Percutaneous-reinforced osteoplasty is currently being investigated as a possible therapeutic procedure for fracture stabilization in high-risk patients, primarily in patients with bone metastases or osteoporosis. For these patients, a percutaneous approach, if structurally sound, can provide a viable method for treating bone fractures without the physiologic stress of anesthesia and open surgery. However, the low strength of fixation is a common limitation that requires further refinement in scaffold design and selection of materials, and may potentially benefit from tissue-engineering-based regenerative approaches. Scaffolds that have tissue regenerative properties and low inflammatory response promote rapid healing at the fracture site and are ideal for percutaneous applications. On the other hand, preclinical mechanical tests of fracture-repaired specimens provide key information on restoration strength and long-term stability and enable further design optimization.
- bone biomechanics
- cementoplasty
- mechanical testing
- percutaneous osteoplasty
1. Introduction
2. Percutaneous-Reinforced Bone Interventions
Percutaneous osteoplasty/cementoplasty (PC) is a minimally invasive technique common in radiology clinics for pain relief, bone consolidation, and stabilization of impending fractures of the (extraspinal) skeletal tissues [18][9]. Under fluoroscopic guidance, cement is injected via catheters and guidewires into the bone through a small hole (“access”) drilled at the skin surface [19,20,21,22,23][10][11][12][13][14]. A block diagram representing percutaneous osteoplasty with reinforcement has been illustrated (Figure 1). Multiple studies have verified the benefits of PC for pain management and stabilization of impending/pathological fractures in osteolytic malignant tumors [24,25,26][15][16][17]. During this procedure, the injected cement percolates into the bone cavity, filling the voids and closing the fracture clefts that provide structural stability, and this mechanism has been primarily associated with pain palliation [23][14].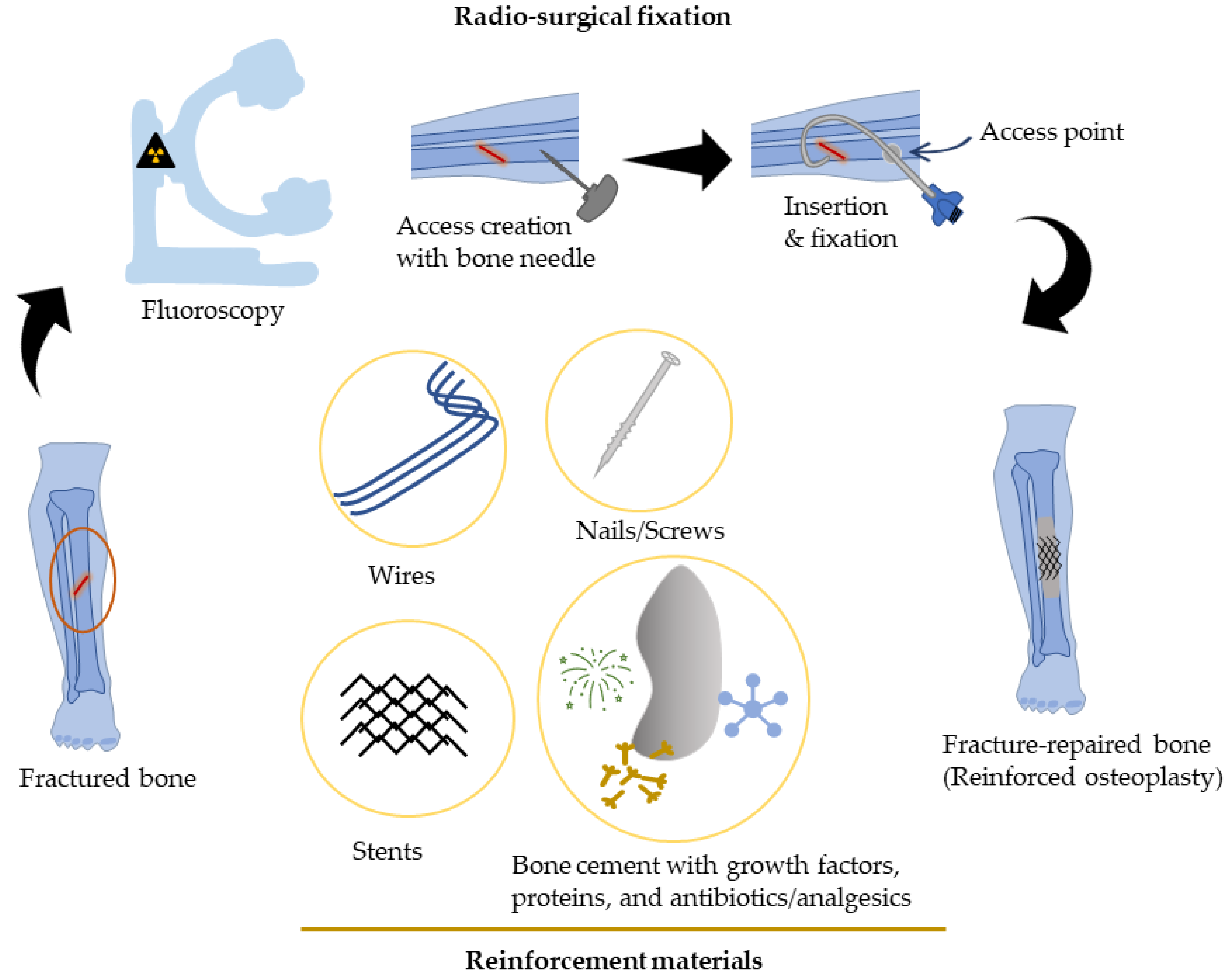
Percutaneous Bone Intervention Procedure
3. Approaches to Improve Strength for Percutaneous Bone Interventions
3.1. Metallic Materials
Scaffolds with material properties (e.g., stiffness) comparable to that of native bone provide better healing activity at the fractured site. These materials should ideally offer low deformations when acted upon by large forces, resulting in low strain and negligible motion (high stability) in the fractured area that encourages enhanced healing. PC with the use of these metallic materials has shown promise in patients with bone metastases. PMMA bone cement has high compressive strength making them ideal for spine applications; however, in the peripheral skeleton where other forces are dominant, e.g., bending, shear, tensile, torsion, etc., it may result in failure necessitating adjunct structural support [55][28]. To overcome this limitation, in one study, a metallic mesh containing 25 to 50 medical-grade stainless steel microneedles was inserted at the site of the metastatic lesion; this was followed by PMMA cement injection to create an overall “rebar” structure for repairing humeral head metastasis [21][12]. The patient had a reduced pain score after the procedure as well as moderate mobility. During the 3-month follow-up, the patient reported a significant drop in pain and improvement in mobility. In a later study, the same concept was applied to patients with femoral metastases; these patients also demonstrated an overall decrease in pain scores and improvements in mobility [13][20].3.2. Regenerative Scaffolds
