Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Kenneth Lundstrom | -- | 1588 | 2022-10-18 20:20:12 | | | |
| 2 | Dean Liu | Meta information modification | 1588 | 2022-10-19 04:38:09 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Lundstrom, K. Alphaviruses in Immunotherapy and Anticancer Therapy. Encyclopedia. Available online: https://encyclopedia.pub/entry/29967 (accessed on 01 March 2026).
Lundstrom K. Alphaviruses in Immunotherapy and Anticancer Therapy. Encyclopedia. Available at: https://encyclopedia.pub/entry/29967. Accessed March 01, 2026.
Lundstrom, Kenneth. "Alphaviruses in Immunotherapy and Anticancer Therapy" Encyclopedia, https://encyclopedia.pub/entry/29967 (accessed March 01, 2026).
Lundstrom, K. (2022, October 18). Alphaviruses in Immunotherapy and Anticancer Therapy. In Encyclopedia. https://encyclopedia.pub/entry/29967
Lundstrom, Kenneth. "Alphaviruses in Immunotherapy and Anticancer Therapy." Encyclopedia. Web. 18 October, 2022.
Copy Citation
Alphaviruses have been engineered as expression vectors for vaccine development and gene therapy. Due to the feature of RNA self-replication, alphaviruses can provide exceptional direct cytoplasmic expression of transgenes based on the delivery of recombinant particles, naked or nanoparticle-encapsulated RNA or plasmid-based DNA replicons.
alphavirus vectors
recombinant particles
RNA delivery
1. Introduction
During the last decade, immunotherapy has become an attractive alternative for cancer therapy [1]. In this context, viral vectors have also proven useful for immunotherapeutic applications [2]. Alphaviruses have frequently been engineered for the overexpression of suitable antigens and immunostimulatory genes for vaccine development and cancer therapy [3]. Additionally, the expression of cytotoxic and antitumor genes has been used for cancer therapy applications. Semliki Forest virus (SFV) [4], Sindbis virus (SIN) [5] and Venezuelan equine encephalitis virus (VEE) [6] are most commonly used for the engineering of expression systems. Additionally, the naturally occurring oncolytic alphavirus M1 [7] and engineered oncolytic versions based on SFV and SIN vectors [8] have been utilized for cancer therapy. The evaluation of efficacy in appropriate animal models has provided proof of concept before conducting clinical trials.
2. Alphavirus Lifecycle and Expression Vector Systems
Alphaviruses possess an enveloped structure of capsid and spike proteins encapsulating a single-stranded RNA (ssRNA) genome of positive polarity [9]. Upon the infection of host cells, the alphavirus ssRNA is released into the cytoplasm, where translation can immediately occur requiring no delivery of RNA to the nucleus as is the case for other RNA viruses such as the influenza virus and DNA viruses (Figure 1). In the cytoplasm, efficient self-replication occurs through a minus-strand RNA template leading to the accumulation of approximately 106 copies of subgenomic RNA per cell. Together with the utilization of the highly efficient 26S subgenomic promoter, high-level expression of viral proteins occurs [10]. The RNA self-replication and high-level expression of alphavirus structural proteins generate high-titer virus progeny. Nucleocapsids comprising the capsid protein harboring full-length alphavirus RNA are transported to the cell surface, where the envelope proteins are attached, and mature viral particles are released by budding.

Figure 1. Schematic presentation of the lifecycle of alphaviruses. Alphaviruses infect host cells by endocytosis through endosomal fusion with the plasma membrane. The positive sense ssRNA is released into the cytoplasm for translation of viral proteins and RNA replication. Full-length ssRNA genomes are packaged into nucleocapsids. The alphavirus envelope proteins are transported to the plasma membrane through the endoplasmic reticulum and Golgi. The nucleocapsids are encircled by the envelope proteins at the plasma membrane and released by budding. ER, endoplasmic reticulum.
