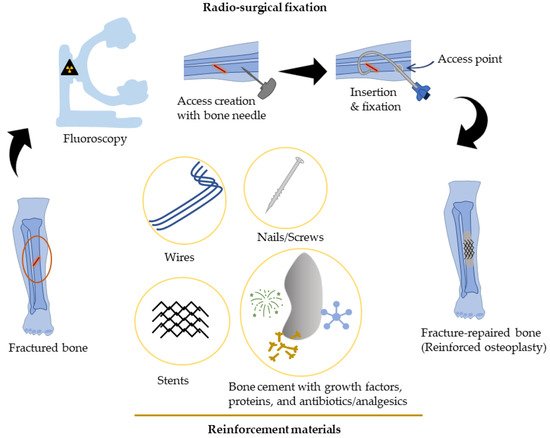
Percutaneous-reinforced osteoplasty is currently being investigated as a possible therapeutic procedure for fracture stabilization in high-risk patients, primarily in patients with bone metastases or osteoporosis. For these patients, a percutaneous approach, if structurally sound, can provide a viable method for treating bone fractures without the physiologic stress of anesthesia and open surgery. However, the low strength of fixation is a common limitation that requires further refinement in scaffold design and selection of materials, and may potentially benefit from tissue-engineering-based regenerative approaches. Scaffolds that have tissue regenerative properties and low inflammatory response promote rapid healing at the fracture site and are ideal for percutaneous applications. On the other hand, preclinical mechanical tests of fracture-repaired specimens provide key information on restoration strength and long-term stability and enable further design optimization.
1. Introduction
Bone disease and traumatic fractures are the most common orthopedic problems worldwide with huge societal and economic effects [
1,
2]. Osteoporosis and bone metastasis are the most common bone diseases responsible for compromised bone strength predisposing individuals to increased risks of fractures [
3,
4]. According to a recent study, in the United States alone, approximately 350,000 people are estimated to die each year from bone metastasis with the highest incidences among people with metastatic disease of the prostate, breast, and kidney [
5]. Fractures occurring in patients with bone metastasis or osteoporosis are difficult to repair as the bone strength and the overall health are severely compromised prohibiting invasive and/or time-consuming surgical procedures. Often these patients have progressed disease and are unable to perform their daily activities independently and live with pain, reduced quality of life, and poor prognosis.
Mechanical testing data provide information about the performance of the device (maximum load, fracture/failure, etc.) under various (simulated) loading conditions, whereas, assays (histopathology, immunohistochemistry, microCT, etc.) involving biological specimens provide information regarding cell/tissue and device interactions, allowing researchers to predict possible complications when used in humans [
6]. It is, therefore, necessary to have a good understanding of the various testing methods and parameters that can be used for relevant mechanical quantification of bone interventional devices. The structural support rendered by the osteoplasty technique and its variations is the cornerstone of pain relief and ambulation therapy making mechanical evaluation crucial to assess the benefits of these procedures. Mechanical characterization of the bone can be achieved at the macroscopic level or the micro/nano level depending on the size of the specimen used for testing [
7,
8]. At the macroscopic level, the whole bone is usually subjected to mechanical testing, which provides an assessment of the bone’s extrinsic parameters such as stiffness or load to failure; these factors are specific to the specimen subjected to the test. On the other hand, tests performed using small-sized specimens are targeted to determine the intrinsic material properties such as stress, strain, elasticity, and ultimate/breaking strength, and these properties are independent of the geometrical specifications of the specimen.
2. Percutaneous-Reinforced Bone Interventions
Percutaneous osteoplasty/cementoplasty (PC) is a minimally invasive technique common in radiology clinics for pain relief, bone consolidation, and stabilization of impending fractures of the (extraspinal) skeletal tissues [
18]. Under fluoroscopic guidance, cement is injected via catheters and guidewires into the bone through a small hole (“access”) drilled at the skin surface [
19,
20,
21,
22,
23]. A block diagram representing percutaneous osteoplasty with reinforcement has been illustrated (
Figure 1). Multiple studies have verified the benefits of PC for pain management and stabilization of impending/pathological fractures in osteolytic malignant tumors [
24,
25,
26]. During this procedure, the injected cement percolates into the bone cavity, filling the voids and closing the fracture clefts that provide structural stability, and this mechanism has been primarily associated with pain palliation [
23].