Regenerative scaffolds allow natural integration of injectates with native bone tissue, inhibit osteolytic activity, and promote bone cell proliferation. In one study, chitosan fiber and calcium phosphate ceramic (CF/CPC) scaffolds were examined for comminuted fracture repair of weight-bearing bones in a canine model [61][29]. Histological examination revealed that the fractures treated with the CF/CPC scaffold showed slow cement resorption and formation of new bone cells after week 4; by week 12, there was partial degradation of the scaffolding material (Figure 42). Mechanical testing demonstrated that bone with scaffolding had a failure strength 3 times stronger than the bone without scaffolding. This suggested that scaffolds can play an important role in bone remodeling and the treatment of fractures.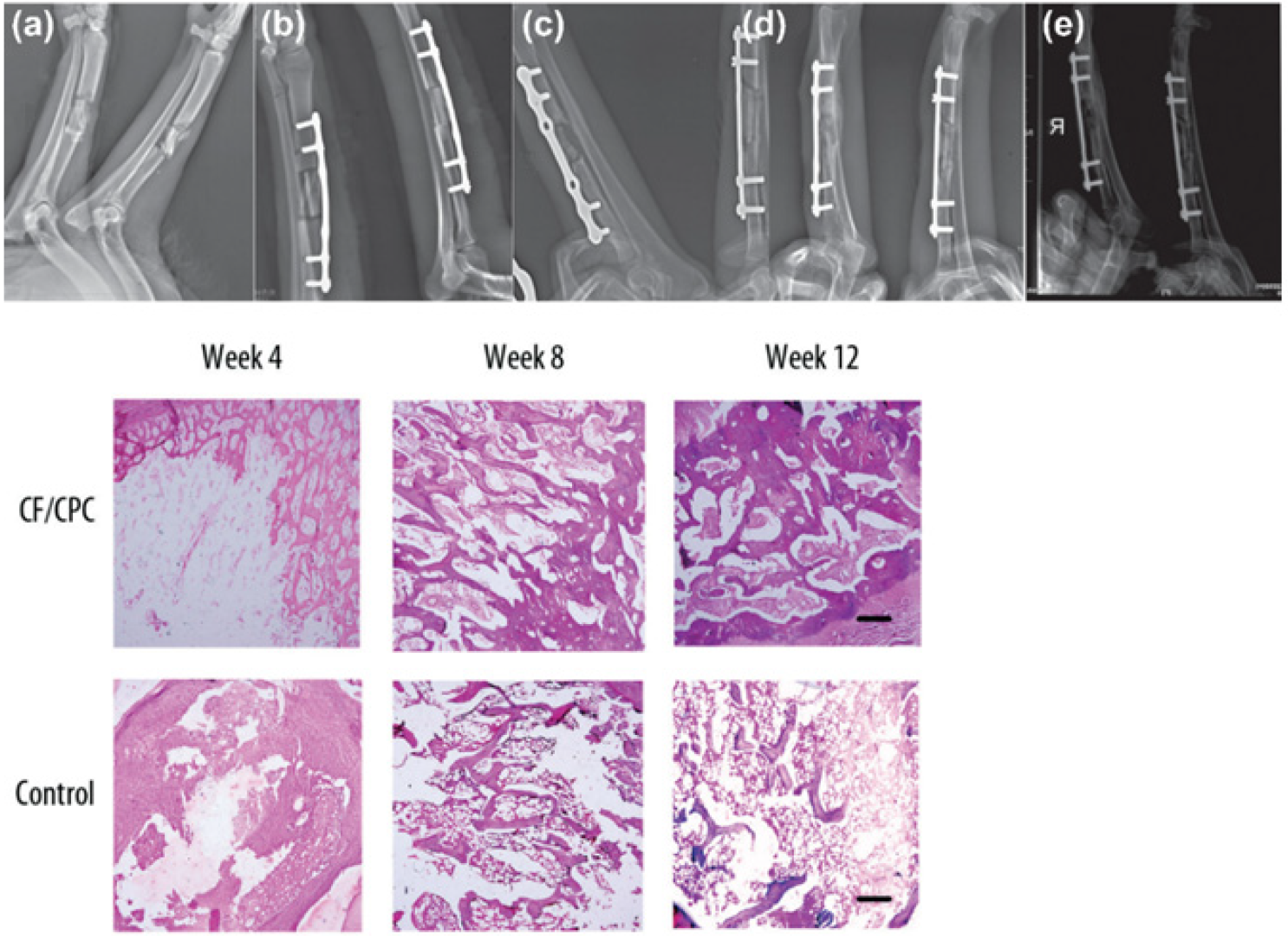
3.3. Bone Morphogenetic Proteins
4. Mechanical Characterization of Bone/Bone Implant Devices
4.1. Flexural Test
The bone in vivo is subjected to multiple forces from daily muscular activity, impact, and gravity that causes bending, torsion, extension, and compression. Because of the natural curvature of the long bone, bone bending is the most common phenomenon induced in vivo when the bone is subjected to these internal loads. To evaluate the bending properties of the bone, one can either choose a 3-point or a 4-point test. With a 3-point test configuration, a shorter gauge specimen can be conveniently examined, whereas, a 4-point test requires a relatively longer gauge specimen. In contrast, the 4-point test has the advantage of simulating a pure bending phenomenon with minimal shear effects (shear: force acting parallel to the material’s cross-section to produce a sliding failure). In the 3-point bend test, there is an inherent influence of shear, which affects the assumptions and outcomes of these tests, e.g., increased deflection/strain or early arrival to failure from low intensity applied force/stress. However, these effects can be reduced if the experiment is designed judiciously. During mechanical testing, a preconditioning stage precedes the main loading stage where low loads are cyclically applied to ensure loading fixtures are in direct contact with the bone surface. This helps to overcome geometrical irregularities common at the bone-fixture interface, which may otherwise lead to specimen instability on the fixture when loads are applied. Whole bone testing using a bend test can provide accurate measurements of its extrinsic properties but the measurement of intrinsic material properties may not be accurate due to geometric irregularities of the specimen and the assumptions involved. A compression or tensional test using a small-sized (cut-out) specimen is recommended in such situations.4.2. Potting Bone Ends to Comply with Four-Point Test and Multidirectional Testing
It can be difficult to evaluate the bone specimen of a small animal in a 4-point bend test configuration because of the short gauge length. When such specimens are subjected to a 4-point bend test, the distance between the internal loading pins tends to be very small, leading to a setup similar to that of a 3-point bend test. Hence, to overcome the limitation, the ends of the bone can be potted in cylindrical or square cups filled with a low melting point bismuth alloy (Wood’s metal/Cerrobend) or bone/dental cement. Subsequently, the loads can be applied directly over the potted surface [80,81][37][38]. This method also helps to securely anchor irregular specimens over the 4-point fixture during testing. As