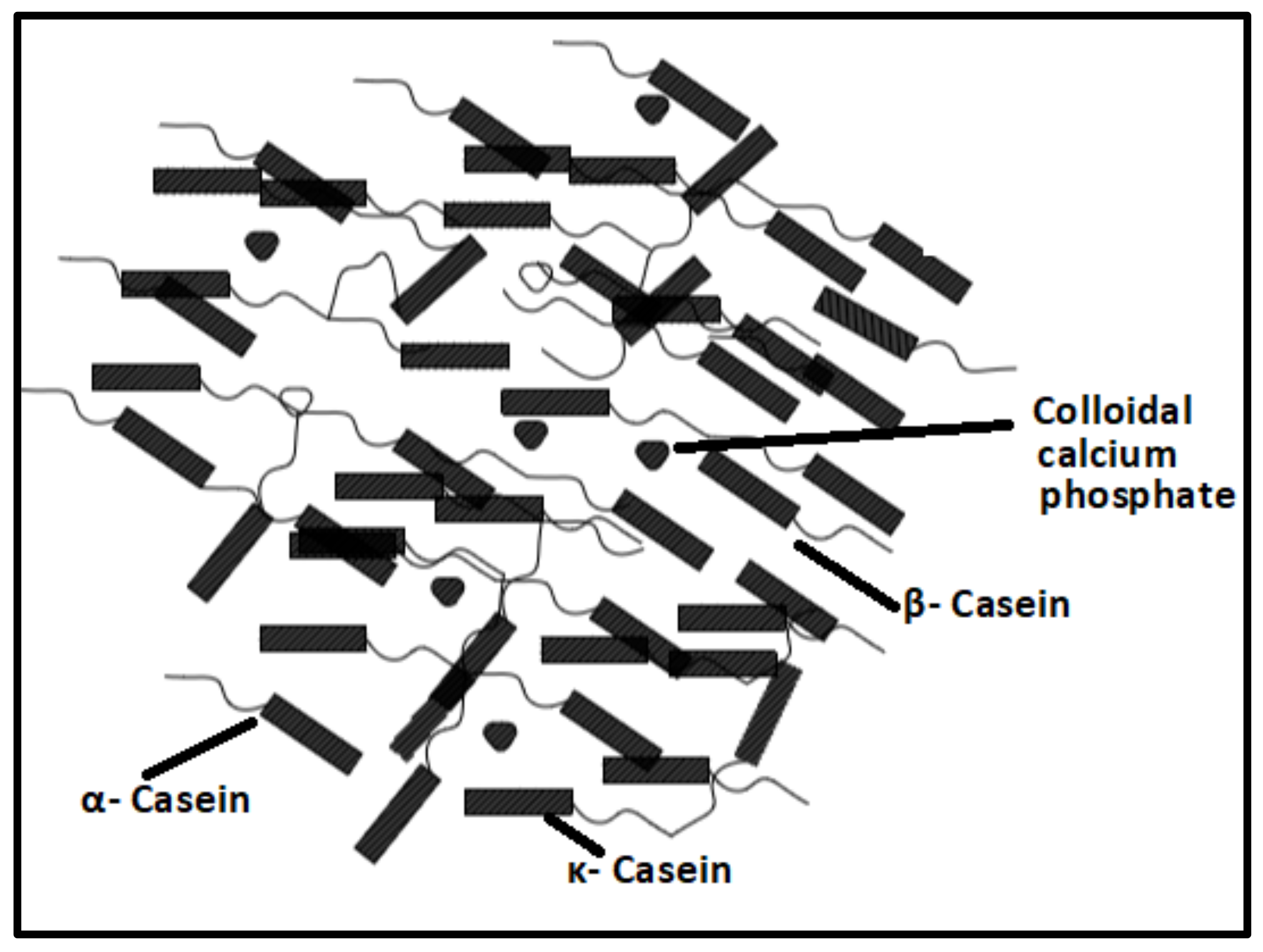
- Horne, D.S. Casein structure, self-assembly and gelation. Curr. Opin. Colloid Interface Sci. 2002, 7, 456–461.
- Sothornvit, R.; Krochta, J.M. Plasticizer effect on mechanical properties of β-lactoglobulin films. J. Food Eng. 2001, 50, 149–155.
- Hristov, P.; Mitkov, I.; Sirakova, D.; Mehandgiiski, I.; Radoslavov, G. Measurement of casein micelle size in raw dairy cattle milk by dynamic light scattering. In Milk Proteins–From Structure to Biological Properties and Health Aspects; Intech: Rijeka, Croatia, 2016.