In the case of expression systems, the focus is on the expression of heterologous genes (Figure 2). In the context of replication-deficient alphavirus particles, the structural protein genes have been replaced by the gene of interest (GoI), and a helper vector is engaged in providing the structural proteins in trans (Figure 2A). Co-transfection of in vitro transcribed RNA from expression and helper vectors into baby hamster kidney (BHK) cells leads to the production of recombinant particles. As the RNA packing signal is located in one of the genes coding for the non-structural proteins (nsPs), in the nsP2 gene of SFV and nsP1 of SIN [11], uniquely RNA from the expression vector is packaged into viral particles, providing expression of the GoI but not the structural protein genes and thereby, eliminating any production of viral progeny. In contrast, introduction of a second 26S subgenomic promoter and the GoI into the full-length alphavirus RNA genome, either downstream of the nsP or the structural protein genes, generates replication-competent particles capable of both high-level GoI expression and viral progeny production (Figure 2B). In addition to the application of recombinant particles, RNA replicons can also be used for GoI expression. As has been demonstrated for the recent BNT162b2 [12] and mRNA-1273 [13] COVID-19 vaccines, RNA-based delivery is highly efficient. However, in contrast to this conventional mRNA approach, alphavirus RNA replicons provide the additional advantage of RNA self-amplification leading to superior expression levels. Moreover, replacement of the SP6 RNA polymerase promoter by a CMV promoter, DNA replicon vectors for GoI expression (Figure 2C) have been engineered for the transfection of cell lines and in vivo administration [14]. The use of DNA replicons eliminates any risk of the production of new virus particles but relies on the less efficient delivery of DNA compared to viral vectors. Moreover, DNA molecules must be delivered to the nucleus for the in vivo transcription of RNA (Figure 2C).

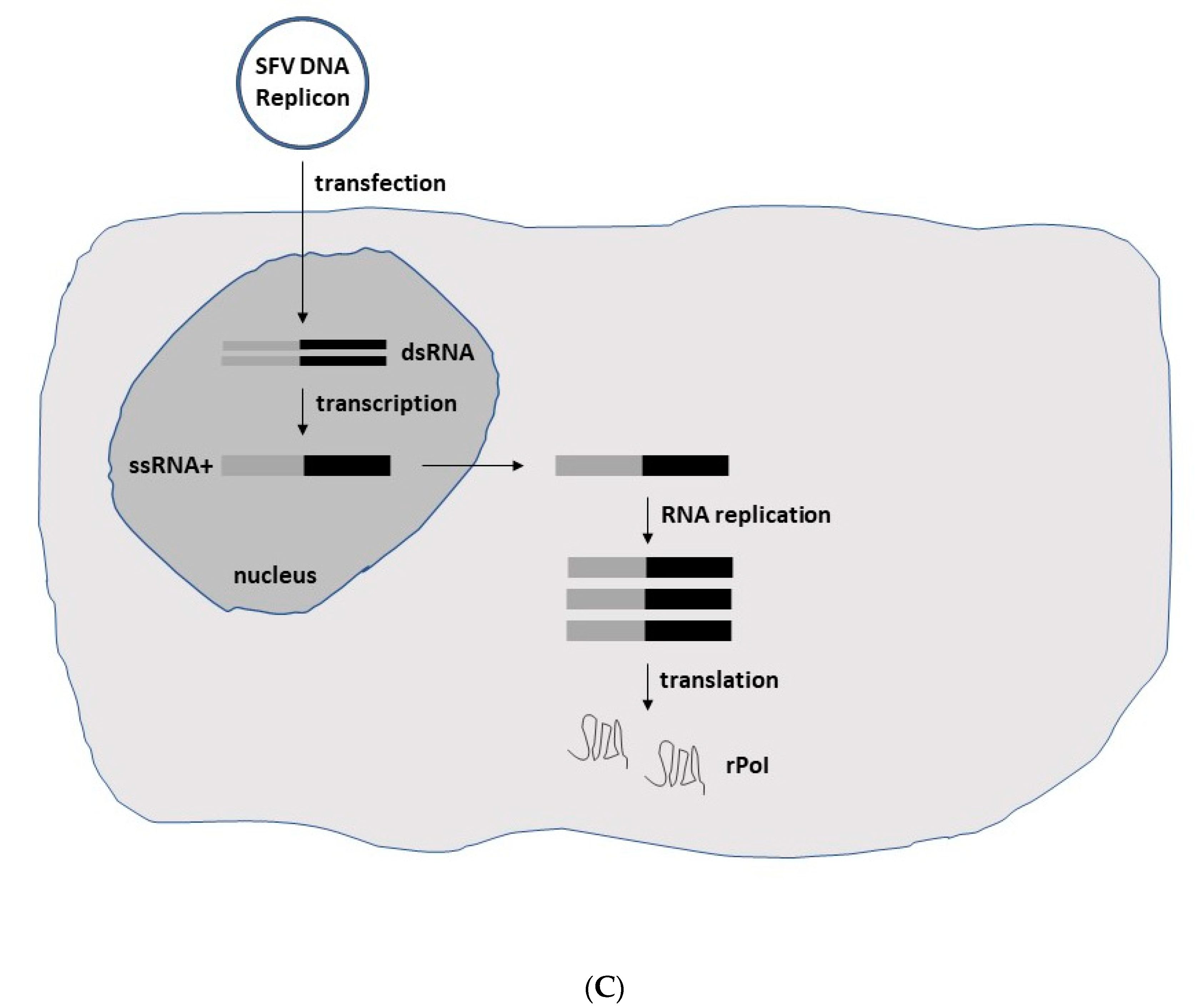
Figure 2. Schematic presentation of SFV expression systems. (A) Replication-deficient recombinant particles. In vitro transcribed RNA molecules from the SFV expression vector carrying the non-structural protein (nsP) genes, replicase genes (replicon) and the gene of interest (GoI) and the structural protein genes (capsid, 6K, envelope E1, E2 and E3) from the helper vector are electroporated or transfected into BHK-21 cells. After RNA replication, only the RNA from the expression vector containing the packaging signal is packaged into nucleocapsids and transported to the plasma membrane, where budding of mature viral particles takes place. Although the generated particles are capable of infecting new host cells, no viral progeny is produced due to the absence of the structural protein genes. However, high-level expression of the recombinant protein of interest (rPoI) takes place (B) Replication-competent recombinant particles. The in vitro transcribed full-length RNA genome with the GoI introduced either downstream of the nsP genes or the structural protein genes is electroporated or transfected into host cells for production of replication-competent viral particles and rPoI expression. (C) DNA replicon vectors. The replacement of the SP6 RNA polymerase promoter by a CMV promoter upstream of the nsP genes allows for direct transfection of host cells for rPoI expression. DNA replicons in the form of DNA plasmids are transfected into host cells, and DNA replicons are delivered to the nucleus. Transcribed ssRNA molecules of positive polarity are delivered to the cytoplasm for RNA replication and expression of the rPoI.
