Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Kenneth Lundstrom and Version 2 by Dean Liu.
Alphaviruses have been engineered as expression vectors for vaccine development and gene therapy. Due to the feature of RNA self-replication, alphaviruses can provide exceptional direct cytoplasmic expression of transgenes based on the delivery of recombinant particles, naked or nanoparticle-encapsulated RNA or plasmid-based DNA replicons.
- alphavirus vectors
- recombinant particles
- RNA delivery
1. Introduction
During the last decade, immunotherapy has become an attractive alternative for cancer therapy [1]. In this context, viral vectors have also proven useful for immunotherapeutic applications [2]. Alphaviruses have frequently been engineered for the overexpression of suitable antigens and immunostimulatory genes for vaccine development and cancer therapy [3]. Additionally, the expression of cytotoxic and antitumor genes has been used for cancer therapy applications. Semliki Forest virus (SFV) [4], Sindbis virus (SIN) [5] and Venezuelan equine encephalitis virus (VEE) [6] are most commonly used for the engineering of expression systems. Additionally, the naturally occurring oncolytic alphavirus M1 [7] and engineered oncolytic versions based on SFV and SIN vectors [8] have been utilized for cancer therapy. The evaluation of efficacy in appropriate animal models has provided proof of concept before conducting clinical trials.
2. Alphavirus Lifecycle and Expression Vector Systems
Alphaviruses possess an enveloped structure of capsid and spike proteins encapsulating a single-stranded RNA (ssRNA) genome of positive polarity [9]. Upon the infection of host cells, the alphavirus ssRNA is released into the cytoplasm, where translation can immediately occur requiring no delivery of RNA to the nucleus as is the case for other RNA viruses such as the influenza virus and DNA viruses (Figure 1). In the cytoplasm, efficient self-replication occurs through a minus-strand RNA template leading to the accumulation of approximately 106 copies of subgenomic RNA per cell. Together with the utilization of the highly efficient 26S subgenomic promoter, high-level expression of viral proteins occurs [10]. The RNA self-replication and high-level expression of alphavirus structural proteins generate high-titer virus progeny. Nucleocapsids comprising the capsid protein harboring full-length alphavirus RNA are transported to the cell surface, where the envelope proteins are attached, and mature viral particles are released by budding.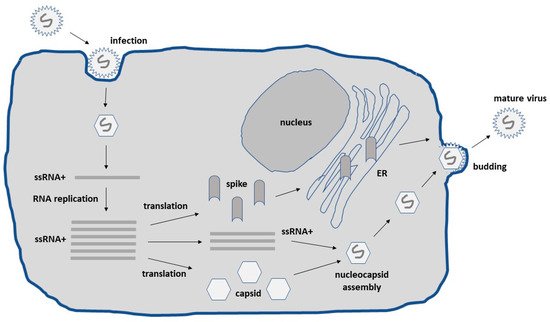
Figure 1. Schematic presentation of the lifecycle of alphaviruses. Alphaviruses infect host cells by endocytosis through endosomal fusion with the plasma membrane. The positive sense ssRNA is released into the cytoplasm for translation of viral proteins and RNA replication. Full-length ssRNA genomes are packaged into nucleocapsids. The alphavirus envelope proteins are transported to the plasma membrane through the endoplasmic reticulum and Golgi. The nucleocapsids are encircled by the envelope proteins at the plasma membrane and released by budding. ER, endoplasmic reticulum.