Figure 1. Block diagram illustrating percutaneous-reinforced osteoplasty procedure. A mid-diaphyseal fracture of the tibia in a strictly “non-surgical” patient is repaired radio-surgically by creating access with a bone biopsy needle followed by insertion of reinforcement material(s) and cement augmentation.
Bone cement has been in regular use for vertebral compression and fracture management in vertebroplasty and kyphoplasty procedures. However, the fracture strength and flexibility of cement-only repaired bone are lower than that of an intact bone. Since long bones are subject to twisting and are more susceptible to fracture in torsion, cement injection alone may not add sufficient mechanical stability. Studies have shown supplemental reinforcement, e.g., cement-filled catheter [
36] or percutaneous osteosynthesis [
37], in conjunction with cementoplasty adds functional improvement and prevents impending pathological fracture in symptomatic patients with extraspinal malignant bone lesions [
13,
38,
39]. Despite potential clinical applications, the advantages of reinforced osteoplasty remain a relatively under-explored area.
Percutaneous fracture fixation and the development of scaffolds to reinforce bone strength are advanced concepts in fracture management and osseous stabilization [
13,
21,
38,
39,
43,
44,
52]. In particular, percutaneous osteoplasty with acrylic bone cement only or in combination with other stiffer materials (reinforcement) that mimic internal fixators have been used to treat bone disease, leading to reduced pain, improved strength, and enhanced mobility [
53,
54]. Kawaii and colleagues have performed percutaneous osteoplasty using a cement-filled catheter and (acrylic) cement augmentation to reunite a painful pathological fracture of the humerus shaft in a patient with metastatic hepatocellular carcinoma [
39]. The procedure was offered because of the patient’s deteriorated health and poor prognosis, which imposed a high risk for open reduction methods. The procedure resulted in immediate pain relief and improved limb mobility; however, the fixation strength was unsatisfactory because of the low durability of the cement-filled catheter.
Percutaneous Bone Intervention Procedure
The scaffolds commonly used in percutaneous repair for proof-of-concept evaluation include bone cement and stents, which can be injected over the wire and safely placed in the region of interest. The procedure of fracture fixation is performed under fluoroscopic guidance. In this procedure, a guidewire is advanced into the bone cavity through access to the cortex. The stent is then deployed at the fracture site, and other reinforcement materials (if any) may be placed within the stent lumen to impart additional strength—mimicking a “rebar” concept of a construction setting. After securely depositing all repair materials in position, cement is injected through the catheter filling the intramedullary canal. Bonding with the injected materials is instantly achieved and results in a rigid scaffold able to undertake varying loads at the fractured site [
44,
52].
3. Approaches to Improve Strength for Percutaneous Bone Interventions
3.1. Metallic Materials
Scaffolds with material properties (e.g., stiffness) comparable to that of native bone provide better healing activity at the fractured site. These materials should ideally offer low deformations when acted upon by large forces, resulting in low strain and negligible motion (high stability) in the fractured area that encourages enhanced healing. PC with the use of these metallic materials has shown promise in patients with bone metastases. PMMA bone cement has high compressive strength making them ideal for spine applications; however, in the peripheral skeleton where other forces are dominant, e.g., bending, shear, tensile, torsion, etc., it may result in failure necessitating adjunct structural support [
55]. To overcome this limitation, in one study, a metallic mesh containing 25 to 50 medical-grade stainless steel microneedles was inserted at the site of the metastatic lesion; this was followed by PMMA cement injection to create an overall “rebar” structure for repairing humeral head metastasis [
21]. The patient had a reduced pain score after the procedure as well as moderate mobility. During the 3-month follow-up, the patient reported a significant drop in pain and improvement in mobility. In a later study, the same concept was applied to patients with femoral metastases; these patients also demonstrated an overall decrease in pain scores and improvements in mobility [
13].
