
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Lynne Sykes | -- | 2322 | 2022-10-28 10:19:36 | | | |
| 2 | Lindsay Dong | -28 word(s) | 2294 | 2022-10-30 15:40:26 | | |
Video Upload Options
Neutrophils are surveillance cells, and the first to react and migrate to sites of inflammation and infection following a chemotactic gradient. Neutrophils play a key role in both sterile inflammation and infection, performing a wide variety of effector functions such as degranulation, phagocytosis, ROS production and release of neutrophil extracellular traps (NETs). Healthy term labour requires a sterile pro-inflammatory process, whereas one of the most common causes of spontaneous preterm birth is microbial driven. Peripheral neutrophilia has long been described during pregnancy, and evidence exists demonstrating neutrophils infiltrating the cervix, uterus and foetal membranes during both term and preterm deliveries. Their presence supports a role in tissue remodelling via their effector functions.
1. Neutrophils and Their Effector Functions
2. Neutrophils during Healthy Pregnancy
2.1. Peripheral Blood Neutrophils in Healthy Pregnancy
2.2. Neutrophils at the Maternal–Foetal Interface in Healthy Pregnancy
3. The Role of Neutrophils in Human Term Labour
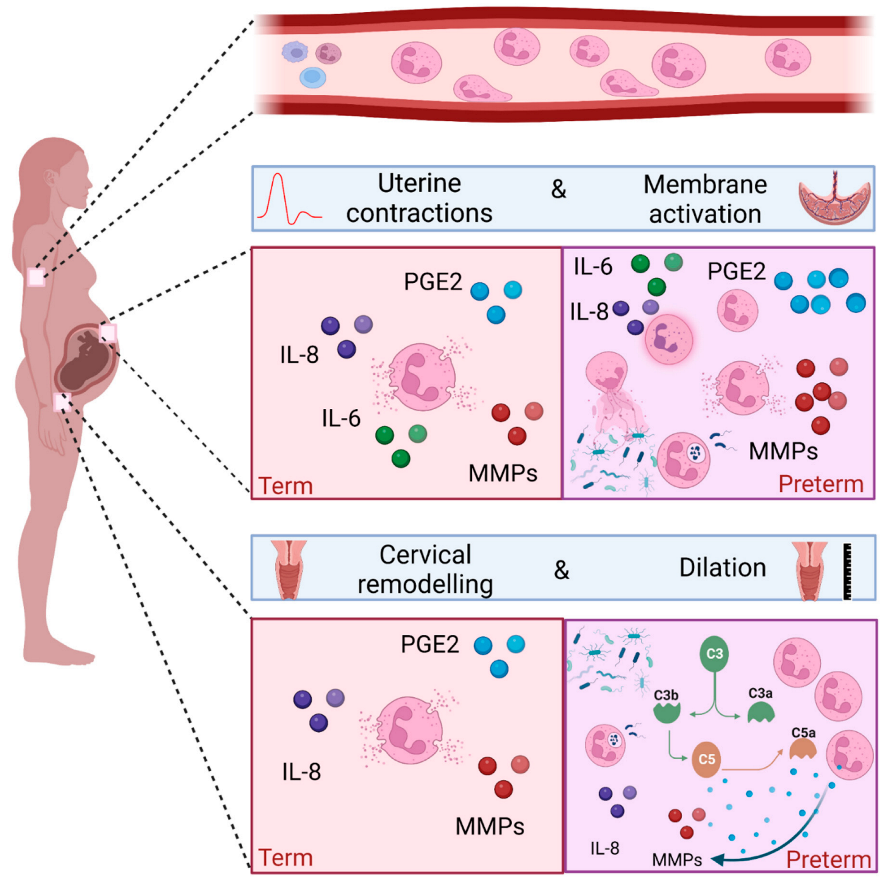
3.1. Peripheral Blood Neutrophils and Term Labour
The onset of labour is consistently associated with neutrophilia [12][41]. Furthermore, peripheral blood neutrophils taken from women in labour show signs of increased activation compared to women not in established labour [11]. In addition, an increase in markers of migration such as CD11a/b and CD62L is seen in vivo, and in vitro studies have confirmed the increased migratory capacity of neutrophils taken from women in labour [12][41].
3.2. Neutrophils and the Uterus in Term Labour
3.3. Neutrophils and the Foetal Membranes in Term Labour
3.4. Neutrophils and Cervical Remodelling in Term Labour
4. The Role of Neutrophils in Preterm Labour
5. Conclusions
References
- Liew, P.X.; Kubes, P. The Neutrophil’s Role During Health and Disease. Physiol. Rev. 2019, 99, 1223–1248.
