
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Mahesh P. Bhat | -- | 1612 | 2022-10-20 04:13:56 | | | |
| 2 | Mahesh P. Bhat | Meta information modification | 1612 | 2022-10-20 11:49:17 | | | | |
| 3 | Catherine Yang | Meta information modification | 1612 | 2022-10-21 02:40:57 | | |
Video Upload Options
Fluoride (F¯) is the most electronegative and lightest member of the halogen group of elements. The contamination of groundwater with inorganic fluoride is due to the withering of underground rocks and also due to increased globalization. According World Health Organisation (WHO) High fluoride concentration in groundwater beyond the permissible limit of 1.5 mg/L is a major threat to human and animal health.
1. Introduction
The selective detection and quantitative analysis of anions and metal ions in real-time using synthetic organic receptors is an emerging and attractive research area across several disciplines including chemistry, biology, and environmental science. Anions play a major role in human life thus, it is important to detect these ions in real-time quantitatively. Hence, there is a need to develop low-cost, simple, efficient, and selective receptors for this purpose. Among various anions, fluoride (F¯) ions are important due to their biological importance, they play an important role in human health and have been extensively used in clinical applications such as in the treatment of osteoporosis, orthodontics, and enamel demineralization. Fluoride ions are also used as a common ingredient in psychiatric, anesthetics, hypnotics drugs, cockroaches, rat poisons, and in nerve gases in military fields. However, excess F¯ ion consumption could lead to skeletal and dental fluorosis, osteosarcoma, thyroid problems, collagen breakdown, and even cancer. Due to its dual nature, qualitative and quantitative analysis of F¯ ions has been an active research area over the last two decades. Therefore, a small quantity of F¯ ions is essential and beneficial to human health. Despite knowing these facts, the continued introduction of F¯ to the environment by excessive usage of fertilizers and industrial wastes is of great concern.
In India, nearly 90 % of the rural population is dependent on groundwater for drinking and domestic purpose. The presence of a high concentration of fluoride in these water bodies makes the population threatened with several health hazards. According to Central Ground Water Board (CGWB), India, high concentrations of fluoride in groundwater have been reported from districts of several states namely, Andhra Pradesh, Telengana, Assam, Bihar, Chhattisgarh, Delhi, Gujarat, Haryana, Jammu & Kashmir, Jharkhand, Karnataka, Kerala, Madhya Pradesh, Maharashtra, Odisha, Punjab, Rajasthan, Tamil Nadu, Uttar Pradesh, and West Bengal. Few areas in India have reported a very high concentration of fluoride exceeding 5 mg/L in groundwater. A general survey of community water supplies has exposed that 25 million people in rural India are consuming high fluoride-contaminated water. About 90 million population including 6 million children in 200 districts in 15 states have been affected by groundwater fluoride-related health problems. Thus, the development of ‘easy-to-use’ and ‘cost-effective’ devices for the detection of groundwater fluoride needs extensive investigation.
2. Research gap
- The majority of the receptors involve complicated molecules which require complicated organic synthesis. Therefore, these receptors are not much cost-effective and environmentally friendly.
- The majority of them are based on fluorometric detection, which requires the help of complicated instrumentation for the detection process.
- Detection of fluoride at ppm level still needs to be explored.
- For real-life applications, the receptors should work in aqueous media.
- The receptors could be utilized for practical applications only when they can be made into some devices for quantitative measurements.
3. Current methods of fluoride ion detection
| Methods |
Advantages |
Disadvantages |
|
Ion-selective electrodes |
Rapid, simple |
Expensive, calibration, precision, fragile, shelf life |
|
Ion-selective electrodes |
Low detection limit, selective |
Expensive, data processing, tedious, long time, not portable |
4. Why colorimetric/fluorometric receptors?
- Simple naked-eye detection
- Easy to handle
- Portable
- No skilled personnel to operate
- Cost-effective
- No sophisticated instrumentations required
5. Objectives
- Designing and synthesizing organic receptors for the colorimetric detection of fluoride in organic media.
- Modification of organic receptors for the colorimetric detection of fluoride in aqueous media.
- Characterization of receptors by various analytical techniques.
- Designing novel ‘easy-to-use’ and ‘cost-effective’ disposable devices for the detection of fluoride in ground water.
- Fabrication of disposable devices.
- Demonstration of ‘in-field’ application of the devices.