3. Alphavirus-Based Immunotherapy for Cancer
In the context of cancers, alphaviruses have been frequently used for prophylactic and therapeutic applications. Immunization with alphavirus vectors overexpressing tumor-associated antigens (TAAs) has been a common approach for cancer vaccine development. This approach has been used to provide both prevention against tumor challenges and tumor regression and eradication. Moreover, overexpression of cytotoxic and antitumor genes has been evaluated for cancer therapy. The delivery of immunostimulatory genes from alphavirus vectors has served the means of cancer immunotherapy. Moreover, alphaviruses induce apoptosis through activation of caspases in infected cells [15], which has resulted in tumor regression after administration of alphaviruses carrying no therapeutic genes and has allowed the use of vectors with reporter genes to verify and localize expression in animal tumor models. Finally, engineered or naturally occurring oncolytic alphaviruses have demonstrated tumor cell-specific killing in animal models [16]. Examples of cancer vaccinations, cancer therapy and immunotherapy are given below and summarized in Table 1.
Table 1. Examples of alphavirus-based vaccines against cancer.
| Cancer | Vector | Finding | Ref |
|---|---|---|---|
| Reporter Genes | |||
| Lung | SFV-EGFP | Tumor regression in mice | [17] |
| Colon | SIN-LacZ | Complete tumor remission | [18] |
| SFV-LacZ RNA | Tumor regression, protection | [19] | |
| TAAs | |||
| Cervical | VEE-HPV-16 E7 | Protection against tumor challenges in mice | [20] |
| SFVenh-HPV E6-E7 | Tumor eradication, long-lasting CTL in mice | [21] | |
| SFV-sHELP-E7SH | Tumor regression, protection in mice | [22] | |
| SFV-HPV E6-E7 DNA | 85% of immunized mice tumor-free | [23] | |
| SFVenh-HPV E6-E7 | Phase I: Immunogenicity in all patients | [24] | |
| Colon | SFV-VEGFR-2 | Inhibition of tumor growth, metastatic spread | [25] |
| SFV-VEGR-2 + SFV-IL-4 | Prolonged survival after coadministration | [25] | |
| VEE-CEA | Phase I: Ag-specific response, long-term survival | [26] | |
| Pancreatic | VEE-CEA | Phase I: Prolonged survival | [27] |
| Melanoma | VEE-TRP-2 + DNA | Superior to plasmid DNA vaccine in mice | [28] |
| VEE-TRP-2 | Humoral immune responses, protection in mice | [29] | |
| VEE-TRP-2 + CTLA-4 mAbs | Tumor regression in 50% of mice | [30] | |
| VEE-TRP-2 + GITR mAbs | Tumor regression in 90% of mice | [30] | |
| SFV-VEGFR-2/IL-12 DNA | Synergistic antitumor activity from combination of | [31] | |
| + SFV-Survivin/β-hCG DNA | DNA replicons | ||
| Ovarian | SFV-OVA + VV-OVA | Immune responses, enhanced antitumor activity | [32] |
| Prostate | VEE-PSMA | Th1-biased immune responses | [33] |
| VEE-PSMA | Phase I: Good safety, weak immunogenicity | [34] | |