- Xie, X.; Shi, Q.; Wu, P.; Zhang, X.; Kambara, H.; Su, J.; Yu, H.; Park, S.-Y.; Guo, R.; Ren, Q.; et al. Single-Cell Transcriptome Profiling Reveals Neutrophil Heterogeneity in Homeostasis and Infection. Nat. Immunol. 2020, 21, 1119–1133.
- Elghetany, M.T. Surface Antigen Changes during Normal Neutrophilic Development: A Critical Review. Blood Cells. Mol. Dis. 2002, 28, 260–274.
- Elghetany, M.T.; Lacombe, F. Physiologic Variations in Granulocytic Surface Antigen Expression: Impact of Age, Gender, Pregnancy, Race, and Stress. J. Leukoc. Biol. 2004, 75, 157–162.
- Lakschevitz, F.S.; Hassanpour, S.; Rubin, A.; Fine, N.; Sun, C.; Glogauer, M. Identification of Neutrophil Surface Marker Changes in Health and Inflammation Using High-Throughput Screening Flow Cytometry. Exp. Cell Res. 2016, 342, 200–209.
- Cloke, T.; Munder, M.; Taylor, G.; Müller, I.; Kropf, P. Characterization of a Novel Population of Low-Density Granulocytes Associated with Disease Severity in HIV-1 Infection. PLoS ONE 2012, 7, e48939.
- Deng, Y.; Ye, J.; Luo, Q.; Huang, Z.; Peng, Y.; Xiong, G.; Guo, Y.; Jiang, H.; Li, J. Low-Density Granulocytes Are Elevated in Mycobacterial Infection and Associated with the Severity of Tuberculosis. PLoS ONE 2016, 11, e0153567.
- Yizengaw, E.; Getahun, M.; Tajebe, F.; Cruz Cervera, E.; Adem, E.; Mesfin, G.; Hailu, A.; Van der Auwera, G.; Yardley, V.; Lemma, M.; et al. Visceral Leishmaniasis Patients Display Altered Composition and Maturity of Neutrophils as Well as Impaired Neutrophil Effector Functions. Front. Immunol. 2016, 7, 517.
- Denny, M.F.; Yalavarthi, S.; Zhao, W.; Thacker, S.G.; Anderson, M.; Sandy, A.R.; McCune, W.J.; Kaplan, M.J. A Distinct Subset of Proinflammatory Neutrophils Isolated from Patients with Systemic Lupus Erythematosus Induces Vascular Damage and Synthesizes Type I Interferons. J. Immunol. 2010, 184, 3284–3297.
- von Dadelszen, P.; Watson, R.W.G.; Noorwali, F.; Marshall, J.C.; Parodo, J.; Farine, D.; Lye, S.J.; Ritchie, J.W.K.; Rotstein, O.D. Maternal Neutrophil Apoptosis in Normal Pregnancy, Preeclampsia, and Normotensive Intrauterine Growth Restriction. Am. J. Obstet. Gynecol. 1999, 181, 408–414.
- Zhang, J.; Shynlova, O.; Sabra, S.; Bang, A.; Briollais, L.; Lye, S.J. Immunophenotyping and Activation Status of Maternal Peripheral Blood Leukocytes during Pregnancy and Labour, Both Term and Preterm. J. Cell. Mol. Med. 2017, 21, 2386–2402.
- Yuan, M.; Jordan, F.; McInnes, I.B.; Harnett, M.M.; Norman, J.E. Leukocytes Are Primed in Peripheral Blood for Activation during Term and Preterm Labour†. Mol. Hum. Reprod. 2009, 15, 713–724.
- Mor, G.; Aldo, P.; Alvero, A.B. The Unique Immunological and Microbial Aspects of Pregnancy. Nat. Rev. Immunol. 2017, 17, 469–482.
- Sykes, L.; MacIntyre, D.A.; Yap, X.J.; Teoh, T.G.; Bennett, P.R. The Th1:Th2 Dichotomy of Pregnancy and Preterm Labour. Mediators Inflamm. 2012, 2012, 967629.
- Racicot, K.; Kwon, J.-Y.; Aldo, P.; Silasi, M.; Mor, G. Understanding the Complexity of the Immune System during Pregnancy. Am. J. Reprod. Immunol. 2014, 72, 107–116.