6. Turmeric, a naturally available colorimetric receptor for the quantitative detection of fluoride[1]
Turmeric (Curcuma longa) an important food ingredient has been used since ancient times for various biological applications, but to date, it has not been considered for the colorimetric detection of anions and cations. In this work, the application of turmeric solution for the colorimetric detection of F− and iron ions was demonstrated. Results showed a significant color change from yellow to blue and yellow to orange upon the addition of F− and iron ions respectively. The detection mechanism was investigated using curcumin, a major component of turmeric powder. The change in color and fluorescence quenching was attributed to the formation of receptor complex with F− and iron ions which resulted in intramolecular charge transfer transition. The mechanism of binding has been confirmed by UV–vis, 1H NMR, and fluorescence titrations. The present detection concept was further explored to develop a low-cost, reusable F− detection kit, which showed its ability to detect F− ions in the both aqueous and organic medium at a very low concentration such as 1 ppm.
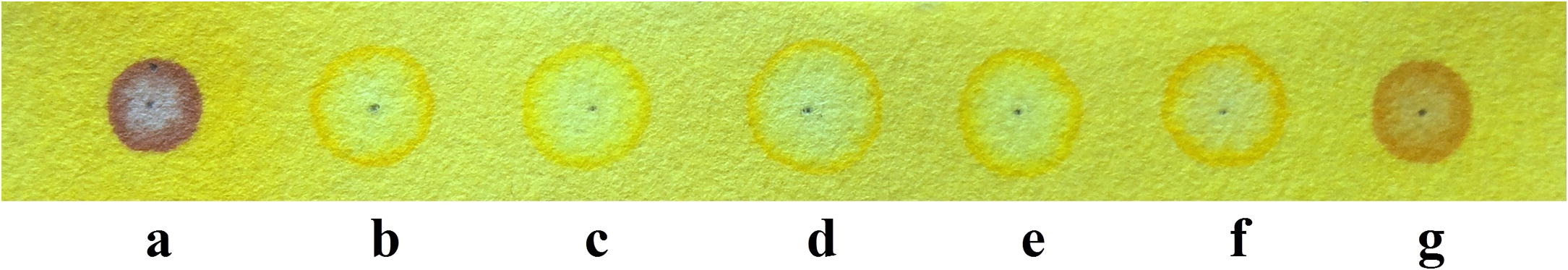
Fig. 1. Turmeric strip showing color change for different anions, (a) F−, (b) Cl−, (c) Br−, (d) I−, (e) HSO4−, (f) H2PO4− and (g) AcO− ions.
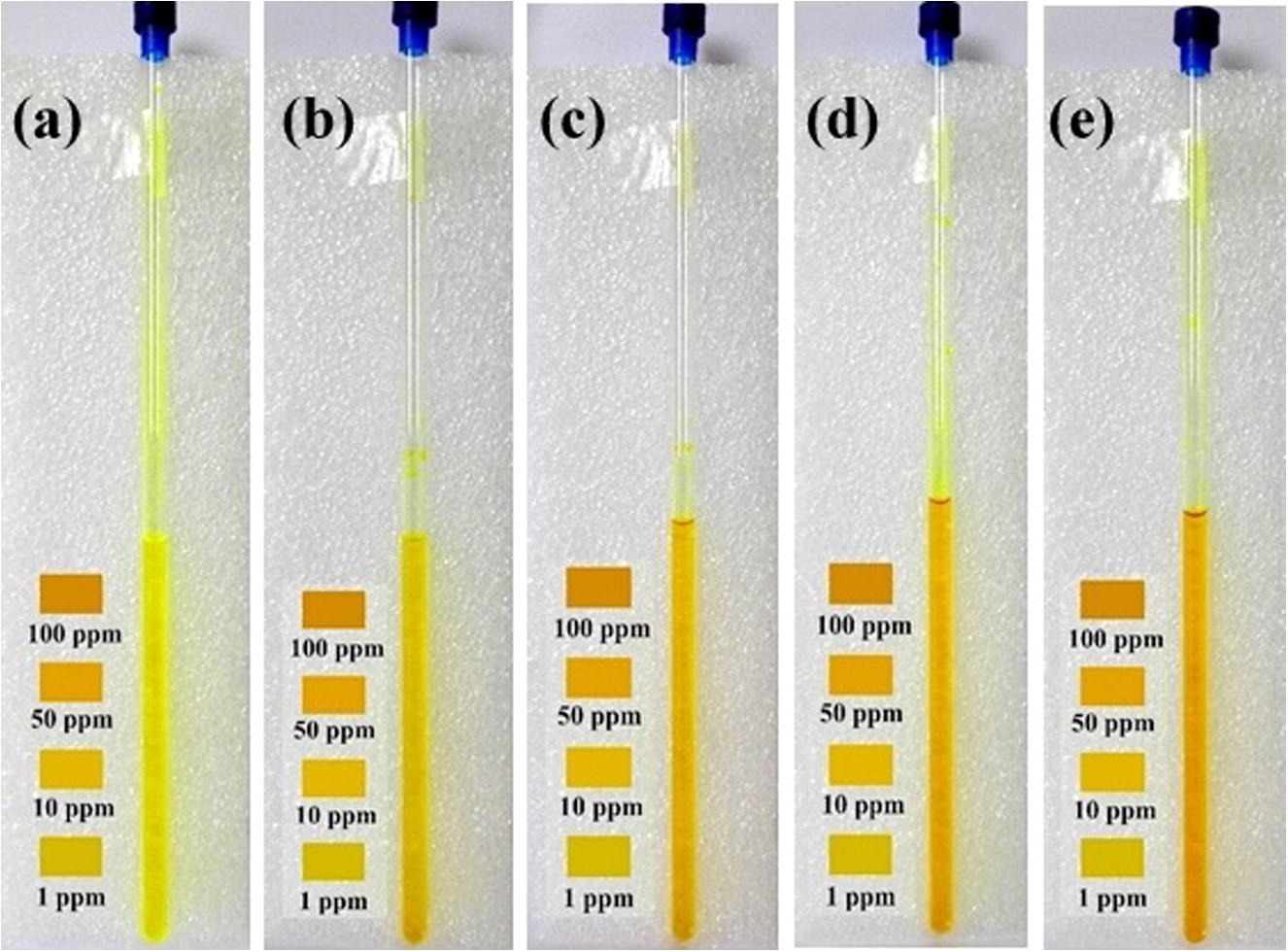
Fig. 2. The real reusable fluoride ions detection kit showing different colors for different concentrations of F− ions present in the aqueous media. (a) curcumin solution, (b) addition of 1 ppm, (c) 10 ppm, (d) 50 ppm, and (e) 100 ppm of F− ions.
