
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Nischal Koirala | -- | 3473 | 2022-10-19 14:47:54 | | | |
| 2 | Lindsay Dong | Meta information modification | 3473 | 2022-10-20 10:17:49 | | |
Video Upload Options
Percutaneous-reinforced osteoplasty is currently being investigated as a possible therapeutic procedure for fracture stabilization in high-risk patients, primarily in patients with bone metastases or osteoporosis. For these patients, a percutaneous approach, if structurally sound, can provide a viable method for treating bone fractures without the physiologic stress of anesthesia and open surgery. However, the low strength of fixation is a common limitation that requires further refinement in scaffold design and selection of materials, and may potentially benefit from tissue-engineering-based regenerative approaches. Scaffolds that have tissue regenerative properties and low inflammatory response promote rapid healing at the fracture site and are ideal for percutaneous applications. On the other hand, preclinical mechanical tests of fracture-repaired specimens provide key information on restoration strength and long-term stability and enable further design optimization.
1. Introduction
2. Percutaneous-Reinforced Bone Interventions
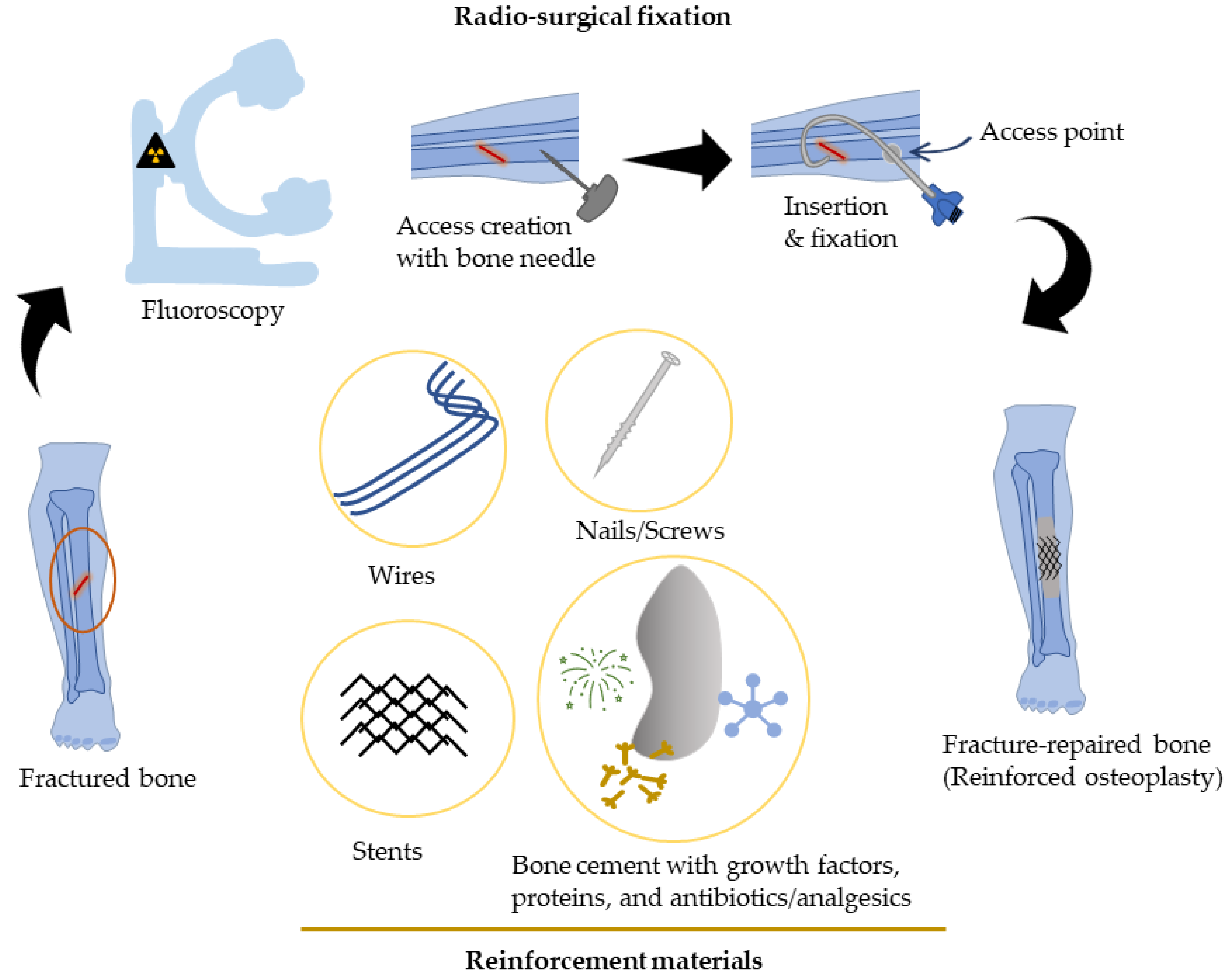
Percutaneous Bone Intervention Procedure
3. Approaches to Improve Strength for Percutaneous Bone Interventions
3.1. Metallic Materials
3.2. Regenerative Scaffolds
3.3. Bone Morphogenetic Proteins
4. Mechanical Characterization of Bone/Bone Implant Devices
4.1. Flexural Test
4.2. Potting Bone Ends to Comply with Four-Point Test and Multidirectional Testing
4.3. Torsional Test
4.4. Hardness/Indentation Test
5. Summary
References
- Cordero, D.M.; Miclau, T.A.; Paul, A.V.; Morshed, S.; Martin, C.; Shearer, D.W. The global burden of musculoskeletal injury in low and lower-middle income countries. OTA Int. Open Access J. Orthop. Trauma 2020, 3, e062.