| VEE-PSA | PSA-specific Abs, delay in tumor growth | [35] | |
| VEE-mSTEAP + pcDNA | Prolonged survival, tumor challenge protection | [36] | |
| VEE-PSCA | Long-term survival of mice | [37] | |
| Cytotoxic and Antitumor Genes | |||
| Glioblastoma | SFV–Endostatin | Tumor growth inhibition, reduced vascularization | [38] |
| Breast | SIN-HER2/neu DNA | Significant tumor growth inhibition, protection | [39] |
| SIN-HER2/neu DNA | 80% less DNA needed compared to plasmid DNA | [40] | |
| VEE-HER2/neu ECD/TM | Complete prevention of tumors in mice | [41] | |
| VEE-HER2/neu ECD/TM | Safe, PR in 1 patient, SD in 2 patients | [42] | |
| Immunostimulation | |||
| Glioblastoma | SFV-IL-18 + rec IL-12 | Superior therapeutic effect of combination | [43] |
| Glioma | SFV-IL-12 | 70–97% tumor volume reduction in rats | [44] |
| Brain | SIN-gp100 + SIN-IL-12 DNA | Superior antitumor activity, prolonged survival | [45] |
| Breast | SFV-IL-12 + LV101 | Superior antitumor activity of combination | [46] |
| Colon | SFVenh-IL-12 | Complete tumor regression, long-term survival | [47] |
| SFV-IL-12 + anti-PD1 | Superior combination therapy in mice | [48] | |
| VEE-IL-12 + VEE-CEA | Superior combination therapy in mice | [49] | |
| Melanoma | SFV-IL-12 + anti-PD1 | Superior combination therapy in mice | [48] |
| LSFV-IL-12 | Phase I: Good safety and tolerability | [50] | |
| Ovarian | SIN-IL12 + Irinotecan | Long-term survival in 35% of mice | [51] |
| Oncolytic Viruses | |||
| Glioblastoma | SFV-VA-EGFP | Long-term survival in 16 out of 17 mice | [52] |
| Prostate | SFV-VA-EGFP | Complete tumor eradication in mice | [53] |
| Lung | SFV-VA-EGFP | Long-term survival in mice | [8] |
| Liver | M1 | Liver tumor targeting in mice | [54] |
| Glioma | M1 | Replication in tumors | [55] |
| Bladder | M1 | Tumor growth inhibition, prolonged survival | [56] |
| Breast | M1 + Doxorubicin | Reduced tumor growth in mice | [7] |
| Pancreatic | M1 + IRE | Tumor growth inhibition, prolonged survival | [57] |
| Cervical | SIN AR339 | Regression of established tumors in mice | [58] |
| Ovarian | SIN AR399 | Ascites formation in metastasis mouse model | [59] |
Abs, antibodies; anti-PD1, immune checkpoint inhibitor; CEA, carcinoembryonic antigen; CTLA-4, CTL antigen-4; GITR, glucocorticoid-induced tumor necrosis factor; HPV, human papilloma virus; IRE, irreversible electroporation; LV101, Salmonella typhimurium AroC strain; mAbs, monoclonal antibodies; mSTEAP, mouse six-transmembrane epithelial antigen of the prostate; OVA. Ovalbumin; PR, partial response; PSA, prostate-specific antigen; PSMA, prostate-specific membrane antigen; rec IL-12, recombinant IL-12; SD, stable disease; SFV, Semliki Forest virus; SIN, Sindbis virus; TRP-2, tyrosine-related protein-2; VEE, Venezuelan equine encephalitis virus; VEGFR-2, vascular endothelial growth factor receptor-2; VV, vaccinia virus.
References
- Riley, R.S.; June, C.H.; Langer, R.; Mitchel, M.J. Delivery technologies for cancer immunotherapy. Nat. Rev. Drug Discov. 2019, 18, 175–196.
- Raja, J.; Ludwig, M.; Gettinger, S.N.; Schalper, K.A.; Kim, H.S. Oncolytic virus immunotherapy: Future prospects for oncology. J. Immunother. Cancer 2018, 6, 140.
- Lundstrom, K. Alphaviruses in Cancer Therapy. Front. Mol. Biosci. 2022, 9, 864781.