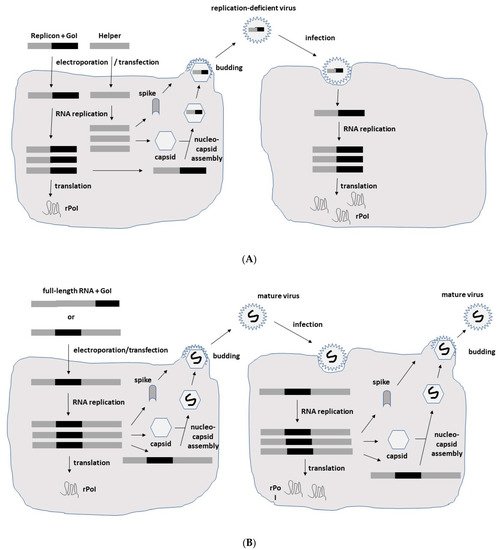
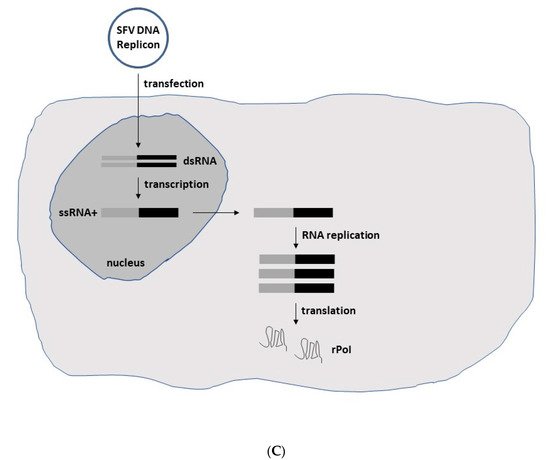
Figure 2. Schematic presentation of SFV expression systems. (A) Replication-deficient recombinant particles. In vitro transcribed RNA molecules from the SFV expression vector carrying the non-structural protein (nsP) genes, replicase genes (replicon) and the gene of interest (GoI) and the structural protein genes (capsid, 6K, envelope E1, E2 and E3) from the helper vector are electroporated or transfected into BHK-21 cells. After RNA replication, only the RNA from the expression vector containing the packaging signal is packaged into nucleocapsids and transported to the plasma membrane, where budding of mature viral particles takes place. Although the generated particles are capable of infecting new host cells, no viral progeny is produced due to the absence of the structural protein genes. However, high-level expression of the recombinant protein of interest (rPoI) takes place (B) Replication-competent recombinant particles. The in vitro transcribed full-length RNA genome with the GoI introduced either downstream of the nsP genes or the structural protein genes is electroporated or transfected into host cells for production of replication-competent viral particles and rPoI expression. (C) DNA replicon vectors. The replacement of the SP6 RNA polymerase promoter by a CMV promoter upstream of the nsP genes allows for direct transfection of host cells for rPoI expression. DNA replicons in the form of DNA plasmids are transfected into host cells, and DNA replicons are delivered to the nucleus. Transcribed ssRNA molecules of positive polarity are delivered to the cytoplasm for RNA replication and expression of the rPoI.
3. Alphavirus-Based Immunotherapy for Cancer
In the context of cancers, alphaviruses have been frequently used for prophylactic and therapeutic applications. Immunization with alphavirus vectors overexpressing tumor-associated antigens (TAAs) has been a common approach for cancer vaccine development. This approach has been used to provide both prevention against tumor challenges and tumor regression and eradication. Moreover, overexpression of cytotoxic and antitumor genes has been evaluated for cancer therapy. The delivery of immunostimulatory genes from alphavirus vectors has served the means of cancer immunotherapy. Moreover, alphaviruses induce apoptosis through activation of caspases in infected cells [15], which has resulted in tumor regression after administration of alphaviruses carrying no therapeutic genes and has allowed the use of vectors with reporter genes to verify and localize expression in animal tumor models. Finally, engineered or naturally occurring oncolytic alphaviruses have demonstrated tumor cell-specific killing in animal models [16]. Examples of cancer vaccinations, cancer therapy and immunotherapy are given below and summarized in Table 1.Table 1. Examples of alphavirus-based vaccines against cancer.