a potential downside, improperly aligned potted ends can introduce inadvertent shear effects but can be managed with custom-designed alignment fixtures, as discussed in [82,83,84][39][40][41]. The bone’s unique geometry and material anisotropy make its bending properties dependent on the testing plane. It may, therefore, be necessary to perform flexural testing in several directions to accurately quantify its mechanical properties. Bramer et al. developed an optimized mechanical testing model to characterize bone properties for use with 4-point testing [80][37]. This test configuration was modified from the test setup described by Foux et al., where the authors used a 3-point testing scheme in 24 directions, perpendicular to the long axis of the bone, to characterize its mechanical properties [81][38]. In the study by Bramer et al., the test specimens were fitted in cylindrical metal cups filled with low melting bismuth alloy [80][37]. The metal cups had 24 grooves corresponding to 24 testing orientations. The specimen was kept in a custom fixture and subjected to nondestructive testing under axial loading in a 4-point bend configuration. During the test, the specimen was retrieved from the fixture, rotated 15°, and replaced in the fixture for testing in the succeeding orientation. This procedure was carried out until testing was completed in 24 directions (360°), i.e., throughout the specimen’s circumference. The mechanical properties were then characterized in terms of stiffness index, area ratio, flatness ratio, and inclination for these orientations.4.3. Torsional Test
Since long bones in the body are continuously subjected to twisting forces, it is important to evaluate the mechanical performance of the bone or bone-implant devices under torsion [85][42]. Torsional testing applies loading to the entire specimen’s length to simulate fractures commonly encountered in clinics. In contrast, compression or flexural test applies concentrated load that may lead to local deformation and the appearance of late fracture or specimen crushing. A torsional test is conducted to obtain useful information such as torsional shear stress or strain, maximum torque, shear modulus, etc.4.4. Hardness/Indentation Test
The bone is a composite structure that obtains unique biomechanical properties from the spatial organization of inorganic (hydroxyapatite crystallites, ~60%) and organic (mostly, type I fibrillar collagen, ~30%) material in a heterogeneous matrix [87][43]. The hierarchical molecular organization of constituent elements at a particular site determines the biological/mechanical properties of the bone (bone quality, fragility, load bearing capacity, etc.), and varies throughout the bone geometry. It is, therefore, important to ascertain the properties of the constituents elements at the micro/nano level in various regions to understand the structural performance of the bone. Bone hardness testing examines the ability to resist deformation when penetrated with an indenter [88][44]. Hardness testing is classified based on the size of the indenter (Brinell, Rockwell, Vickers, Knoop) employed for testing and the hardness value typically varies according to the sectional region that is indented [89][45]. Hardness testing provides a better understating of the strength of the bone (bone quality) in an in vivo environment and is particularly useful for bone-related research.5. Summary