3.2. Regenerative Scaffolds
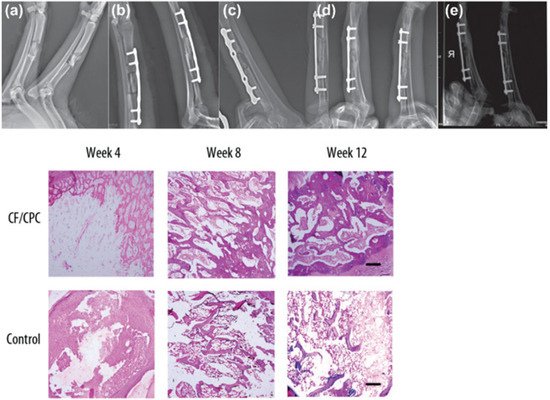
Regenerative scaffolds allow natural integration of injectates with native bone tissue, inhibit osteolytic activity, and promote bone cell proliferation. In one study, chitosan fiber and calcium phosphate ceramic (CF/CPC) scaffolds were examined for comminuted fracture repair of weight-bearing bones in a canine model [
61]. Histological examination revealed that the fractures treated with the CF/CPC scaffold showed slow cement resorption and formation of new bone cells after week 4; by week 12, there was partial degradation of the scaffolding material (
Figure 4). Mechanical testing demonstrated that bone with scaffolding had a failure strength 3 times stronger than the bone without scaffolding. This suggested that scaffolds can play an important role in bone remodeling and the treatment of fractures.
Figure 4. Chitosan fiber and calcium phosphate ceramics (CF/CPC) scaffold for fracture repair in weight-bearing long bones (radiuses) [
61]. Upper panel: (
a) X-ray images of both radii in adult dogs, right radius received CF/CPC scaffold, left radius were untreated (blank control). Radiographic images post-implantation at various time points in the experimental group: (
b) 0 weeks, (
c) 4 weeks, (
d) 8 weeks, (
e) 12 weeks. Lower panel: Histological examination of the bone-defect area tissue at 4-, 8-, and 12 weeks after surgery. The experimental group showed time-dependent slow resorption of cement and the formation of new bone tissues. In contrast, no biological activity occurred in the control group.
The choice of bone cement for fracture repair has a significant impact on the strength and quality of the repair. Calcium phosphate or magnesium phosphate cement may be used as an alternative to acrylic (PMMA) bone cement and is being investigated for bone scaffolding and bone tissue engineering [
62,
63,
64,
65]. These scaffolds facilitate osteogenesis and osseointegration, which are suitable for bone healing and regeneration purposes. However, calcium phosphate cement has low mechanical strength and low resorption rate [
66]; magnesium phosphate cement has a high exothermic setting reaction, inducing local thermal necrosis and the possible release of harmful ions [
67].
3.3. Bone Morphogenetic Proteins
The delivery of bone morphogenetic proteins (BMPs) to the fractured sites using carrier materials such as natural or synthetic polymers, inorganic materials, or composite materials favors tissue regeneration and remodeling resulting in improved healing response [
68]. The availability of BMP in the scaffold allows migration, proliferation, and differentiation of regenerative cells in the vicinity of the injury. One study evaluated the efficacy of bone healing in a nonhuman primate fibular osteotomy model using human BMP-2 in various carrier matrixes [
64]. The investigation found that BMP injected in calcium phosphate paste accelerated bone healing by approximately 40% compared to the healing of untreated osteotomy sites. With this combination, the mean torsion stiffness and maximum torque were equal to that of the intact fibula at 10 weeks versus torsion stiffness and maximum torque values of approximately 55% and 58%, respectively, for untreated osteotomy sites. Histological examination at this time point displayed bridging of the osteotomy sites with the bone for all carrier matrices. These results affirm that the incorporation and delivery of various biological factors to the compromised site significantly alters the healing and regeneration response and improves the outcome of percutaneous interventional strategies.
4. Mechanical Characterization of Bone/Bone Implant Devices
4.1. Flexural Test
The bone in vivo is subjected to multiple forces from daily muscular activity, impact, and gravity that causes bending, torsion, extension, and compression. Because of the natural curvature of the long bone, bone bending is the most common phenomenon induced in vivo when the bone is subjected to these internal loads. To evaluate the bending properties of the bone, one can either choose a 3-point or a 4-point test. With a 3-point test configuration, a shorter gauge specimen can be conveniently examined, whereas, a 4-point test requires a relatively longer gauge specimen. In contrast, the 4-point test has the advantage of simulating a pure bending phenomenon with minimal shear effects (shear: force acting parallel to the material’s cross-section to produce a sliding failure). In the 3-point bend test, there is an inherent influence of shear, which affects the assumptions and outcomes of these tests, e.g., increased deflection/strain or early arrival to failure from low intensity applied force/stress. However, these effects can be reduced if the experiment is designed judiciously.
