] and unsurprisingly this coincides with an increase in cell adhesion molecule expression to aid transmigration [
]. Expression of the chemoattractant
mRNA was found to be higher in myometrium of women at term during labour compared to term not in labour, which may explain in part the increased abundance of neutrophils during labour [
]. Consistent with this, a parallel increase in IL-8 concentrations and neutrophil counts are seen in the lower uterine segment in women who are in active labour. This is also associated with increased concentrations of matrix metalloproteinases 8 and 9 [
].
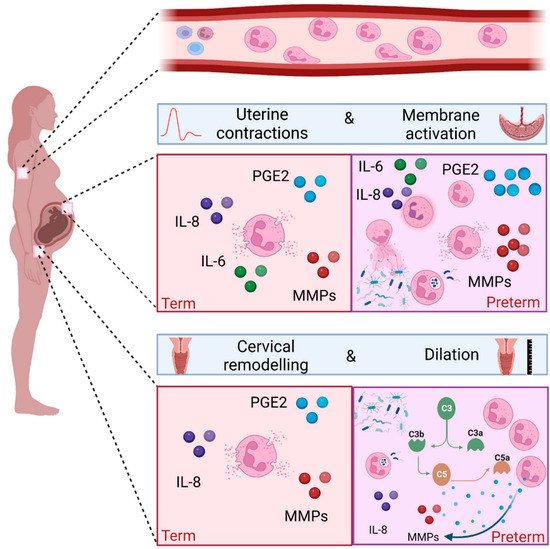
Leukocytes, including neutrophils, are known to infiltrate foetal membranes at the time of term labour [
61,
62], and this is accompanied by an increase in the concentrations of pro-inflammatory cytokines such as IL-1β, IL-6, IL-8 [
61,
62], and pro-labour mediators such as COX-2 and PGE2 synthases [
67,
68].
To facilitate vaginal delivery, cervical dilation is one of the processes that occurs during labour. It is a complex process involving softening, effacement and ripening of the cervix. Biochemical changes are required, including a decline in collagen synthesis, an increase in collagenase activity, local immune cell infiltration, and increased concentrations of cytokines and prostaglandins [
69]. Several studies have demonstrated cervical infiltration of neutrophils from biopsies taken at the time of labour [
52,
61,
66,
70]. Furthermore, the content of the cervical mucous plug is rich in neutrophils [
71]. There are conflicting opinions about the importance of cervical neutrophils in causing the cervical changes required for labour, since not all human and murine studies have demonstrated increased neutrophil density in the presence of cervical ripening [
52,
72].
4. The Role of Neutrophils in Preterm Labour
Preterm birth (PTB) is defined as a birth occurring before 37 completed weeks of pregnancy and can be further classified as extremely preterm (<28 weeks), very preterm (28–32 weeks), and moderate to late preterm (32–36 weeks). There are 15 million babies born preterm each year, with global rates averaging 10%, although significant variations (5–18%) exist depending on geographical location [
76]. PTB is the biggest cause of childhood mortality under the age of 5, with morbidity and mortality increasing with decreasing gestational age at delivery [
77]. Roughly two thirds of births are spontaneous, with women presenting either with preterm prelabour rupture of membranes (PPROM), or with uterine contractions and cervical dilation. The causes of preterm birth are multifactorial, but the most common causal factors are infection and/or inflammation. Extreme and very preterm birth are most likely to have evidence of infection and/or inflammation, and babies born with evidence of foetal inflammatory response have a worse prognosis for any given gestational age.
Since neutrophils play a major role in infection and inflammation, they are also likely to play a key role in infection and inflammation in the context of preterm birth. The most common source of intrauterine infection is ascending pathogenic microbes from the cervical-vaginal interface [
78]. The presence of intrauterine infection is associated with neutrophil infiltration and pro-inflammatory cytokine production [
79]. PPROM often presents with or leads to clinical signs of chorioamnionitis, with neutrophil invasion of the chorioamnion being the hallmark of histological chorioamnionitis [
80]. Evidence also exists to support the role of neutrophils in driving local inflammation at the cervical-vaginal interface in women who deliver preterm [
7,
81,
82,
83].