Percutaneous osteoplasty with reinforcement is emerging as a new therapeutic model for patients with induced or impending fractures from bone metastases or osteoporosis. These patients often present with compromised bone strength or weak health (strictly “non-surgical” patients) that prevents them from undergoing invasive surgical fixation procedures under general anesthesia. The supplementation is required as cement augmentation alone lacks sufficient strength for bone union and stabilization, esp. weight-bearing bones. Due to an obvious lack of qualified materials for reinforcement-osteoplasty, researchers have improvised radio-surgical use materials for proof-of-concept verification with limited success [12,13,48][20][46][47]. Reinforcement osteoplasty involves strengthening bone toughness with durability-awarding materials that mimic “rebar” in the background of cementing material. Because of the percutaneous nature of the application, materials need to be sized accordingly so that they can fit and be delivered via a small opening or “access” made through the skin into the cortical region. The requirements of reinforcement implants can be a major design/ technical challenge as it requires them to be biocompatible, flexible (to allow insertion at an angle), and miniature as well as sturdy (after deployment at the target site) to render resilient support. Failure to fulfill these criteria may produce no appreciable results. For instance, authors reported no added benefit with osteoplasty (PMMA cement) alone or in combination with Kirschner wires (K-wires) to resist bending stress in a cadaveric human diaphyseal model possibly from the use of suboptimal composite materials [48][47]. The volume of cement injected also plays a key role in determining the strength of fixation. Because of the enormous built-in back pressure and the quick onset of polymerization, it may not be usually feasible to manually inject cement volumes greater than 6–8 mL in the form of a single cohesive ball. In such scenarios, the use of an automated hydraulic-force cement injector [96,97][48][49] or a robotic injection device as described by Garnon et al. [98][50] may be preferred. In vitro mechanical assessment provides insights into the feasibility of these novel procedures and devices that are designed to undertake loads and provide stability for a wide array of orthopedic applications. Various test methods have been developed to characterize the mechanical properties of these devices, and the intended use and location in vivo determine the types of tests that need to be executed and the parameters to look for. PMMA bone cement has superior axial compressive strength and capacity to withstand compression in flat bones like the spine and hip [40][51]. However, it has low torsional, shear, and bending stress handling capacity [36][18] and carries a risk of secondary fracture when applied to overcome long bone neoplastic defects. Studies report 8–9% of secondary long bone fractures in metastatic patients following osteoplasty [30][52]. In the event of secondary fracture, further fixation is almost impractical because of the permanent closure of the internal void from cement filling. Calcium phosphate cement may be preferable over PMMA bone cement for fracture repair in the given context because of its superior biological and osteogenic properties [99][53]. However, the inferior mechanical strength of calcium phosphate cement over PMMA cement requires further research to address this limitation. Percutaneous osteoplasty with a range of adjunctive reinforcement is implemented on a case-by-case basis in clinics for the consolidation of long bone fractures (impending/pathological) and has produced encouraging results [13,37][19][20]. However, these studies lack adequate preclinical biomechanical characterization