During mechanical testing, a preconditioning stage precedes the main loading stage where low loads are cyclically applied to ensure loading fixtures are in direct contact with the bone surface. This helps to overcome geometrical irregularities common at the bone-fixture interface, which may otherwise lead to specimen instability on the fixture when loads are applied. Whole bone testing using a bend test can provide accurate measurements of its extrinsic properties but the measurement of intrinsic material properties may not be accurate due to geometric irregularities of the specimen and the assumptions involved. A compression or tensional test using a small-sized (cut-out) specimen is recommended in such situations.
4.2. Potting Bone Ends to Comply with Four-Point Test and Multidirectional Testing
It can be difficult to evaluate the bone specimen of a small animal in a 4-point bend test configuration because of the short gauge length. When such specimens are subjected to a 4-point bend test, the distance between the internal loading pins tends to be very small, leading to a setup similar to that of a 3-point bend test. Hence, to overcome the limitation, the ends of the bone can be potted in cylindrical or square cups filled with a low melting point bismuth alloy (Wood’s metal/Cerrobend) or bone/dental cement. Subsequently, the loads can be applied directly over the potted surface [
80,
81]. This method also helps to securely anchor irregular specimens over the 4-point fixture during testing. As a potential downside, improperly aligned potted ends can introduce inadvertent shear effects but can be managed with custom-designed alignment fixtures, as discussed in [
82,
83,
84].
The bone’s unique geometry and material anisotropy make its bending properties dependent on the testing plane. It may, therefore, be necessary to perform flexural testing in several directions to accurately quantify its mechanical properties. Bramer et al. developed an optimized mechanical testing model to characterize bone properties for use with 4-point testing [
80]. This test configuration was modified from the test setup described by Foux et al., where the authors used a 3-point testing scheme in 24 directions, perpendicular to the long axis of the bone, to characterize its mechanical properties [
81]. In the study by Bramer et al., the test specimens were fitted in cylindrical metal cups filled with low melting bismuth alloy [
80]. The metal cups had 24 grooves corresponding to 24 testing orientations. The specimen was kept in a custom fixture and subjected to nondestructive testing under axial loading in a 4-point bend configuration. During the test, the specimen was retrieved from the fixture, rotated 15°, and replaced in the fixture for testing in the succeeding orientation. This procedure was carried out until testing was completed in 24 directions (360°), i.e., throughout the specimen’s circumference. The mechanical properties were then characterized in terms of stiffness index, area ratio, flatness ratio, and inclination for these orientations.
4.3. Torsional Test
Since long bones in the body are continuously subjected to twisting forces, it is important to evaluate the mechanical performance of the bone or bone-implant devices under torsion [
85]. Torsional testing applies loading to the entire specimen’s length to simulate fractures commonly encountered in clinics. In contrast, compression or flexural test applies concentrated load that may lead to local deformation and the appearance of late fracture or specimen crushing. A torsional test is conducted to obtain useful information such as torsional shear stress or strain, maximum torque, shear modulus, etc.
4.4. Hardness/Indentation Test
The bone is a composite structure that obtains unique biomechanical properties from the spatial organization of inorganic (hydroxyapatite crystallites, ~60%) and organic (mostly, type I fibrillar collagen, ~30%) material in a heterogeneous matrix [
87]. The hierarchical molecular organization of constituent elements at a particular site determines the biological/mechanical properties of the bone (bone quality, fragility, load bearing capacity, etc.), and varies throughout the bone geometry. It is, therefore, important to ascertain the properties of the constituents elements at the micro/nano level in various regions to understand the structural performance of the bone. Bone hardness testing examines the ability to resist deformation when penetrated with an indenter [
88]. Hardness testing is classified based on the size of the indenter (Brinell, Rockwell, Vickers, Knoop) employed for testing and the hardness value typically varies according to the sectional region that is indented [
89]. Hardness testing provides a better understating of the strength of the bone (bone quality) in an in vivo environment and is particularly useful for bone-related research.