- Liljestrom, P.; Garoff, H. A new generation of animal cell expression vectors based on the Semliki Forest virus replicon. Biotechnology 1991, 9, 1356–1361.
- Xiong, C.; Levis, R.; Shen, P.; Schlesinger, S.; Rice, C.M.; Huang, H.V. Sindbis virus: An efficient, broad host range vector for gene expression in animal cells. Science 1989, 243, 1188–1191.
- Davis, N.L.; Willis, L.V.; Smith, J.F.; Johnston, R.F. In vitro synthesis of infectious Venezuelan equine encephalitis virus RNA from a cDNA clone: Analysis of a viable deletion mutant. Virology 1989, 171, 189–204.
- Zhang, J.; Liu, Y.; Tan, J.; Zhang, Y.; Wong, C.-W.; Lin, Z.; Liu, X.; Sander, M.; Yang, X.; Liang, L.; et al. Necroptotic Virotherapy of Oncolytic Alphavirus M1 Cooperated with Doxorubicin Displays Promising Therapeutic Efficacy in TNBC. Oncogene 2021, 40, 4783–4795.
- Määttä, A.M.; Mäkinen, K.; Ketola, A.; Liimatainen, T.; Yongabi, F.N.; Vähä-Koskela, M. Replication Competent Semliki Forest Virus Prolongs Survival in Experimental Lung Cancer. Int. J. Cancer 2008, 123, 1704–1711.
- Strauss, J.H.; Strauss, E.G. The alphaviruses: Gene expression, replication and evolution. Microbiol. Rev. 1994, 58, 491–562.
- Frolov, I.; Hoffman, T.A.; Pragal, B.M.; Dryga, S.A.; Huang, H.; Schlesinger, S.; Rice, C.M. Alphavirus-based expression vectors: Strategies and applications. Proc. Natl. Acad. Sci. USA 1996, 93, 11371–11377.
- Frolova, E.; Frolov, I.; Schlesinger, S. Packaging Signals in Alphaviruses. J. Virol. 1997, 71, 248–258.
- Lamb, Y.N. BNT162b2 mRNA COVID-19 vaccine: First approval. Drugs 2021, 81, 495–501.
- Oliver, S.E.; Gargano, J.W.; Marin, M.; Wallace, M.; Curran, K.G.; Chamberland, M.; McClung, N.; Campos-Outcalt, D.; Morgan, R.L.; Mbaeyi, S.; et al. The advisory committee on immunization practices’ interim recommendation for use of Moderna COVID-19 vaccine—United States, December 2020. MMWR Morb. Mortal. Wkly. Rep. 2021, 69, 1653–1656.
- DiCiommo, D.P.; Bremner, R. Rapid, high level protein production using DNA-based Semliki Forest virus vectors. J. Biol. Chem. 1998, 273, 18060–18066.
- Olkkonen, V.M.; Liljestrom, P.; Garoff, H.; Simons, K.; Dotti, C.G. Expression of heterologous proteins in cultured rat hippocampal neurons using the Semliki Forest virus vector. J. Neurosc. Res. 1993, 35, 445–451.
- Fukuhara, H.; Ino, Y.; Todo, T. Oncolytic virus therapy: A new era of cancer treatment at dawn. Cancer Sci. 2016, 107, 1373–1379.
- Murphy, A.M.; Morris-Downes, M.M.; Sheahan, B.J.; Atkins, G.J. Inhibition of human lung carcinoma cell growth by apoptosis induction using Semliki Forest virus recombinant particles. Gene Ther. 2000, 7, 1477–1482.
- Granot, T.; Yamanashi, Y.; Meruelo, D. Sindbis viral vectors transiently deliver tumor-associated antigens to lymph nodes and elicit diversified antitumor CD8+ T-cell immunity. Mol. Ther. 2014, 22, 112–122.
- Ying, H.; Zaks, T.Z.; Wang, R.-F.; Irvine, K.R.; Kammula, U.S.; Marincola, F.M. Cancer therapy using a self-replicating RNA vaccine. Nat. Med. 1999, 5, 823–827.
- Velders, M.P.; McElhiney, S.; Cassetti, M.C.; Eiben, G.L.; Higgins, T.; Kovacs, G.R. Eradication of established tumors by vaccination with Venezuelan equine encephalitis virus replicon particles delivering human papillomavirus 16 E7 RNA. Cancer Res. 2001, 61, 7861–7867.