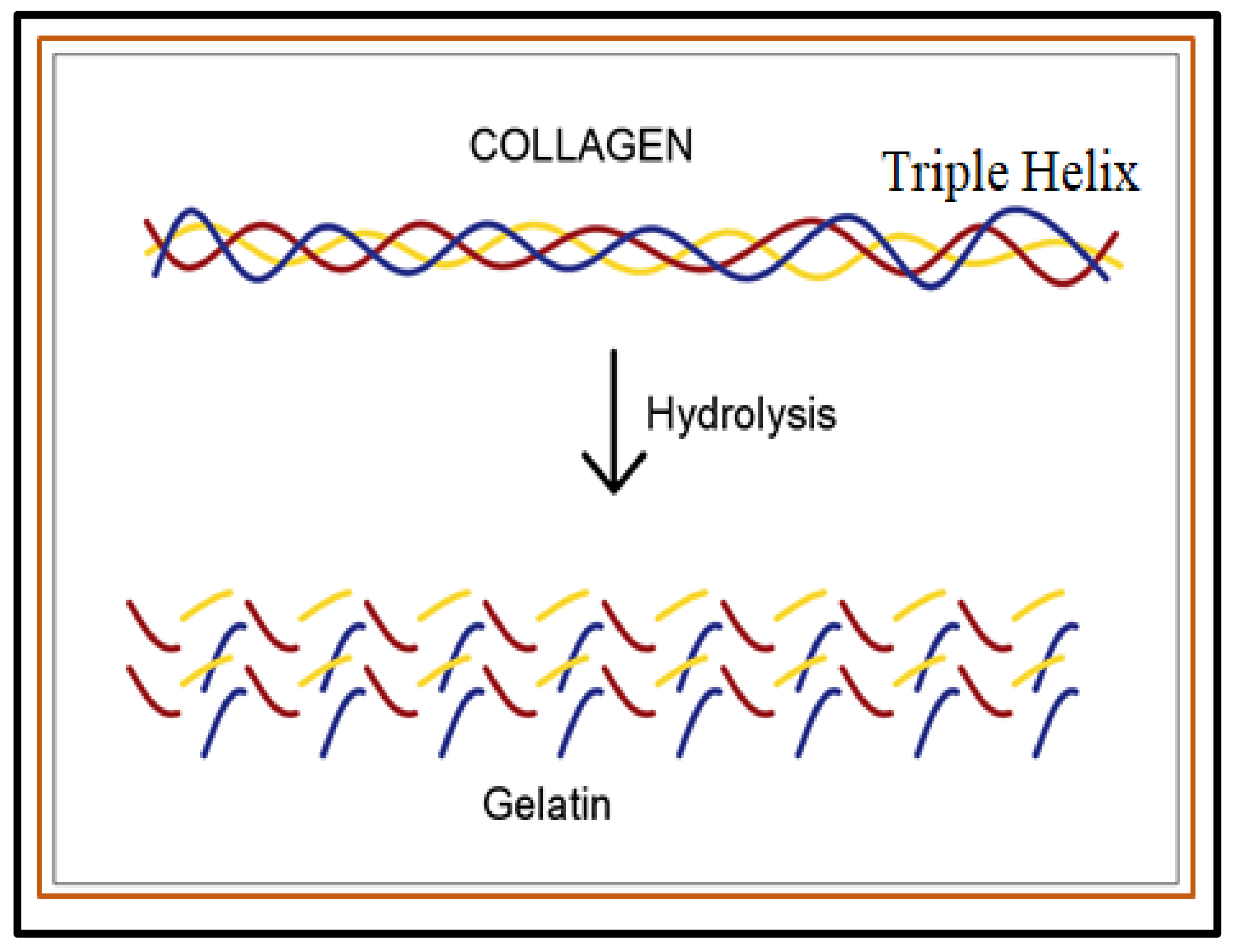
- Sibilla, S.; Godfrey, M.; Brewer, S.; Budh-Raja, A.; Genovese, L. An overview of the beneficial effects of hydrolysed collagen as a nutraceutical on skin properties: Scientific background and clinical studies. Open Nutraceuticals J. 2015, 8, 29–42.
- Schmidt, M.M.; Dornelles, R.C.P.; Mello, R.O.; Kubota, E.H.; Mazutti, M.A.; Kempka, A.P.; Demiate, I.M. Collagen extraction process. Int. Food Res. J. 2016, 23, 913–922.
- Bigi, A.; Panzavolta, S.; Rubini, K. Relationship between triple-helix content and mechanical properties of gelatin films. Biomaterials 2004, 25, 5675–5680.