- O’Hara, N.N.; Isaac, M.; Slobogean, G.P.; Klazinga, N.S. The socioeconomic impact of orthopaedic trauma: A systematic review and meta-analysis. PLoS ONE 2020, 15, e0227907.
- Sozen, T.; Ozisik, L.; Basaran, N.C. An overview and management of osteoporosis. Eur. J. Rheumatol. 2017, 4, 46–56.
- Lipton, A.; Uzzo, R.; Amato, R.J.; Ellis, G.K.; Hakimian, B.; Roodman, G.D.; Smith, M.R. The Science and Practice of Bone Health in Oncology: Managing Bone Loss and Metastasis in Patients With Solid Tumors. J. Natl. Compr. Cancer Netw. 2009, 7 (Suppl. S7), S1–S29.
- Huang, J.-F.; Shen, J.; Li, X.; Rengan, R.; Silvestris, N.; Wang, M.; Derosa, L.; Zheng, X.; Belli, A.; Zhang, X.-L.; et al. Incidence of patients with bone metastases at diagnosis of solid tumors in adults: A large population-based study. Ann. Transl. Med. 2020, 8, 482.
- Androjna, C.; Yee, C.S.; White, C.R.; Waldorff, E.I.; Ryaby, J.T.; Zborowski, M.; Alliston, T.; Midura, R.J. A comparison of alendronate to varying magnitude PEMF in mitigating bone loss and altering bone remodeling in skeletally mature osteoporotic rats. Bone 2021, 143, 115761.
- Bailey, S.; Vashishth, D. Mechanical Characterization of Bone: State of the Art in Experimental Approaches—What Types of Experiments Do People Do and How Does One Interpret the Results? Curr. Osteoporos. Rep. 2018, 16, 423–433.
- Nyman, J.S.; Granke, M.; Singleton, R.C.; Pharr, G.M. Tissue-Level Mechanical Properties of Bone Contributing to Fracture Risk. Curr. Osteoporos. Rep. 2016, 14, 138–150.
- Tian, Q.; Cheng, Y.; Wu, C. Percutaneous Osteoplasty for Extraspinal Metastases. J. Interv. Med. 2018, 1, 137–142.
- Katsanos, K.; Sabharwal, T.; Adam, A. Percutaneous Cementoplasty. Semin. Interv. Radiol. 2010, 27, 137–147.
- Tian, Q.-H.; Wu, C.-G.; Gu, Y.-F.; He, C.-J.; Li, M.-H.; Cheng, Y.-D. Combination Radiofrequency Ablation and Percutaneous Osteoplasty for Palliative Treatment of Painful Extraspinal Bone Metastasis: A Single-Center Experience. J. Vasc. Interv. Radiol. 2014, 25, 1094–1100.