- Daemen, T.; Riezebos-Brilman, A.; Bungener, L.; Regts, J.; Dontje, B.; Wilschut, J. Eradication of established HPV16-transformed tumours after immunisation with recombinant Semliki Forest virus expressing a fusion protein of E6 and E7. Vaccine 2003, 21, 1082–1088.
- Ip, P.P.; Boerma, A.; Walczak, M.; Oosterhuis, K.; Haanen, J.B.; Schumacher, T.N.; Nijman, H.W.; Daemen, T. Antigen design enhances the immunogenicity of Semliki Forest virus-based therapeutic human papillomavirus vaccines. Gene Ther. 2015, 22, 560–567.
- Van de Wall, S.; Ljungberg, K.; Ip, P.P.; Boerma, A.; Knudsen, M.L.; Nijman, H.W.; Peter, L.; Daemen, T. Potent therapeutic efficacy of an alphavirus replicon DNA vaccine expressing human papilloma virus E6 and E7 antigens. Oncoimmunology 2018, 7, e1487913.
- Komdeur, F.L.; Singh, A.; Van de Wall, S.; Meulenberg, J.J.M.; Boerma, A.; Hoogeboom, B.N.; Paijens, S.T.; Oyarce, C.; de Bruyn, M.; Schuuring, E.; et al. First-in-human phase I clinical trial of an SFV based RNA replicon cancer vaccine against HPV-induced cancers. Mol. Ther. 2021, 29, 611–625.
- Lyons, J.A.; Sheahan, B.J.; Galbraith, S.E.; Mehra, R.; Atkins, G.J.; Fleeton, M.N. Inhibition of angiogenesis by a Semliki Forest virus vector expressing VEGFR-2 reduces tumour growth and metastasis in mice. Gene Ther. 2007, 14, 503–513.
- Crosby, E.J.; Hobeika, A.C.; Niedzweicki, D.; Rushing, C.; Hsu, D.; Berglund, P.; Smith, J.; Osada, T.; Gwin, W.R., III; Hartman, Z.C.; et al. Long-term survival of patients with stage III colon cancer treated with VRP-CEA(6D), an alphavirus vector that increases the CD8+ effector memory T cell to Treg ration. J. Immunother. Cancer 2020, 8, e001662.
- Morse, M.A.; Hobelka, A.C.; Osada, T.; Berglund, P.; Hubby, B.; Negri, S.; Niedzwiecki, D.; Devi, G.R.; Burnett, B.K.; Clay, T.M.; et al. An alphavirus vector overcomes the presence of neutralizing antibodies and elevated numbers of Tregs to induce immune responses in humans with advanced cancer. J. Clin. Investig. 2010, 120, 3234–3241.
- Goldberg, S.M.; Bartido, S.M.; Gardner, J.P.; Guevara-Patino, J.A.; Montgomery, S.C.; Perales, M.-A.; Maughan, M.F.; Dempsey, J.; Donovan, G.P.; Olson, W.C.; et al. Comparison of two cancer vaccines targeting tyrosinase: Plamid DNA and recombinant alphavirus replicon particles. Clin. Cancer Res. 2005, 11, 8114–8121.
- Avogadri, F.; Merghoub, T.; Maughan, M.F.; Hirschhorn-Cymerman, D.; Morris, J.; Ritter, E. Alphavirus replicon particles expressing TRP-2 provide potent therapeutic effect on melanoma through activation of humoral and cellular immunity. PLoS ONE 2010, 5, e12670.
- Avogadri, F.; Zappasodi, R.; Yang, A.; Budhu, S.; Malandro, N.; Hisrchhorn-Cymerman, D. Combination of alphavirus replicon particle-based vaccination with immunomodulatory antibodies: Therapeutic activity in the B16 melanoma mouse model and immune correlates. Cancer Immunol. Res. 2014, 2, 448–458.
- Yin, X.; Wang, W.; Zhu, X.; Wang, Y.; Wu, S.; Wang, Z. Synergistic antitumor efficacy of combined DNA vaccines targeting tumor cells and angiogenesis. Biochem. Biophys. Res. Comm. 2015, 465, 239–244.
- Zhang, Y.Q.; Tsai, Y.C.; Monie, A.; Wu, T.C.; Hung, C.F. Enhancing the therapeutic effect against ovarian cancer through a combination of viral oncolysis and antigen-specific immunotherapy. Mol. Ther. 2010, 18, 692–699.
- Durso, R.J.; Andjelic, S.; Gardner, J.P.; Margitich, D.J.; Donovan, G.P.; Arrigale, R.R.; Wang, X.; Maughan, M.F.; Talarico, T.L.; Olmsted, R.A.; et al. A novel alphavirus vaccine encoding prostate-specific membrane antigen elicits potent cellular and humoral immune responses. Clin. Cancer Res. 2007, 13, 3999–4008.