- Etxabide, A.; Leceta, I.; Cabezudo, S.; Guerrero, P.; de la Caba, K. Sustainable fish gelatin films: From food processing waste to compost. ACS Sustain. Chem. Eng. 2016, 4, 4626–4634.
- Ramos, M.; Valdés, A.; Beltran, A.; Garrigós, M.C. Gelatin-based films and coatings for food packaging applications. Coatings 2016, 6, 41.
- Dash, R.; Mukherjee, S.; Kundu, S. Isolation, purification and characterization of silk protein sericin from cocoon peduncles of tropical tasar silkworm, Antheraea mylitta. Int. J. Biol. Macromol. 2006, 38, 255–258.
- Sothornvit, R.; Chollakup, R.; Potjanart, S. Extracted sericin from silk waste for film formation. Songklanakarin J. Sci. Technol. 2010, 32, 17–22.
- Zhang, Y.-Q. Applications of natural silk protein sericin in biomaterials. Biotechnol. Adv. 2002, 20, 91–100.
- Yildirim, M.; Hettiarachchy, N.S. Properties of films produced by cross-linking whey proteins and 11s globulin using transglutaminase. J. Food Sci. 1998, 63, 248–252.
- Motoki, M.; Aso, H.; Seguro, K.; Nio, N. ALPHA.s1-Casein film prepared using transglutaminase. Agric. Biol. Chem. 1987, 51, 993–996.
- Ghosh, A.; Ali, M.A.; Dias, G.J. Effect of cross-linking on microstructure and physical performance of casein protein. Biomacromolecules 2009, 10, 1681–1688.
- McHugh, T.H.; Krochta, J.M. Sorbitol- vs glycerol-plasticized whey protein edible films: Integrated oxygen permeability and tensile property evaluation. J. Agric. Food Chem. 1994, 42, 841–845.
- Wieser, H. Chemistry of gluten proteins. Food Microbiol. 2007, 24, 115–119.
- Shewry, P.R.; Tatham, A.S.; Forde, J.; Kreis, M.; Miflin, B.J. The classification and nomenclature of wheat gluten proteins: A reassessment. J. Cereal Sci. 1986, 4, 97–106.
- Mojumdar, S.C.; Moresoli, C.; Simon, L.C.; Legge, R.L. Edible wheat gluten (WG) protein films. J. Therm. Anal. Calorim. 2011, 104, 929–936.
- Micard, V.; Belamri, R.; Morel, M.H.; Guilbert, S. Properties of chemically and physically treated wheat gluten films. J. Agric. Food Chem. 2000, 48, 2948–2953.
- Ma, C.Y. Soybean|soy concentrates and isolates. In Reference Module in Food Science; Elsevier: Amsterdam, The Netherlands, 2015.
- Wang, Y.; Padua, G.W. Nanoscale characterization of zein self-assembly. Langmuir 2012, 28, 2429–2435.
- Paraman, I.; Lamsal, B.P. Recovery and characterization of α-zein from corn fermentation coproducts. J. Agric. Food Chem. 2011, 59, 3071–3077.
- Parris, N.; Dickey, L.C. Extraction and solubility characteristics of zein proteins from dry-milled corn. J. Agric. Food Chem. 2001, 49, 3757–3760.
- Hernández-Muñoz, P.; Kanavouras, A.; Ng, P.K.; Gavara, R. Development and characterization of biodegradable films made from wheat gluten protein fractions. J. Agric. Food Chem. 2003, 51, 7647–7654.
- Rhim, J.-W.; Lee, J.H.; Ng, P.K.W. Mechanical and barrier properties of biodegradable soy protein isolate-based films coated with polylactic acid. LWT—Food Sci. Technol. 2007, 40, 232–238.
- Shi, K.; Yu, H.; Lakshmana Rao, S.; Lee, T.C. Improved mechanical property and water resistance of zein films by plasticization with tributyl citrate. J. Agric. Food Chem. 2012, 60, 5988–5993.
- Kurita, K. Chitin and Chitosan: Functional biopolymers from marine crustaceans. Mar. Biotechnol. 2006, 8, 203.
- Kabalak, M.; Aracagök, D.; Torun, M. Extraction, characterization and comparison of chitins from large bodied four Coleoptera and Orthoptera species. Int. J. Biol. Macromol. 2019, 145, 402–409.
- Aranday-García, R.; Saimoto, H.; Shirai, K.; Ifuku, S. Chitin biological extraction from shrimp wastes and its fibrillation for elastic nanofiber sheets preparation. Carbohydr. Polym. 2019, 213, 112–120.