and the outcomes may be limited to short-term gains. Further studies are needed to evaluate the long-term benefits of these procedures. Lack of osteointegration with the native tissues and non-osteoconductive properties of the implant are the most common causes of infections and imminent failures; therefore, research efforts need to be directed to find optimal solutions with a focus on physical, mechanical, and biochemical factors present in vivo. The challenges can be partly addressed with the development of next-generation bone cement composed of various osteogenic growth factors and possibly antitumor/anti-inflammatory drugs that can positively impact molecular and cellular processes and allow bonding and integration with the skeletal tissues in the targeted region without inflammation. The transformation of bone cement into an osteosynthetic material instead of being limited to a space filler heralds new bone growth and may alleviate concerns related to poor stress handling capability. Extraosseous cement leakage during percutaneous osteoplasty procedures is also a common concern that can potentially cause inflammation, pain, and tissue injury, and can be overcome with the use of an optimally viscous cement and following a cautious surgical approach [100][54]. While there is a clear lack of sufficient clinical studies to support percutaneous reinforcement, the potential benefits in terms of pain relief, mechanical stability, and early facilitation of weight-bearing bones with bioengineered scaffold should not be discounted. Proper guidance on patient selection, surgical efficacy, and related complications based on the outcomes of large-scale studies and longer follow-ups are awaited. Moving forward, emphasis needs to be given to combination treatment that includes novel biomaterials with biocompatible and bioactive properties that can provide synergy in supplementing bone strength by aiding new bone formation, restoring anatomical defects and physiological function, and pain regression for percutaneous applications.References
- Cordero, D.M.; Miclau, T.A.; Paul, A.V.; Morshed, S.; Martin, C.; Shearer, D.W. The global burden of musculoskeletal injury in low and lower-middle income countries. OTA Int. Open Access J. Orthop. Trauma 2020, 3, e062.
- O’Hara, N.N.; Isaac, M.; Slobogean, G.P.; Klazinga, N.S. The socioeconomic impact of orthopaedic trauma: A systematic review and meta-analysis. PLoS ONE 2020, 15, e0227907.
- Sozen, T.; Ozisik, L.; Basaran, N.C. An overview and management of osteoporosis. Eur. J. Rheumatol. 2017, 4, 46–56.
- Lipton, A.; Uzzo, R.; Amato, R.J.; Ellis, G.K.; Hakimian, B.; Roodman, G.D.; Smith, M.R. The Science and Practice of Bone Health in Oncology: Managing Bone Loss and Metastasis in Patients With Solid Tumors. J. Natl. Compr. Cancer Netw. 2009, 7 (Suppl. S7), S1–S29.
- Huang, J.-F.; Shen, J.; Li, X.; Rengan, R.; Silvestris, N.; Wang, M.; Derosa, L.; Zheng, X.; Belli, A.; Zhang, X.-L.; et al. Incidence of patients with bone metastases at diagnosis of solid tumors in adults: A large population-based study. Ann. Transl. Med. 2020, 8, 482.
- Androjna, C.; Yee, C.S.; White, C.R.; Waldorff, E.I.; Ryaby, J.T.; Zborowski, M.; Alliston, T.; Midura, R.J. A comparison of alendronate to varying magnitude PEMF in mitigating bone loss and altering bone remodeling in skeletally mature osteoporotic rats. Bone 2021, 143, 115761.
- Bailey, S.; Vashishth, D. Mechanical Characterization of Bone: State of the Art in Experimental Approaches—What Types of Experiments Do People Do and How Does One Interpret the Results? Curr. Osteoporos. Rep. 2018, 16, 423–433.