- Slovin, S.F.; Kehoe, M.; Durso, R.; Fernandez, C.; Olson, W.; Gao, J.P.; Israel, R.; Scher, H.I.; Morris, S. A phase I dose escalation trial of vaccine replicon particles (VRP) expressing prostate-specific membrane antigen (PSMA) in subjects with prostate cancer. Vaccine 2013, 31, 943–949.
- Riabov, V.; Tretyakova, I.; Alexander, R.B.; Pushko, P.; Klyushnenkova, E.N. Anti-tumor effect of the alphavirus-based virus-like particle vector expressing prostate-specific antigen in a HLA-DR transgenic mouse model of prostate cancer. Vaccine 2015, 33, 5386–5395.
- Garcia-Hernandez, M.L.; Gray, A.; Hubby, B.; Kast, W.M. In vivo effects of vaccination with six-transmembrane epithelial antigen of the prostate: A candidate antigen for treating prostate cancer. Cancer Res. 2007, 67, 1344–1351.
- Garcia-Hernandez, M.L.; Gray, A.; Hubby, B.; Klinger, O.J.; Kast, W.M. Prostate stem cell antigen vaccination induces a long-term protective immune response against prostate cancer in the absence of autoimmunity. Cancer Res. 2008, 68, 861–869.
- Yamanaka, R.; Zullo, S.A.; Ramsey, J.; Onodera, M.; Tanaka, R.; Blaese, M. Induction of therapeutic antitumor anti-angiogenesis by intratumoral injection of genetically engineered endostatin-producing Semliki Forest virus. Cancer Gene Ther. 2001, 8, 796–802.
- Wang, X.; Wang, J.P.; Rao, X.M.; Price, J.E.; Zhou, H.S.; Lachman, L.B. Prime-boost vaccination with plasmid and adenovirus gene vaccines control HER2/neu+ metastatic breast cancer in mice. Breast Cancer Res. 2005, 7, R580–R588.
- Lachman, L.B.; Rao, X.M.; Kremer, R.H.; Ozpolat, B.; Kirjakova, G.; Price, J.E. DNA vaccination against neu reduces breast cancer incidence and metastasis in mice. Cancer Gene Ther. 2001, 8, 259–268.
- Wang, X.; Wang, J.-P.; Maughan, M.F.; Lachman, L.B. Alphavirus replicon particles containing the gene for HER2/neu inhibit breast cancer growth and tumorigenesis. Breast Cancer Res. 2005, 7, R145–R155.
- Crosby, E.J.; Gwin, W.; Blackwell, K.; Marcom, P.K.; Chang, S.; Maecker, H.T.; Broadwater, G.; Hyslop, T.; Kim, S.; Rogatko, A.; et al. Vaccine-induced memory CD8(+) T cells provide clinical benefit in HER2 expressing breast cancer: A mouse to human translational study. Clin. Cancer Res. 2019, 25, 2725–2736.
- Yamanaka, R.; Tsuchiya, N.; Yajima, N.; Honma, J.; Hasegawa, H.; Tanaka, R.; Ramsey, J.; Blaese, R.M.; Xanthopoulos, K.G. Induction of an antitumor immunological response by an intratumoral injection of dendritic cells pulsed with genetically engineered Semliki Forest virus to produce interleukin-18 combined with the systemic administration of interleukin-12. J. Neurosurg. 2003, 99, 746–753.
- Roche, F.P.; Sheahan, B.J.; O’Mara, S.M.; Atkins, G.J. Semliki Forest virus-mediated gene therapy of the RG2 rat glioma. Neuropathol. Appl. Neurobiol. 2010, 36, 648–660.
- Yamanaka, R.; Xanthopoulos, K.G. Induction of antigen-specific immune responses against malignant brain tumors by intramuscular injection of sindbis DNA encoding gp100 and IL-18. DNA Cell Biol. 2005, 24, 317–324.
- Kramer, M.G.; Masner, M.; Casales, E.; Moreno, M.; Smerdou, C.; Chabalgoity, J.A. Neoadjuvant administration of Semliki Forest virus expressing interleukin-12 combined with attenuated Salmonella eradicates breast cancer metastasis and achieves long-term survival in immunocompetent mice. BMC Cancer 2015, 15, 620.
- Rodriguez-Madoz, J.R.; Prieto, J.; Smerdou, C. Semliki Forest virus vectors engineered to express higher IL-12 levels induce efficient elimination of murine colon adenocarcinomas. Mol. Ther. 2005, 12, 153–163.