6. A reversible fluoride chemosensor for the development of multi-input molecular logic gates[2]
A potential rhodamine-derived chemosensor was synthesized for the quantitative colorimetric detection of F− ions in real-time and to design multiple-input logic gates at the molecular level. Bottom-up fabrication techniques to design logic gates using chemical inputs have been initiated for a decade now and have shown immense promise to make the size of microprocessors even smaller, as the chemical signals are at the molecular level. The chemosensor exhibited a change in color from pale yellow to dark yellow for F− ions. However, upon adding Cu2+ ions to the same solution, pink coloration was exhibited. These changes in color and absorbance were used to develop chemical input logic gates. Further, the color change trend could be reversed for sixteen cycles with the alternating addition of F− and Cu2+ ions. Moreover, the chemosensor was used to design combinational three-input logic gates by adding Cu2+, F−, and Ni2+ ions. In addition, UV-vis titrations and 1H NMR titrations were carried out to understand the color change and binding mechanism. Furthermore, the chemosensor was investigated for its ability to detect F− ions in real samples.
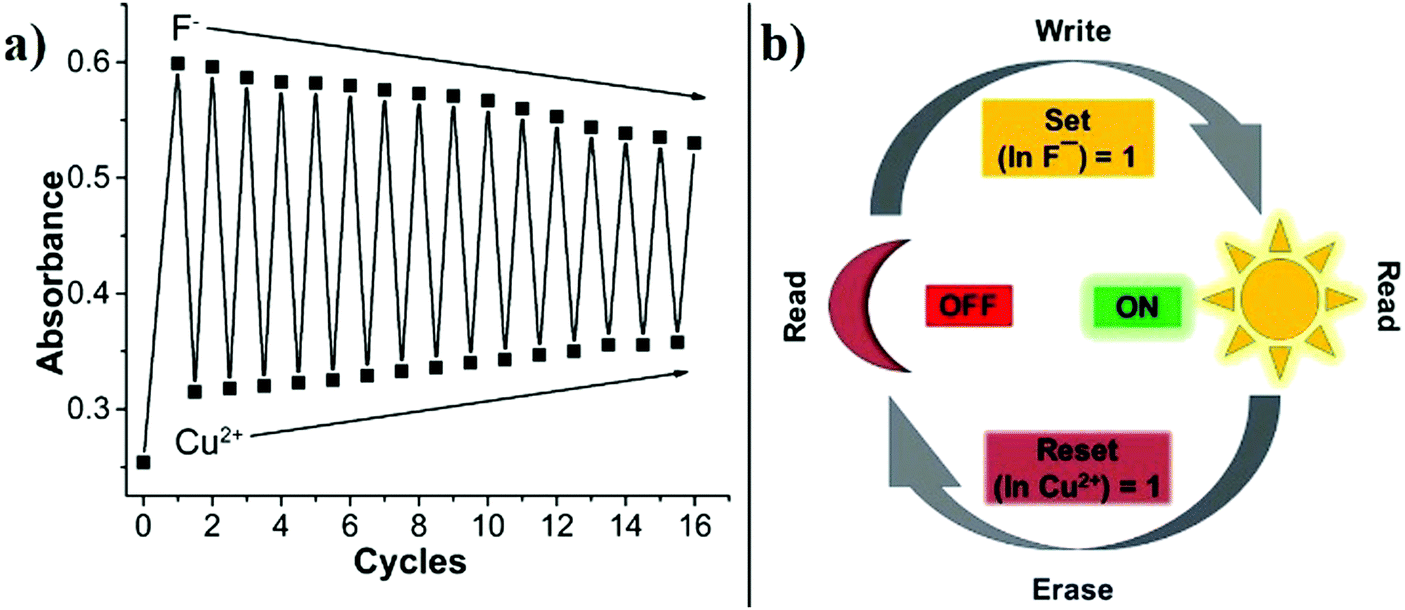
Fig. 3. (a) Color change of (i) the chemosensor (M2) upon adding (ii) Cu2+ ions (M2 + Cu2+), (iii) F− ions (M2 + Cu2+ + F−), and (iv) Ni2+ ions (M2 + Cu2+ + F− + Ni2+). (b) The UV-vis spectral changes after adding Cu2+, F−, and Ni2+ ions to the chemosensor.

Fig. 4. Color changes of surface-modified diatoms after the addition of F− and Cu2+ ions.
7. New optofluidic-based lab-on-a-chip device for the real-time fluoride analysis[3]