- Kelekis, A.; Filippiadis, D.K.; Kelekis, N.L.; Martin, J.-B. Percutaneous Augmented Osteoplasty of the Humeral Bone Using a Combination of MicroNeedles Mesh and Cement. J. Vasc. Interv. Radiol. 2015, 26, 595–597.
- Seo, S.-S.; Park, J.-Y.; Kim, H.-J.; Yoon, J.-W.; Park, S.-H.; Kim, K.-H. Percutaneous osteoplasty for the treatment of a painful osteochondral lesion of the talus: A case report and literature review. Pain Phys. 2012, 15, E743–E748.
- Anselmetti, G.C. Osteoplasty: Percutaneous Bone Cement Injection beyond the Spine. Semin. Interv. Radiol. 2010, 27, 199–208.
- Shi, G.; Liu, Q.; Chen, H.; Feng, F.; Jia, P.; Bao, L.; Tang, H. Percutaneous osteoplasty for the management of a humeral head metastasis. Medicine 2019, 98, e15727.
- Liu, H.-F.; Wu, C.-G.; Tian, Q.-H.; Wang, T.; Yi, F. Application of Percutaneous Osteoplasty in Treating Pelvic Bone Metastases: Efficacy and Safety. Cardiovasc. Interv. Radiol. 2019, 42, 1738–1744.
- Shi, G.; Tang, H. Percutaneous osteoplasty for the management of a pubic bone metastasis. Orthopade 2019, 48, 704–707.
- Lei, M.; Liu, Y.; Yang, S.; Jiang, W.; Cao, Y.; Liu, S. Percutaneous cementoplasty for painful osteolytic distal femur metastases: A case report. J. Pain Res. 2016, 9, 859–863.
- Deschamps, F.; Farouil, G.; Hakime, A.; Teriitehau, C.; Barah, A.; de Baere, T. Percutaneous Stabilization of Impending Pathological Fracture of the Proximal Femur. Cardiovasc. Interv. Radiol. 2012, 35, 1428–1432.
- Kelekis, A.; Filippiadis, D.; Anselmetti, G.; Brountzos, E.; Mavrogenis, A.; Papagelopoulos, P.; Martin, J.-B. Percutaneous Augmented Peripheral Osteoplasty in Long Bones of Oncologic Patients for Pain Reduction and Prevention of Impeding Pathologic Fracture: The Rebar Concept. Cardiovasc. Interv. Radiol. 2016, 39, 90–96.
- Kim, Y.-I.; Kang, H.G.; Kim, T.S.; Kim, S.-K.; Kim, J.H.; Kim, H.S. Palliative percutaneous stabilization of lower extremity for bone metastasis using flexible nails and bone cement. Surg. Oncol. 2014, 23, 192–198.
- Kawai, N.; Sato, M.; Iwamoto, T.; Tanihata, H.; Minamiguti, H.; Nakata, K. Percutaneous Osteoplasty with Use of a Cement-filled Catheter for a Pathologic Fracture of the Humerus. J. Vasc. Interv. Radiol. 2007, 18, 805–809.
- Nakata, K.; Kawai, N.; Sato, M.; Cao, G.; Sahara, S.; Sonomura, T.; Takasaka, I.; Minamiguchi, H.; Nakai, M. Bone Marrow Nails Created by Percutaneous Osteoplasty for Long Bone Fracture: Comparisons Among Acrylic Cement Alone, Acrylic-Cement–Filled Bare Metallic Stent, and Acrylic-Cement–Filled Covered Metallic Stent. Cardiovasc. Interv. Radiol. 2011, 34, 609–614.
- Nakata, K.; Kawai, N.; Sato, M.; Cao, G.; Sahara, S.; Tanihata, H.; Takasaka, I.; Minamiguchi, H.; Nakai, T. Percutaneous Osteoplasty with a Bone Marrow Nail for Fractures of Long Bones: Experimental Study. J. Vasc. Interv. Radiol. 2010, 21, 1436–1441.
- Koirala, N.; Duffy, S.; McLennan, G. A biomechanical testing model for evaluating the feasibility of percutaneous osteoplasty in weight-bearing bones. J. Vasc. Interv. Radiol. 2016, 27, S135.