- Nyman, J.S.; Granke, M.; Singleton, R.C.; Pharr, G.M. Tissue-Level Mechanical Properties of Bone Contributing to Fracture Risk. Curr. Osteoporos. Rep. 2016, 14, 138–150.
- Tian, Q.; Cheng, Y.; Wu, C. Percutaneous Osteoplasty for Extraspinal Metastases. J. Interv. Med. 2018, 1, 137–142.
- Katsanos, K.; Sabharwal, T.; Adam, A. Percutaneous Cementoplasty. Semin. Interv. Radiol. 2010, 27, 137–147.
- Tian, Q.-H.; Wu, C.-G.; Gu, Y.-F.; He, C.-J.; Li, M.-H.; Cheng, Y.-D. Combination Radiofrequency Ablation and Percutaneous Osteoplasty for Palliative Treatment of Painful Extraspinal Bone Metastasis: A Single-Center Experience. J. Vasc. Interv. Radiol. 2014, 25, 1094–1100.
- Kelekis, A.; Filippiadis, D.K.; Kelekis, N.L.; Martin, J.-B. Percutaneous Augmented Osteoplasty of the Humeral Bone Using a Combination of MicroNeedles Mesh and Cement. J. Vasc. Interv. Radiol. 2015, 26, 595–597.
- Seo, S.-S.; Park, J.-Y.; Kim, H.-J.; Yoon, J.-W.; Park, S.-H.; Kim, K.-H. Percutaneous osteoplasty for the treatment of a painful osteochondral lesion of the talus: A case report and literature review. Pain Phys. 2012, 15, E743–E748.
- Anselmetti, G.C. Osteoplasty: Percutaneous Bone Cement Injection beyond the Spine. Semin. Interv. Radiol. 2010, 27, 199–208.
- Shi, G.; Liu, Q.; Chen, H.; Feng, F.; Jia, P.; Bao, L.; Tang, H. Percutaneous osteoplasty for the management of a humeral head metastasis. Medicine 2019, 98, e15727.
- Liu, H.-F.; Wu, C.-G.; Tian, Q.-H.; Wang, T.; Yi, F. Application of Percutaneous Osteoplasty in Treating Pelvic Bone Metastases: Efficacy and Safety. Cardiovasc. Interv. Radiol. 2019, 42, 1738–1744.
- Shi, G.; Tang, H. Percutaneous osteoplasty for the management of a pubic bone metastasis. Orthopade 2019, 48, 704–707.
- Lei, M.; Liu, Y.; Yang, S.; Jiang, W.; Cao, Y.; Liu, S. Percutaneous cementoplasty for painful osteolytic distal femur metastases: A case report. J. Pain Res. 2016, 9, 859–863.
- Deschamps, F.; Farouil, G.; Hakime, A.; Teriitehau, C.; Barah, A.; de Baere, T. Percutaneous Stabilization of Impending Pathological Fracture of the Proximal Femur. Cardiovasc. Interv. Radiol. 2012, 35, 1428–1432.
- Kelekis, A.; Filippiadis, D.; Anselmetti, G.; Brountzos, E.; Mavrogenis, A.; Papagelopoulos, P.; Martin, J.-B. Percutaneous Augmented Peripheral Osteoplasty in Long Bones of Oncologic Patients for Pain Reduction and Prevention of Impeding Pathologic Fracture: The Rebar Concept. Cardiovasc. Interv. Radiol. 2016, 39, 90–96.
- Kim, Y.-I.; Kang, H.G.; Kim, T.S.; Kim, S.-K.; Kim, J.H.; Kim, H.S. Palliative percutaneous stabilization of lower extremity for bone metastasis using flexible nails and bone cement. Surg. Oncol. 2014, 23, 192–198.
- Kawai, N.; Sato, M.; Iwamoto, T.; Tanihata, H.; Minamiguti, H.; Nakata, K. Percutaneous Osteoplasty with Use of a Cement-filled Catheter for a Pathologic Fracture of the Humerus. J. Vasc. Interv. Radiol. 2007, 18, 805–809.
- Nakata, K.; Kawai, N.; Sato, M.; Cao, G.; Sahara, S.; Sonomura, T.; Takasaka, I.; Minamiguchi, H.; Nakai, M. Bone Marrow Nails Created by Percutaneous Osteoplasty for Long Bone Fracture: Comparisons Among Acrylic Cement Alone, Acrylic-Cement–Filled Bare Metallic Stent, and Acrylic-Cement–Filled Covered Metallic Stent. Cardiovasc. Interv. Radiol. 2011, 34, 609–614.