A PDMS (Polydimethylsiloxane) microfluidic channel coupled with UV–vis fiber-optic spectrometer and the new synthesized colorimetric probe was integrated into an optofluidic-based Lab-on-a-chip device for highly sensitive and real-time quantitative measurements of fluoride ions (F¯). An ‘S-shaped microchannel in a microfluidic device was designed to act as a microreactor to facilitate the continuous reaction between synthesized colorimetric probe (sensor) and F¯ ions. Following this reaction, the UV–vis optical probe in the downstream detection zone of the microfluidic device was used to capture their spectrum and present as F¯ concentration in real-time conditions. An initial study of the developed colorimetric probe with multi-color change with several binding and chromophore groups such as –OH, –NH, and –NO2 groups confirmed its high sensitivity and selectivity for F¯ ions with a detection limit of 0.79 ppm. The performance of the developed optofluidic device was evaluated for the selective, sensitive detection of F¯ ions including real samples out-performing conventional methods. The technology has advantages such as low sample consumption, rapid analysis, high sensitivity, and portability. The presented new Lab-on-a-chip device provides many competitive advantages for the real-time analysis of F¯ ions needed across broad sectors.
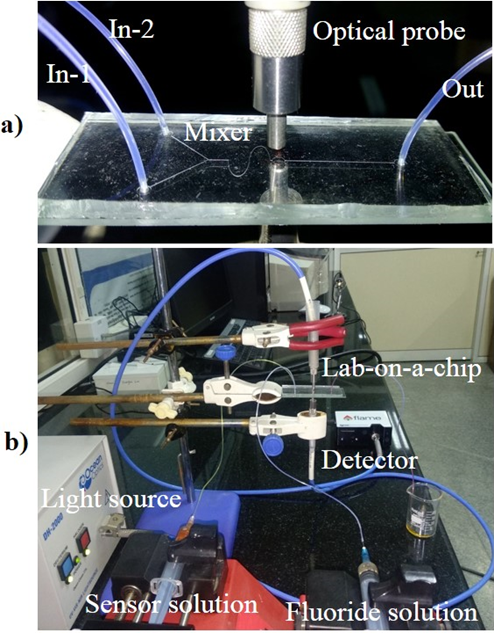
Fig. 5. a) Microfluidic chip showing various regions, UV–vis fiber optics aligned with the pre-designed detection zone of the microchannel for the determination of F¯ ions. b) Working set-up of a lab-on-a-chip device for quantitative detection of F¯ ion.