- Mifsut, D.; Renovell, P.; Gomar, F.; Saravia, M. Percutaneous osteoplasty in treatment of bone lymphangiomatosis. Indian J. Orthop. 2013, 47, 515–518.
- Tian, Q.; He, C.; Xiao, Q. Clinical Study of Percutaneous Osteoplasty with and without Interventional Internal Fixation for Impending Pathological Fracture of the Proximal Femur. Chin. J. Radiol. 2015, 49, 52–56.
- Kelekis, A.; Martin, J.-B.; Anselmetti, G.; Filipiadis, D. Regarding “Percutaneous Augmented Peripheral Osteoplasty in Long Bones of Oncologic Patients for Pain Reduction and Prevention of Impeding Pathologic Fracture: The Rebar Concept”: Reply. Cardiovasc. Interv. Radiol. 2016, 39, 479–480.
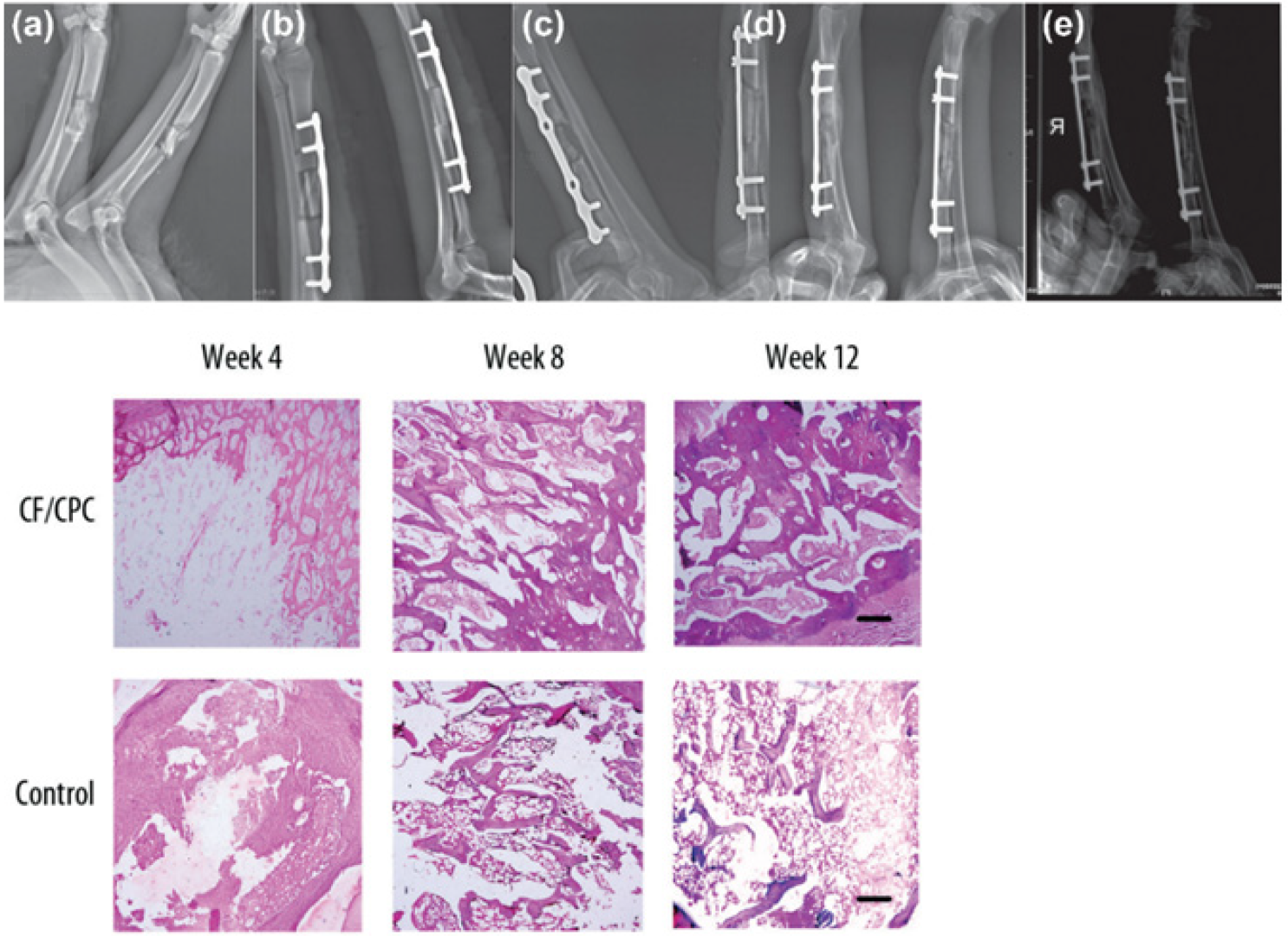
- Huang, S.-L.; Wen, B.; Bian, W.-G.; Yan, H.-W. Reconstruction of comminuted long-bone fracture using CF/CPC scaffolds manufactured by rapid prototyping. Med. Sci. Monit. 2012, 18, BR435–BR440.