- Aghaeepour, N.; Ganio, E.A.; Mcilwain, D.; Tsai, A.S.; Tingle, M.; Van Gassen, S.; Gaudilliere, D.K.; Baca, Q.; McNeil, L.; Okada, R.; et al. An Immune Clock of Human Pregnancy. Sci. Immunol. 2017, 2, eaan2946.
- Griffin, J.F.T.; Beck, I. A Longitudinal Study of Leucocyte Numbers and Mitogenesis during the Last Ten Weeks of Human Pregnancy. J. Reprod. Immunol. 1983, 5, 239–247.
- Luppi, P.; Haluszczak, C.; Betters, D.; Richard, C.A.H.; Trucco, M.; DeLoia, J.A. Monocytes Are Progressively Activated in the Circulation of Pregnant Women. J. Leukoc. Biol. 2002, 72, 874–884.
- Chandra, S.; Tripathi, A.K.; Mishra, S.; Amzarul, M.; Vaish, A.K. Physiological Changes in Hematological Parameters During Pregnancy. Indian J. Hematol. Blood Transfus. 2012, 28, 144–146.
- Lampé, R.; Kövér, Á.; Szűcs, S.; Pál, L.; Árnyas, E.; Ádány, R.; Póka, R. Phagocytic Index of Neutrophil Granulocytes and Monocytes in Healthy and Preeclamptic Pregnancy. J. Reprod. Immunol. 2015, 107, 26–30.
- Pramanik, S.S.; Pramanik, T.; Mondal, S.C.; Chanda, R. Number, Maturity and Phagocytic Activity of Neutrophils in the Three Trimesters of Pregnancy. East. Mediterr. Health J. 2007, 13, 862–864.
- Sacks, G.P.; Studena, K.; Sargent, I.L.; Redman, C.W.G. Normal Pregnancy and Preeclampsia Both Produce Inflammatory Changes in Peripheral Blood Leukocytes Akin to Those of Sepsis. Am. J. Obstet. Gynecol. 1998, 179, 80–86.
- Naccasha, N.; Gervasi, M.-T.; Chaiworapongsa, T.; Berman, S.; Yoon, B.H.; Maymon, E.; Romero, R. Phenotypic and Metabolic Characteristics of Monocytes and Granulocytes in Normal Pregnancy and Maternal Infection. Am. J. Obstet. Gynecol. 2001, 185, 1118–1123.
- Köstlin, N.; Kugel, H.; Spring, B.; Leiber, A.; Marmé, A.; Henes, M.; Rieber, N.; Hartl, D.; Poets, C.F.; Gille, C. Granulocytic Myeloid Derived Suppressor Cells Expand in Human Pregnancy and Modulate T-Cell Responses. Eur. J. Immunol. 2014, 44, 2582–2591.
- Farias-Jofre, M.; Romero, R.; Galaz, J.; Xu, Y.; Tao, L.; Demery-Poulos, C.; Arenas-Hernandez, M.; Bhatti, G.; Liu, Z.; Kawahara, N.; et al. Pregnancy Tailors Endotoxin-Induced Monocyte and Neutrophil Responses in the Maternal Circulation. Inflamm. Res. 2022, 71, 653–668.
- Bert, S.; Ward, E.J.; Nadkarni, S. Neutrophils in Pregnancy: New Insights into Innate and Adaptive Immune Regulation. Immunology 2021, 164, 665–676.
- Gomez-Lopez, N.; Romero, R.; Xu, Y.; Miller, D.; Leng, Y.; Panaitescu, B.; Silva, P.; Faro, J.; Alhousseini, A.; Gill, N.; et al. The Immunophenotype of Amniotic Fluid Leukocytes in Normal and Complicated Pregnancies. Am. J. Reprod. Immunol. 2018, 79, e12827.
- Thomson, A.J.; Telfer, J.F.; Young, A.; Campbell, S.; Stewart, C.J.; Cameron, I.T.; Greer, I.A.; Norman, J.E. Leukocytes Infiltrate the Myometrium during Human Parturition: Further Evidence That Labour Is an Inflammatory Process. Hum. Reprod. Oxf. Engl. 1999, 14, 229–236.
- Sakamoto, Y.; Moran, P.; Bulmer, J.N.; Searle, R.F.; Robson, S.C. Macrophages and Not Granulocytes Are Involved in Cervical Ripening. J. Reprod. Immunol. 2005, 66, 161–173.
- Word, R.A.; Li, X.-H.; Hnat, M.; Carrick, K. Dynamics of Cervical Remodeling during Pregnancy and Parturition: Mechanisms and Current Concepts. Semin. Reprod. Med. 2007, 25, 069–079.