- Nakata, K.; Kawai, N.; Sato, M.; Cao, G.; Sahara, S.; Tanihata, H.; Takasaka, I.; Minamiguchi, H.; Nakai, T. Percutaneous Osteoplasty with a Bone Marrow Nail for Fractures of Long Bones: Experimental Study. J. Vasc. Interv. Radiol. 2010, 21, 1436–1441.
- Koirala, N.; Duffy, S.; McLennan, G. A biomechanical testing model for evaluating the feasibility of percutaneous osteoplasty in weight-bearing bones. J. Vasc. Interv. Radiol. 2016, 27, S135.
- Mifsut, D.; Renovell, P.; Gomar, F.; Saravia, M. Percutaneous osteoplasty in treatment of bone lymphangiomatosis. Indian J. Orthop. 2013, 47, 515–518.
- Tian, Q.; He, C.; Xiao, Q. Clinical Study of Percutaneous Osteoplasty with and without Interventional Internal Fixation for Impending Pathological Fracture of the Proximal Femur. Chin. J. Radiol. 2015, 49, 52–56.
- Kelekis, A.; Martin, J.-B.; Anselmetti, G.; Filipiadis, D. Regarding “Percutaneous Augmented Peripheral Osteoplasty in Long Bones of Oncologic Patients for Pain Reduction and Prevention of Impeding Pathologic Fracture: The Rebar Concept”: Reply. Cardiovasc. Interv. Radiol. 2016, 39, 479–480.
- Huang, S.-L.; Wen, B.; Bian, W.-G.; Yan, H.-W. Reconstruction of comminuted long-bone fracture using CF/CPC scaffolds manufactured by rapid prototyping. Med. Sci. Monit. 2012, 18, BR435–BR440.
- McDonald, E.; Chu, T.; Tufaga, M.; Marmor, M.; Singh, R.; Yetkinler, D.; Matityahu, A.; Buckley, J.M.; McClellan, R.T. Tibial Plateau Fracture Repairs Augmented With Calcium Phosphate Cement Have Higher In Situ Fatigue Strength Than Those With Autograft. J. Orthop. Trauma 2011, 25, 90–95.
- Libicher, M.; Hillmeier, J.; Liegibel, U.; Sommer, U.; Pyerin, W.; Vetter, M.; Meinzer, H.-P.; Grafe, I.; Meeder, P.; Nöldge, G.; et al. Osseous integration of calcium phosphate in osteoporotic vertebral fractures after kyphoplasty: Initial results from a clinical and experimental pilot study. Osteoporos. Int. 2006, 17, 1208–1215.
- Seeherman, H.J.; Bouxsein, M.; Kim, H.; Li, R.; Li, X.J.; Aiolova, M.; Wozney, J.M. Recombinant Human Bone Morphogenetic Protein-2 Delivered in an Injectable Calcium Phosphate Paste Accelerates Osteotomy-Site Healing in a Nonhuman Primate Model. J. Bone Jt. Surg. 2004, 86, 1961–1972.
- Sharifi, D.; Soroori, S.; Hasaraki, S.; Jafari, N. Radiographic Evaluations of the Tetra-Calcium Phosphate and Diacalcium Phosphate with Bone Plate in Osseo-Integration of Bone Repair in Rabbit. Am. J. Anim. Vet. Sci. 2009, 4, 80–84.
- Ambard, A.J.; Mueninghoff, L. Calcium Phosphate Cement: Review of Mechanical and Biological Properties. J. Prosthodont. 2006, 15, 321–328.
- Mestres, G.; Ginebra, M.-P. Novel magnesium phosphate cements with high early strength and antibacterial properties. Acta Biomater. 2011, 7, 1853–1861.
- Seeherman, H.; Wozney, J.M. Delivery of bone morphogenetic proteins for orthopedic tissue regeneration. Cytokine Growth Factor Rev. 2005, 16, 329–345.
- Bramer, J.A.; Barentsen, R.H.; Elst, M.V.; Lange, E.S.; Patka, P.; Haarman, H.J. Representative assessment of long bone shaft biomechanical properties: An optimized testing method. J. Biomech. 1998, 31, 741–745.
- Foux, A.; Black, R.C.; Uhthoff, H.K. Quantitative Measures for Fracture Healing: An In-Vitro Biomechanical Study. J. Biomech. Eng. 1990, 112, 401–406.
- Dragomir-Daescu, D.; Rezaei, A.; Uthamaraj, S.; Rossman, T.; Bronk, J.T.; Bolander, M.; Lambert, V.; McEligot, S.; Entwistle, R.; Giambini, H.; et al. Proximal Cadaveric Femur Preparation for Fracture Strength Testing and Quantitative CT-based Finite Element Analysis. J. Vis. Exp. 2017, 121, e54925.