Inflammation in the absence of infection, also referred to as sterile inflammation, has also been widely reported in the context of preterm birth [
84,
85,
86]. DAMPs, also known as alarmins, are endogenous molecules that send a danger message as part of a response to inflammation. Alarmins that have been commonly associated with preterm labour include high mobility group box 1 (HMGB1), IL-1α, and cell free DNA. HMGB1 [
87,
88] and IL-1 α [
89,
90] concentrations are higher in amniotic fluid of women who have evidence of sterile inflammation and deliver preterm. Cell free DNA activates Toll-like receptor 9 (TLR-9), and animal models support the concept of cell free DNA leading to preterm delivery via leukocyte migration and inflammation at the maternal–foetal interface [
91,
92].
Products of the microbiota can be translocated into the circulation, priming and enhancing neutrophil’s function [
108]. The use of peripheral blood neutrophil concentrations as a predictor of preterm birth has been explored in women presenting in threatened preterm labour and PPROM, using the neutrophil-to-lymphocyte ratio (NLR) and total neutrophil counts [
109,
110]. Several studies show an increase in neutrophil counts and the NLR in women who subsequently deliver preterm [
12,
111]. Peripheral blood neutrophils exhibit a more activated immunophenotype in women who deliver preterm. Gervasi et al. collected peripheral blood from women who had a healthy pregnancy and subsequent term labour and compared the granulocyte phenotype with women who delivered preterm. Using flow cytometry, they identified that granulocytes expressed higher levels of CD11b, CD15 and CD66 in women who delivered preterm [
112].
The neutrophils migrate towards a chemotactic gradient, with in vitro evidence demonstrating the chemotactic effect of both unstimulated and LPS stimulated foetal membranes [
118]. Conditioned media from LPS stimulated foetal membranes leads to the release of cytokines, chemokines, and reactive oxygen species from neutrophils, as well as neutrophil degranulation and NET release [
118].
No significant differences were seen in neutrophil concentrations between women who delivered at term compared to preterm, however the study was likely to be underpowered for this outcome as only six women delivered preterm. RNA-seq was performed on cervical neutrophils from a subset of the cohort (n = 9). Despite small numbers, the expression of genes involved in neutrophil activation and degranulation negatively correlated with the presence of
G. vaginalis and positively correlated with the presence of
L. iners in matched vaginal swabs. We have shown that in women at high risk of preterm birth, neutrophils are more likely to be present at the cervical-vaginal interface if the microbial composition is one of high risk of preterm birth (CST III/
L. iners, or CST IV, diverse), compared to low risk (CST I/
L. crispatus, CST II/
L. gasseri, CST V/
L. jensenii) [
107]. Furthermore, in women that have detectable live cervical neutrophils, there are higher concentrations of pro-inflammatory mediators such as C3b, C1q and C4b in the cervical-vaginal fluid. Taken together, these data suggest a plausible role for cervical neutrophils in microbial driven cervical shortening and PTB [
107].
5. Conclusions
Neutrophils are polymorphonuclear cells and are the most predominant circulating innate immune cells. They play a key role in both inflammation and infection, with effector functions that lead to direct and indirect cell death and microbial clearance. Activated neutrophils secrete pro-inflammatory mediators such as cytokines, proteases and collagenases, and pro-labour mediators such as COX-2 and PGE2. These mediators are required for the physiological processes of healthy term labour; cervical remodelling, uterine contractility, and foetal membrane rupture. However, in cases of microbial driven preterm birth, the premature recruitment of neutrophils into the cervix, uterus and foetal membranes, combined with increased activation, are likely to play a key role in triggering PPROM and PTB.