- Quetglas, J.I.; Labiano, S.; Aznar, M.A.; Bolanos, E.; Azpilikueta, A.; Rodriguez, I.; Casales, E.; Sánchez-Paulete, A.R.; Segura, V.; Smerdou, C.; et al. Virotherapy with a Semliki Forest Virus-Based Vector Encoding IL12 Synergizes with PD-1/PD-L1 Blockade. Cancer Immunol. Res. 2015, 3, 449–454.
- Osada, T.; Berglund, P.; Morse, M.A.; Hubby, B.; Lewis, W.; Niedzwiecki, D.; Yang, X.Y.; Hobeika, A.; Burnett, B.; Devi, G.R.; et al. Co-delivery of antigen and IL-12 by Venezuelan equine encephalitis virus replicon particles enhances antigen-specific immune responses and anti-tumor effects. Cancer Immunol. Immunother. 2012, 61, 1941–1951.
- Ren, H.; Boulikas, T.; Lundstrom, K.; Söling, P.C.; Warnke, P.C.; Rainov, N.G. Immunogene therapy of recurrent glioblastoma multiforme with a liposomally encapsulated replication-incompetent Semliki Forest virus vector carrying the human interleukin-12 gene—A phase I/II clinical protocol. J. Neurooncol. 2003, 64, 147–154.
- Granot, T.; Meruelo, D. The role of natural killer cells in combinatorial anti-cancer therapy using Sindbis viral vector and irinotecan. Cancer Gene Ther. 2012, 19, 588–591.
- Heikkilä, J.E.; Vähä-Koskela, M.J.; Ruotsalainen, J.J.; Martikainen, M.W.; Stanford, M.M.; McCart, J.A.; Bell, J.C.; Hinkkanen, A.E. Intravenously administered alphavirus vector VA7 eradicates orthotopic human glioma xenografts in nude mice. PLoS ONE 2010, 5, e8603.
- Martikainen, M.; Ruotsalainen, J.; Tuomela, J.; Härkönen, P.; Essand, M.; Heikkilä, J.E.; Hinkkanen, A. Oncolytic alphavirus SFV-A7 efficiently eradicates subcutaneous and orthotopic prostate tumours in mice. Br. J. Cancer 2017, 117, 51–55.
- Zhu, W.; Liang, J.; Tan, J.; Guo, L.; Cai, J.; Hu, J.; Yan, G.; Liu, Y.; Zhang, J.; Song, D.; et al. Real-Time Visualization and Quantification of Oncolytic M1 Virus In Vitro and In Vivo. Hum. Gene Ther. 2021, 32, 158–165.
- Cai, J.; Zhu, W.; Lin, Y.; Zhang, S.; Chen, X.; Gong, S.; He, S.; Hu, J.; Yan, G.; Liang, J. Systematic Characterization of the Biodistribution of the Oncolytic Virus M1. Hum. Gene Ther. 2020, 31, 1203–1213.
- Hu, C.; Liu, Y.; Lin, Y.; Liang, J.K.; Zhong, W.W.; Li, K.; Huang, W.; Wang, D.; Yan, G.; Zhu, W.; et al. Intravenous injections of the oncolytic virus M1 as a novel therapy for muscle-invasive bladder cancer. Cell Death Dis. 2018, 9, 274.
- Sun, S.; Liu, Y.; He, C.; Hu, W.; Liu, W.; Huang, X.; Wu, J.; Xie, F.; Chen, C.; Wang, J.; et al. Combining Nanoknife with M1 oncolytic virus enhances anticancer activity in pancreatic cancer. Cancer Lett. 2021, 502, 9–24.
- Wang, Y.; Huang, H.; Zou, H.; Tian, X.; Hu, J.; Qiu, P.; Hu, H.; Yan, G. Liposome Encapsulation of Oncolytic Virus M1 To Reduce Immunogenicity and Immune Clearance in Vivo. Mol. Pharm. 2019, 16, 779–785.
- Unno, Y.; Shino, Y.; Kondo, F.; Igarashi, N.; Wang, G.; Shimura, R.; Yamaguchi, T.; Asano, T.; Saisho, H.; Sekiya, S.; et al. Oncolytic virotherapy for cervical and ovarian cancer cells by Sindbis virus strain AR339. Clin. Cancer Res. 2005, 11, 4553–4560.
More
Information
Subjects:
Virology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
809
Revisions:
2 times
(View History)
Update Date:
19 Oct 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