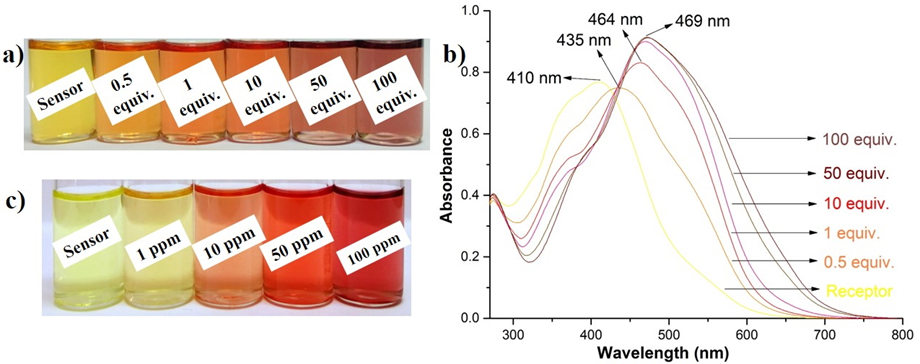
Fig. 6. a, b) Color change and UV-vis absorption changes of the colorimetric probe (2.5×10-5 M) in DMSO:H2O (8:2, v/v) medium upon the addition of various concentrations of F¯ ions in TBA form. c) Color change of the colorimetric probe (2.5×10-5 M) in DMSO with the addition of various concentrations of NaF.
8. Colorimetric Receptors for the Detection of Biologically Important Anions and Their Application in Designing Molecular Logic Gate[4]
Three organic receptors were synthesized for the colorimetric detection of environmentally hazardous fluoride and acetate ions. Receptor R1 showed the highest selectivity and sensitivity for F− ions. R1 showed reversibility of five cycles which was utilized to design a molecular logic gate. Receptor R2 exhibited equal selectivity and sensitivity for F− and AcO− ions. Receptor R3 showed more selectivity and sensitivity for AcO− ions. Receptors were studied for UV-vis and NMR titration experiments to propose the binding mechanism behind the detection process.

Fig. 7. Colorimetric study of the receptor (2.5×10−5 M) in DMSO upon the addition of various concentrations of F− in TBA salt form (a) receptor R1, (b) 1 equiv., (c) 5 equiv., (d) 10 equiv., (e) 50 equiv.
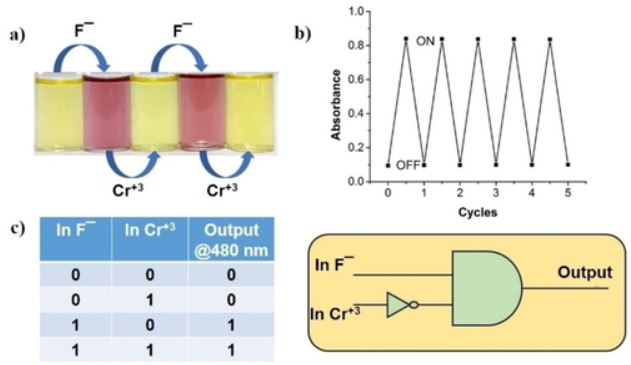
Fig. 8. (a) Color change of the receptor R1 upon alternate addition of F− and Cr3+; (b) Reversible switching cycles of UV-vis absorption (λ=488 nm) by alternate addition of F− and Cr+3 ions; (c) The sequential logic circuit for the receptor R1 and its truth table.
References
- Mahesh P. Bhat; Madhuprasad; Pravin Patil; S.K. Nataraj; Tariq Altalhi; Ho-Young Jung; Dusan Losic; Mahaveer D. Kurkuri; Turmeric, naturally available colorimetric receptor for quantitative detection of fluoride and iron. Chemical Engineering Journal 2016, 303, 14-21, 10.1016/j.cej.2016.05.113.
- Mahesh P. Bhat; Madhuprasad Kigga; Harshith Govindappa; Pravin Patil; Ho-Young Jung; Jingxian Yu; Mahaveer Kurkuri; A reversible fluoride chemosensor for the development of multi-input molecular logic gates. New Journal of Chemistry 2019, 43, 12734-12743, 10.1039/c9nj03399h.
- Mahesh P. Bhat; Mahaveer Kurkuri; Dusan Losic; Madhuprasad Kigga; Tariq Altalhi; New optofluidic based lab-on-a-chip device for the real-time fluoride analysis. Analytica Chimica Acta 2021, 1159, 338439, 10.1016/j.aca.2021.338439.
- Mahesh P. Bhat; Shraddha Vinayak; Jingxian Yu; Ho‐Young Jung; Mahaveer Kurkuri; Colorimetric Receptors for the Detection of Biologically Important Anions and Their Application in Designing Molecular Logic Gate. ChemistrySelect 2020, 5, 13135-13143, 10.1002/slct.202003147.