- Ivarsson, B.J.; Genovese, D.; Crandall, J.R.; Bolton, J.R.; Untaroiu, C.D.; Bose, D. The Tolerance of the Femoral Shaft in Combined Axial Compression and Bending Loading. Stapp. Car. Crash J. 2009, 53, 251–290.
- Inacio, J.V.; Cristino, D.; Hast, M.W.; Dailey, H. An Adaptable Ct-Derived 3D-Printed Alignment Fixture Minimizes Errors in Whole-Bone Biomechanical Testing. J. Biomech. Eng. 2021, 143, 111006.
- Jepsen, K.J.; Silva, M.; Vashishth, D.; Guo, X.E.; van der Meulen, M.C.H. Establishing Biomechanical Mechanisms in Mouse Models: Practical Guidelines for Systematically Evaluating Phenotypic Changes in the Diaphyses of Long Bones. J. Bone Miner. Res. 2015, 30, 951–966.
- Feng, X. Chemical and Biochemical Basis of Cell-Bone Matrix Interaction in Health and Disease. Curr. Chem. Biol. 2009, 3, 189–196.
- Wu, W.; Zhu, Y.; Chen, W.; Li, S.; Yin, B.; Wang, J.; Zhang, X.; Liu, G.; Hu, Z.; Zhang, Y. Bone Hardness of Different Anatomical Regions of Human Radius and its Impact on the Pullout Strength of Screws. Orthop. Surg. 2019, 11, 270–276.
- Broitman, E. Indentation Hardness Measurements at Macro-, Micro-, and Nanoscale: A Critical Overview. Tribol. Lett. 2016, 65, 23.
- Koirala, N.; McLennan, G. Percutaneous reinforced osteoplasty for long bone metastases: A feasibility study. Skelet. Radiol. 2020, 49, 375–382.
- Cazzato, R.L.; Koch, G.; Garnon, J.; Ramamurthy, N.; Jégu, J.; Clavert, P.; Gangi, A. Biomechanical effects of osteoplasty with or without Kirschner wire augmentation on long bone diaphyses undergoing bending stress: Implications for percutaneous imaging-guided consolidation in cancer patients. Eur. Radiol. Exp. 2019, 3, 4.
- Anselmetti, G.C.; Zoarski, G.; Manca, A.; Masala, S.; Eminefendic, H.; Russo, F.; Regge, D. Percutaneous Vertebroplasty and Bone Cement Leakage: Clinical Experience with a New High-Viscosity Bone Cement and Delivery System for Vertebral Augmentation in Benign and Malignant Compression Fractures. Cardiovasc. Interv. Radiol. 2008, 31, 937–947.
- Georgy, B.A. Feasibility, Safety and Cement Leakage in Vertebroplasty of Osteoporotic and Malignant Compression Fractures Using Ultra-Viscous Cement and Hydraulic Delivery System. Pain Phys. 2012, 15, 223–228.
- Garnon, J.; Meylheuc, L.; Harrer, L.; Koch, G.; Gangi, A.; Bayle, B. Injection Device for Percutaneous Osteoplasty. In New Trends in Medical and Service Robotics; Rauter, G., Cattin, P.C., Zam, A., Riener, R., Carbone, G., Pisla, D., Eds.; Mechanisms and Machine Science; Springer International Publishing: Cham, Switzerland, 2021; Volume 93, pp. 81–88. ISBN 978-3-030-58103-9.
- Webb, J.C.J.; Spencer, R.F. The role of polymethylmethacrylate bone cement in modern orthopaedic surgery. J. Bone Jt. Surgery. Br. Vol. 2007, 89, 851–857.
- Cazzato, R.L.; Garnon, J.; Dalili, D.; Autrusseau, P.-A.; Auloge, P.; De Marini, P.; Buy, X.; Palussiere, J.; Gangi, A. Percutaneous osteoplasty in long bones: Current status and assessment of outcomes. Tech. Vasc. Interv. Radiol. 2022, 25, 100803.
- Wong, S.K.; Wong, Y.H.; Chin, K.-Y.; Ima-Nirwana, S. A Review on the Enhancement of Calcium Phosphate Cement with Biological Materials in Bone Defect Healing. Polymers 2021, 13, 3075.
- Zhang, K.; Shen, Y.; Ren, Y.; Zou, D. Prevention and treatment of bone cement-related complications in patients receiving percutaneous kyphoplasty. Int. J. Clin. Exp. Med. 2015, 8, 2371–2377.