- McDonald, E.; Chu, T.; Tufaga, M.; Marmor, M.; Singh, R.; Yetkinler, D.; Matityahu, A.; Buckley, J.M.; McClellan, R.T. Tibial Plateau Fracture Repairs Augmented With Calcium Phosphate Cement Have Higher In Situ Fatigue Strength Than Those With Autograft. J. Orthop. Trauma 2011, 25, 90–95.
- Libicher, M.; Hillmeier, J.; Liegibel, U.; Sommer, U.; Pyerin, W.; Vetter, M.; Meinzer, H.-P.; Grafe, I.; Meeder, P.; Nöldge, G.; et al. Osseous integration of calcium phosphate in osteoporotic vertebral fractures after kyphoplasty: Initial results from a clinical and experimental pilot study. Osteoporos. Int. 2006, 17, 1208–1215.
- Seeherman, H.J.; Bouxsein, M.; Kim, H.; Li, R.; Li, X.J.; Aiolova, M.; Wozney, J.M. Recombinant Human Bone Morphogenetic Protein-2 Delivered in an Injectable Calcium Phosphate Paste Accelerates Osteotomy-Site Healing in a Nonhuman Primate Model. J. Bone Jt. Surg. 2004, 86, 1961–1972.
- Sharifi, D.; Soroori, S.; Hasaraki, S.; Jafari, N. Radiographic Evaluations of the Tetra-Calcium Phosphate and Diacalcium Phosphate with Bone Plate in Osseo-Integration of Bone Repair in Rabbit. Am. J. Anim. Vet. Sci. 2009, 4, 80–84.
- Ambard, A.J.; Mueninghoff, L. Calcium Phosphate Cement: Review of Mechanical and Biological Properties. J. Prosthodont. 2006, 15, 321–328.
- Mestres, G.; Ginebra, M.-P. Novel magnesium phosphate cements with high early strength and antibacterial properties. Acta Biomater. 2011, 7, 1853–1861.
- Seeherman, H.; Wozney, J.M. Delivery of bone morphogenetic proteins for orthopedic tissue regeneration. Cytokine Growth Factor Rev. 2005, 16, 329–345.
- Bramer, J.A.; Barentsen, R.H.; Elst, M.V.; Lange, E.S.; Patka, P.; Haarman, H.J. Representative assessment of long bone shaft biomechanical properties: An optimized testing method. J. Biomech. 1998, 31, 741–745.
- Foux, A.; Black, R.C.; Uhthoff, H.K. Quantitative Measures for Fracture Healing: An In-Vitro Biomechanical Study. J. Biomech. Eng. 1990, 112, 401–406.
- Dragomir-Daescu, D.; Rezaei, A.; Uthamaraj, S.; Rossman, T.; Bronk, J.T.; Bolander, M.; Lambert, V.; McEligot, S.; Entwistle, R.; Giambini, H.; et al. Proximal Cadaveric Femur Preparation for Fracture Strength Testing and Quantitative CT-based Finite Element Analysis. J. Vis. Exp. 2017, 121, e54925.
- Ivarsson, B.J.; Genovese, D.; Crandall, J.R.; Bolton, J.R.; Untaroiu, C.D.; Bose, D. The Tolerance of the Femoral Shaft in Combined Axial Compression and Bending Loading. Stapp. Car. Crash J. 2009, 53, 251–290.