- Agbaje, O.B.A.; Shir, I.B.; Zax, D.B.; Schmidt, A.; Jacob, D.E. Biomacromolecules within bivalve shells: Is chitin abundant? Acta Biomater. 2018, 80, 176–187.
- King, C.; Shamshina, J.L.; Gurau, G.; Berton, P.; Khan, N.F.A.F.; Rogers, R.D. A platform for more sustainable chitin films from an ionic liquid process. Green Chem. 2016, 19, 117–126.
- Madeleine-Perdrillat, C.; Karbowiak, T.; Raya, J.; Gougeon, R.; Bodart, P.R.; Debeaufort, F. Water-induced local ordering of chitosan polymer chains in thin layer films. Carbohydr. Polym. 2015, 118, 107–114.
- Ma, X.; Qiao, C.; Wang, X.; Yao, J.; Xu, J. Structural characterization and properties of polyols plasticized chitosan films. Int. J. Biol. Macromol. 2019, 135, 240–245.
- Guerrero, P.; Muxika, A.; Zarandona, I.; De La Caba, K. Crosslinking of chitosan films processed by compression molding. Carbohydr. Polym. 2019, 206, 820–826.
- Lörcks, J. Properties and applications of compostable starch-based plastic material. Polym. Degrad. Stab. 1998, 59, 245–249.
- Zhang, Y.; Rempel, C.; McLaren, D. Chapter 16—Thermoplastic starch. In Innovations in Food Packaging, 2nd ed.; Han, J.H., Ed.; Academic Press: San Diego, CA, USA, 2014; pp. 391–412.
- Talja, R.A.; Helén, H.; Roos, Y.H.; Jouppila, K. Effect of various polyols and polyol contents on physical and mechanical properties of potato starch-based films. Carbohydr. Polym. 2007, 67, 288–295.
- Jimenez, A.; Fabra, M.J.; Talens, P.; Chiralt, A. Edible and biodegradable starch films: A review. Food Bioprocess Technol. 2012, 5, 2058–2076.
- O’Sullivan, A.C. Cellulose: The structure slowly unravels. Cellulose 1997, 4, 173–207.
- Spence, K.L.; Venditti, R.A.; Habibi, Y.; Rojas, O.J.; Pawlak, J.J. The effect of chemical composition on microfibrillar cellulose films from wood pulps: Mechanical processing and physical properties. Bioresour. Technol. 2010, 101, 5961–5968.
- Zhao, Y.; Moser, C.; Lindström, M.E.; Henriksson, G.; Li, J. Cellulose nanofibers from softwood, hardwood, and tunicate: Preparation–structure–film performance interrelation. ACS Appl. Mater. Interfaces 2017, 9, 13508–13519.
- Saucedo-Pompa, S.; Rojas-Molina, R.; Aguilera-Carbó, A.F.; Saenz-Galindo, A.; de La Garza, H.; Jasso-Cantú, D.; Aguilar, C.N. Edible film based on candelilla wax to improve the shelf life and quality of avocado. Food Res. Int. 2009, 42, 511–515.
- Donhowe, G.; Fennema, O. Water vapor and oxygen permeability of wax films. J. Am. Oil Chem. Soc. 1993, 70, 867–873.
- Melvin, C.; Jewell, E.; de Vooys, A.; Lammers, K.; Murray, N.M. Surface and adhesion characteristics of current and next generation steel packaging materials. J. Packag. Technol. Res. 2018, 2, 93–103.
- Grassino, A.; Pezzani, A.; Squitieri, G. Characterisation of different types of lacquers used in food packaging: Lacquer adhesion tests. Acta Aliment. —Acta Aliment. 2007, 36, 27–37.
- Allman, A.; Jewell, E.; de Vooys, A.; Hayes, R. Inter-layer adhesion performance of steel packaging materials for food cans under retort conditions. J. Packag. Technol. Res. 2018, 2, 115–124.
- Zuo, H.; Cao, Z.; Shu, J.; Xu, D.; Zhong, J.; Zhao, J.; Wang, T.; Chen, Y.; Gao, F.; Shen, L. Effect of structure on the properties of ambient-cured coating films prepared via a Michael addition reaction based on an acetoacetate-modified castor oil prepared by thiol-ene coupling. Prog. Org. Coat. 2019, 135, 27–33.
- Ruiz, M.M.; Schroeder, W.F.; Hoppe, C.E. The use of a fatty acid/β-Hydroxyester blend to enhance the surface hydrophilicity of crosslinked poly(ethylene glycol) coatings. Prog. Org. Coat. 2019, 135, 313–320.