- Myers, D.A. The Recruitment and Activation of Leukocytes into the Immune Cervix: Further Support That Cervical Remodeling Involves an Immune and Inflammatory Mechanism. Biol. Reprod. 2012, 87, 107.
- Golightly, E.; Jabbour, H.N.; Norman, J.E. Endocrine Immune Interactions in Human Parturition. Mol. Cell. Endocrinol. 2011, 335, 52–59.
- Liao, J.B.; Buhimschi, C.S.; Norwitz, E.R. Normal Labor: Mechanism and Duration. Obstet. Gynecol. Clin. N. Am. 2005, 32, 145–164.
- Terzidou, V. Biochemical and Endocrinological Preparation for Parturition. Best Pract. Res. Clin. Obstet. Gynaecol. 2007, 21, 729–756.
- Sennström, M.B.; Ekman, G.; Westergren-Thorsson, G.; Malmström, A.; Byström, B.; Endrésen, U.; Mlambo, N.; Norman, M.; Ståbi, B.; Brauner, A. Human Cervical Ripening, an Inflammatory Process Mediated by Cytokines. Mol. Hum. Reprod. 2000, 6, 375–381.
- Challis, J.R.; Lockwood, C.J.; Myatt, L.; Norman, J.E.; Strauss, J.F.; Petraglia, F. Inflammation and Pregnancy. Reprod. Sci. 2009, 16, 206–215.
- Wright, H.L.; Moots, R.J.; Bucknall, R.C.; Edwards, S.W. Neutrophil Function in Inflammation and Inflammatory Diseases. Rheumatology 2010, 49, 1618–1631.
- Osman, I.; Young, A.; Ledingham, M.A.; Thomson, A.J.; Jordan, F.; Greer, I.A.; Norman, J.E. Leukocyte Density and Pro-inflammatory Cytokine Expression in Human Fetal Membranes, Decidua, Cervix and Myometrium before and during Labour at Term. Mol. Hum. Reprod. 2003, 9, 41–45.
- Young, A.; Thomson, A.J.; Ledingham, M.; Jordan, F.; Greer, I.A.; Norman, J.E. Immunolocalization of Proinflammatory Cytokines in Myometrium, Cervix, and Fetal Membranes During Human Parturition at Term1. Biol. Reprod. 2002, 66, 445–449.
- Sakamoto, Y.; Moran, P.; Searle, R.F.; Bulmer, J.N.; Robson, S.C. Interleukin-8 Is Involved in Cervical Dilatation but Not in Prelabour Cervical Ripening. Clin. Exp. Immunol. 2004, 138, 151–157.
- Delgado, I.; Neubert, R.; Dudenhausen, J.W. Changes in White Blood Cells during Parturition in Mothers and Newborn. Gynecol. Obstet. Investig 1994, 38, 227–235.
- Bollopragada, S.; Youssef, R.; Jordan, F.; Greer, I.; Norman, J.; Nelson, S. Term Labor Is Associated with a Core Inflammatory Response in Human Fetal Membranes, Myometrium, and Cervix. Am. J. Obstet. Gynecol. 2009, 200, 104.e1–104.e11.
- Winkler, M.; Fischer, D.-C.; Ruck, P.; Marx, T.; Kaiserling, E.; Oberpichler, A.; Tschesche, H.; Rath, W. Parturition at Term: Parallel Increases in Interleukin-8 and Proteinase Concentrations and Neutrophil Count in the Lower Uterine Segment. Hum. Reprod. 1999, 14, 1096–1100.
- Osman, I.; Young, A.; Jordan, F.; Greer, I.A.; Norman, J.E. Leukocyte Density and Proinflammatory Mediator Expression in Regional Human Fetal Membranes and Decidua Before and During Labot at Term. J. Soc. Gynecol. Investig. 2006, 13, 97–103.
- Takahashi, N.; Okuno, T.; Fujii, H.; Makino, S.; Takahashi, M.; Ohba, M.; Saeki, K.; Itakura, A.; Takeda, S.; Yokomizo, T. Up-Regulation of Cytosolic Prostaglandin E Synthase in Fetal-Membrane and Amniotic Prostaglandin E2 Accumulation in Labor. PLoS ONE 2021, 16, e0250638.
- Norman, J.E. Cervical Function and Prematurity. Best Pract. Res. Clin. Obstet. Gynaecol. 2007, 21, 791–806.