- Inacio, J.V.; Cristino, D.; Hast, M.W.; Dailey, H. An Adaptable Ct-Derived 3D-Printed Alignment Fixture Minimizes Errors in Whole-Bone Biomechanical Testing. J. Biomech. Eng. 2021, 143, 111006.
- Jepsen, K.J.; Silva, M.; Vashishth, D.; Guo, X.E.; van der Meulen, M.C.H. Establishing Biomechanical Mechanisms in Mouse Models: Practical Guidelines for Systematically Evaluating Phenotypic Changes in the Diaphyses of Long Bones. J. Bone Miner. Res. 2015, 30, 951–966.
- Feng, X. Chemical and Biochemical Basis of Cell-Bone Matrix Interaction in Health and Disease. Curr. Chem. Biol. 2009, 3, 189–196.
- Wu, W.; Zhu, Y.; Chen, W.; Li, S.; Yin, B.; Wang, J.; Zhang, X.; Liu, G.; Hu, Z.; Zhang, Y. Bone Hardness of Different Anatomical Regions of Human Radius and its Impact on the Pullout Strength of Screws. Orthop. Surg. 2019, 11, 270–276.
- Broitman, E. Indentation Hardness Measurements at Macro-, Micro-, and Nanoscale: A Critical Overview. Tribol. Lett. 2016, 65, 23.
- Koirala, N.; McLennan, G. Percutaneous reinforced osteoplasty for long bone metastases: A feasibility study. Skelet. Radiol. 2020, 49, 375–382.
- Cazzato, R.L.; Koch, G.; Garnon, J.; Ramamurthy, N.; Jégu, J.; Clavert, P.; Gangi, A. Biomechanical effects of osteoplasty with or without Kirschner wire augmentation on long bone diaphyses undergoing bending stress: Implications for percutaneous imaging-guided consolidation in cancer patients. Eur. Radiol. Exp. 2019, 3, 4.
- Anselmetti, G.C.; Zoarski, G.; Manca, A.; Masala, S.; Eminefendic, H.; Russo, F.; Regge, D. Percutaneous Vertebroplasty and Bone Cement Leakage: Clinical Experience with a New High-Viscosity Bone Cement and Delivery System for Vertebral Augmentation in Benign and Malignant Compression Fractures. Cardiovasc. Interv. Radiol. 2008, 31, 937–947.
- Georgy, B.A. Feasibility, Safety and Cement Leakage in Vertebroplasty of Osteoporotic and Malignant Compression Fractures Using Ultra-Viscous Cement and Hydraulic Delivery System. Pain Phys. 2012, 15, 223–228.
- Garnon, J.; Meylheuc, L.; Harrer, L.; Koch, G.; Gangi, A.; Bayle, B. Injection Device for Percutaneous Osteoplasty. In New Trends in Medical and Service Robotics; Rauter, G., Cattin, P.C., Zam, A., Riener, R., Carbone, G., Pisla, D., Eds.; Mechanisms and Machine Science; Springer International Publishing: Cham, Switzerland, 2021; Volume 93, pp. 81–88. ISBN 978-3-030-58103-9.
- Webb, J.C.J.; Spencer, R.F. The role of polymethylmethacrylate bone cement in modern orthopaedic surgery. J. Bone Jt. Surgery. Br. Vol. 2007, 89, 851–857.
- Cazzato, R.L.; Garnon, J.; Dalili, D.; Autrusseau, P.-A.; Auloge, P.; De Marini, P.; Buy, X.; Palussiere, J.; Gangi, A. Percutaneous osteoplasty in long bones: Current status and assessment of outcomes. Tech. Vasc. Interv. Radiol. 2022, 25, 100803.
- Wong, S.K.; Wong, Y.H.; Chin, K.-Y.; Ima-Nirwana, S. A Review on the Enhancement of Calcium Phosphate Cement with Biological Materials in Bone Defect Healing. Polymers 2021, 13, 3075.
- Zhang, K.; Shen, Y.; Ren, Y.; Zou, D. Prevention and treatment of bone cement-related complications in patients receiving percutaneous kyphoplasty. Int. J. Clin. Exp. Med. 2015, 8, 2371–2377.