5. Summary
Percutaneous osteoplasty with reinforcement is emerging as a new therapeutic model for patients with induced or impending fractures from bone metastases or osteoporosis. These patients often present with compromised bone strength or weak health (strictly “non-surgical” patients) that prevents them from undergoing invasive surgical fixation procedures under general anesthesia. The supplementation is required as cement augmentation alone lacks sufficient strength for bone union and stabilization, esp. weight-bearing bones. Due to an obvious lack of qualified materials for reinforcement-osteoplasty, researchers have improvised radio-surgical use materials for proof-of-concept verification with limited success [
12,
13,
48]. Reinforcement osteoplasty involves strengthening bone toughness with durability-awarding materials that mimic “rebar” in the background of cementing material. Because of the percutaneous nature of the application, materials need to be sized accordingly so that they can fit and be delivered via a small opening or “access” made through the skin into the cortical region. The requirements of reinforcement implants can be a major design/ technical challenge as it requires them to be biocompatible, flexible (to allow insertion at an angle), and miniature as well as sturdy (after deployment at the target site) to render resilient support. Failure to fulfill these criteria may produce no appreciable results. For instance, authors reported no added benefit with osteoplasty (PMMA cement) alone or in combination with Kirschner wires (K-wires) to resist bending stress in a cadaveric human diaphyseal model possibly from the use of suboptimal composite materials [
48]. The volume of cement injected also plays a key role in determining the strength of fixation. Because of the enormous built-in back pressure and the quick onset of polymerization, it may not be usually feasible to manually inject cement volumes greater than 6–8 mL in the form of a single cohesive ball. In such scenarios, the use of an automated hydraulic-force cement injector [
96,
97] or a robotic injection device as described by Garnon et al. [
98] may be preferred.
In vitro mechanical assessment provides insights into the feasibility of these novel procedures and devices that are designed to undertake loads and provide stability for a wide array of orthopedic applications. Various test methods have been developed to characterize the mechanical properties of these devices, and the intended use and location in vivo determine the types of tests that need to be executed and the parameters to look for. PMMA bone cement has superior axial compressive strength and capacity to withstand compression in flat bones like the spine and hip [
40]. However, it has low torsional, shear, and bending stress handling capacity [
36] and carries a risk of secondary fracture when applied to overcome long bone neoplastic defects. Studies report 8–9% of secondary long bone fractures in metastatic patients following osteoplasty [
30]. In the event of secondary fracture, further fixation is almost impractical because of the permanent closure of the internal void from cement filling. Calcium phosphate cement may be preferable over PMMA bone cement for fracture repair in the given context because of its superior biological and osteogenic properties [
99]. However, the inferior mechanical strength of calcium phosphate cement over PMMA cement requires further research to address this limitation.
Percutaneous osteoplasty with a range of adjunctive reinforcement is implemented on a case-by-case basis in clinics for the consolidation of long bone fractures (impending/pathological) and has produced encouraging results [
13,
37]. However, these studies lack adequate preclinical biomechanical characterization and the outcomes may be limited to short-term gains. Further studies are needed to evaluate the long-term benefits of these procedures. Lack of osteointegration with the native tissues and non-osteoconductive properties of the implant are the most common causes of infections and imminent failures; therefore, research efforts need to be directed to find optimal solutions with a focus on physical, mechanical, and biochemical factors present in vivo. The challenges can be partly addressed with the development of next-generation bone cement composed of various osteogenic growth factors and possibly antitumor/anti-inflammatory drugs that can positively impact molecular and cellular processes and allow bonding and integration with the skeletal tissues in the targeted region without inflammation. The transformation of bone cement into an osteosynthetic material instead of being limited to a space filler heralds new bone growth and may alleviate concerns related to poor stress handling capability. Extraosseous cement leakage during percutaneous osteoplasty procedures is also a common concern that can potentially cause inflammation, pain, and tissue injury, and can be overcome with the use of an optimally viscous cement and following a cautious surgical approach [
100]. While there is a clear lack of sufficient clinical studies to support percutaneous reinforcement, the potential benefits in terms of pain relief, mechanical stability, and early facilitation of weight-bearing bones with bioengineered scaffold should not be discounted. Proper guidance on patient selection, surgical efficacy, and related complications based on the outcomes of large-scale studies and longer follow-ups are awaited. Moving forward, emphasis needs to be given to combination treatment that includes novel biomaterials with biocompatible and bioactive properties that can provide synergy in supplementing bone strength by aiding new bone formation, restoring anatomical defects and physiological function, and pain regression for percutaneous applications.
This entry is adapted from the peer-reviewed paper 10.3390/jcm11195572