- Bokström, H.; Brännström, M.; Alexandersson, M.; Norström, A. Leukocyte Subpopulations in the Human Uterine Cervical Stroma at Early and Term Pregnancy. Hum. Reprod. 1997, 12, 586–590.
- Hein, M.; Petersen, A.C.; Helmig, R.B.; Uldbjerg, N.; Reinholdt, J. Immunoglobulin Levels and Phagocytes in the Cervical Mucus Plug at Term of Pregnancy. Acta Obstet. Gynecol. Scand. 2005, 84, 734–742.
- Timmons, B.C.; Mahendroo, M.S. Timing of Neutrophil Activation and Expression of Proinflammatory Markers Do Not Support a Role for Neutrophils in Cervical Ripening in the Mouse1. Biol. Reprod. 2006, 74, 236–245.
- Blencowe, H.; Cousens, S.; Oestergaard, M.Z.; Chou, D.; Moller, A.-B.; Narwal, R.; Adler, A.; Vera Garcia, C.; Rohde, S.; Say, L.; et al. National, Regional, and Worldwide Estimates of Preterm Birth Rates in the Year 2010 with Time Trends since 1990 for Selected Countries: A Systematic Analysis and Implications. Lancet 2012, 379, 2162–2172.
- Liu, L.; Oza, S.; Hogan, D.; Perin, J.; Rudan, I.; Lawn, J.E.; Cousens, S.; Mathers, C.; Black, R.E. Global, Regional, and National Causes of Child Mortality in 2000–13, with Projections to Inform Post-2015 Priorities: An Updated Systematic Analysis. Lancet 2015, 385, 430–440.
- Agrawal, V.; Hirsch, E. Intrauterine Infection and Preterm Labor. Semin. Fetal. Neonatal Med. 2012, 17, 12–19.
- Kim, C.J.; Romero, R.; Chaemsaithong, P.; Chaiyasit, N.; Yoon, B.H.; Kim, Y.M. Acute Chorioamnionitis and Funisitis: Definition, Pathologic Features, and Clinical Significance. Am. J. Obstet. Gynecol. 2015, 213, S29–S52.
- Steel, J.H.; O’Donoghue, K.; Kennea, N.L.; Sullivan, M.H.F.; Edwards, A.D. Maternal Origin of Inflammatory Leukocytes in Preterm Fetal Membranes, Shown by Fluorescence in Situ Hybridisation. Placenta 2005, 26, 672–677.
- Yamada, T.; Minakami, H.; Matsubara, S.; Yatsuda, T.; Sato, I. Changes in Polymorphonuclear Leukocytes in the Vagina of Patients with Preterm Labor. Gynecol. Obstet. Investig 1998, 45, 32–34.
- Hezelgrave, N.L.; Seed, P.T.; Chin-Smith, E.C.; Ridout, A.E.; Shennan, A.H.; Tribe, R.M. Cervicovaginal Natural Antimicrobial Expression in Pregnancy and Association with Spontaneous Preterm Birth. Sci. Rep. 2020, 10, 12018.
- Whitworth, M.K.; Pafilis, I.; Vince, G.; Quenby, S. Cervical Leukocyte Sub-Populations in Idiopathic Preterm Labour. J. Reprod. Immunol. 2007, 75, 48–55.
- Stranik, J.; Kacerovsky, M.; Andrys, C.; Soucek, O.; Bolehovska, R.; Holeckova, M.; Matulova, J.; Jacobsson, B.; Musilova, I. Intra-Amniotic Infection and Sterile Intra-Amniotic Inflammation Are Associated with Elevated Concentrations of Cervical Fluid Interleukin-6 in Women with Spontaneous Preterm Labor with Intact Membranes. J. Matern. Fetal Neonatal Med. 2021, 1–9.
- Gomez-Lopez, N.; Romero, R.; Varrey, A.; Leng, Y.; Miller, D.; Done, B.; Xu, Y.; Bhatti, G.; Motomura, K.; Gershater, M.; et al. RNA Sequencing Reveals Diverse Functions of Amniotic Fluid Neutrophils and Monocytes/Macrophages in Intra-Amniotic Infection. J. Innate Immun. 2021, 13, 63–82.
- Nadeau-Vallée, M.; Obari, D.; Palacios, J.; Brien, M.-È.; Duval, C.; Chemtob, S.; Girard, S. Sterile Inflammation and Pregnancy Complications: A Review. Reproduction 2016, 152, R277–R292.
- Romero, R.; Miranda, J.; Chaiworapongsa, T.; Korzeniewski, S.J.; Chaemsaithong, P.; Gotsch, F.; Dong, Z.; Ahmed, A.I.; Yoon, B.H.; Hassan, S.S.; et al. Prevalence and Clinical Significance of Sterile Intra-Amniotic Inflammation in Patients with Preterm Labor and Intact Membranes. Am. J. Reprod. Immunol. 2014, 72, 458–474.
- Baumbusch, M.A.; Buhimschi, C.S.; Oliver, E.A.; Zhao, G.; Thung, S.; Rood, K.; Buhimschi, I.A. High Mobility Group-Box 1 (HMGB1) Levels Are Increased in Amniotic Fluid of Women with Intra-Amniotic Inflammation-Determined Preterm Birth, and the Source May Be the Damaged Fetal Membranes. Cytokine 2016, 81, 82–87.
- Figueroa, R.; Garry, D.; Elimian, A.; Patel, K.; Sehgal, P.B.; Tejani, N. Evaluation of Amniotic Fluid Cytokines in Preterm Labor and Intact Membranes. J. Matern. Fetal Neonatal Med. 2005, 18, 241–247.
- Romero, R.; Brody, D.T.; Oyarzun, E.; Mazor, M.; King Wu, Y.; Hobbins, J.C.; Durum, S.K. Infection and Labor: III. Interleukin-1: A Signal for the Onset of Parturition. Am. J. Obstet. Gynecol. 1989, 160, 1117–1123.
- Thaxton, J.E.; Romero, R.; Sharma, S. TLR9 Activation Coupled to IL-10 Deficiency Induces Adverse Pregnancy Outcomes. J. Immunol. 2009, 183, 1144–1154.
- Scharfe-Nugent, A.; Corr, S.C.; Carpenter, S.B.; Keogh, L.; Doyle, B.; Martin, C.; Fitzgerald, K.A.; Daly, S.; O’Leary, J.J.; O’Neill, L.A.J. TLR9 Provokes Inflammation in Response to Fetal DNA: Mechanism for Fetal Loss in Preterm Birth and Preeclampsia. J. Immunol. 2012, 188, 5706–5712.
- Clarke, T.B.; Davis, K.M.; Lysenko, E.S.; Zhou, A.Y.; Yu, Y.; Weiser, J.N. Recognition of Peptidoglycan from the Microbiota by Nod1 Enhances Systemic Innate Immunity. Nat. Med. 2010, 16, 228–231.
- Ozel, A.; Alici Davutoglu, E.; Yurtkal, A.; Madazli, R. How Do Platelet-to-Lymphocyte Ratio and Neutrophil-to-Lymphocyte Ratio Change in Women with Preterm Premature Rupture of Membranes, and Threaten Preterm Labour? J. Obstet. Gynaecol. 2020, 40, 195–199.
- Lakshmi, M.P.A.S.; Sravani, V.L. Role of Neutrophil-Lymphocyte Ratio in Determining the Outcomes of Preterm Premature Rupture of Membranes. Int. J. Reprod. Contracept. Obstet. Gynecol. 2021, 10, 1617–1620.
- Vakili, S.; Torabinavid, P.; Tabrizi, R.; Shojazadeh, A.; Asadi, N.; Hessami, K. The Association of Inflammatory Biomarker of Neutrophil-to-Lymphocyte Ratio with Spontaneous Preterm Delivery: A Systematic Review and Meta-Analysis. Mediators Inflamm. 2021, 2021, 6668381.
- Gervasi, M.-T.; Chaiworapongsa, T.; Naccasha, N.; Blackwell, S.; Yoon, B.H.; Maymon, E.; Romero, R. Phenotypic and Metabolic Characteristics of Maternal Monocytes and Granulocytes in Preterm Labor with Intact Membranes. Am. J. Obstet. Gynecol. 2001, 185, 1124–1129.
- Tong, M.; Potter, J.A.; Mor, G.; Abrahams, V.M. Lipopolysaccharide-Stimulated Human Fetal Membranes Induce Neutrophil Activation and Release of Vital Neutrophil Extracellular Traps. J. Immunol. 2019, 203, 500–510.
- Molina, B.; Bayar, E.; Lee, Y.S.; Muller, I.; Botto, M.; MacIntyre, D.; Bennett, P.R.; Kropf, P.; Sykes, L. Cervicovaginal Inflammation and Neutrophils Infiltration/Activation in Women at High-Risk of Prematurity. BJOG Int. J. Obstet. Gynaecol. 2022, 129, 47–62.