| Cancer | Vector | Finding | Ref |
|---|---|---|---|
| Reporter Genes | |||
| Lung | SFV-EGFP | Tumor regression in mice | [17] |
| Colon | SIN-LacZ | Complete tumor remission | [18] |
| SFV-LacZ RNA | Tumor regression, protection | [19] | |
| TAAs | |||
| Cervical | VEE-HPV-16 E7 | Protection against tumor challenges in mice | [20] |
| SFVenh-HPV E6-E7 | Tumor eradication, long-lasting CTL in mice | [21] | |
| SFV-sHELP-E7SH | Tumor regression, protection in mice | [22] | |
| SFV-HPV E6-E7 DNA | 85% of immunized mice tumor-free | [23] | |
| SFVenh-HPV E6-E7 | Phase I: Immunogenicity in all patients | [24] | |
| Colon | SFV-VEGFR-2 | Inhibition of tumor growth, metastatic spread | [25] |
| SFV-VEGR-2 + SFV-IL-4 | Prolonged survival after coadministration | [25] | |
| VEE-CEA | Phase I: Ag-specific response, long-term survival | [26] | |
| Pancreatic | VEE-CEA | Phase I: Prolonged survival | [27] |
| Melanoma | VEE-TRP-2 + DNA | Superior to plasmid DNA vaccine in mice | [28] |
| VEE-TRP-2 | Humoral immune responses, protection in mice | [29] | |
| VEE-TRP-2 + CTLA-4 mAbs | Tumor regression in 50% of mice | [30] | |
| VEE-TRP-2 + GITR mAbs | Tumor regression in 90% of mice | [30] | |
| SFV-VEGFR-2/IL-12 DNA | Synergistic antitumor activity from combination of | [31] | |
| + SFV-Survivin/β-hCG DNA | DNA replicons | ||
| Ovarian | SFV-OVA + VV-OVA | Immune responses, enhanced antitumor activity | [32] |
| Prostate | VEE-PSMA | Th1-biased immune responses | [33] |
| VEE-PSMA | Phase I: Good safety, weak immunogenicity | [34] | |
| VEE-PSA | PSA-specific Abs, delay in tumor growth | [35] | |
| VEE-mSTEAP + pcDNA | Prolonged survival, tumor challenge protection | [36] | |
| VEE-PSCA | Long-term survival of mice | [37] | |
| Cytotoxic and Antitumor Genes | |||
| Glioblastoma | SFV–Endostatin | Tumor growth inhibition, reduced vascularization | [38] |
| Breast | SIN-HER2/neu DNA | Significant tumor growth inhibition, protection | [39] |
| SIN-HER2/neu DNA | 80% less DNA needed compared to plasmid DNA | [40] | |
| VEE-HER2/neu ECD/TM | Complete prevention of tumors in mice | [41] | |
| VEE-HER2/neu ECD/TM | Safe, PR in 1 patient, SD in 2 patients | [42] | |
| Immunostimulation | |||
| Glioblastoma | SFV-IL-18 + rec IL-12 | Superior therapeutic effect of combination | [43] |
| Glioma | SFV-IL-12 | 70–97% tumor volume reduction in rats | [44] |
| Brain | SIN-gp100 + SIN-IL-12 DNA | Superior antitumor activity, prolonged survival | [45] |
| Breast | SFV-IL-12 + LV101 | Superior antitumor activity of combination | [46] |
| Colon | SFVenh-IL-12 | Complete tumor regression, long-term survival | [47] |
| SFV-IL-12 + anti-PD1 | Superior combination therapy in mice | [48] | |
| VEE-IL-12 + VEE-CEA | Superior combination therapy in mice | [49] | |
| Melanoma | SFV-IL-12 + anti-PD1 | Superior combination therapy in mice | [48] |
| LSFV-IL-12 | Phase I: Good safety and tolerability | [50] | |
| Ovarian | SIN-IL12 + Irinotecan | Long-term survival in 35% of mice | [51] |
| Oncolytic Viruses | |||
| Glioblastoma | SFV-VA-EGFP | Long-term survival in 16 out of 17 mice | [52] |
| Prostate | SFV-VA-EGFP | Complete tumor eradication in mice | [53] |
| Lung | SFV-VA-EGFP | Long-term survival in mice | [8] |
| Liver | M1 | Liver tumor targeting in mice | [54] |
| Glioma | M1 | Replication in tumors | [55] |
| Bladder | M1 | Tumor growth inhibition, prolonged survival | [56] |
| Breast | M1 + Doxorubicin | Reduced tumor growth in mice | [7] |
| Pancreatic | M1 + IRE | Tumor growth inhibition, prolonged survival | [57] |
| Cervical | SIN AR339 | Regression of established tumors in mice | [58] |
| Ovarian | SIN AR399 | Ascites formation in metastasis mouse model | [59] |
Abs, antibodies; anti-PD1, immune checkpoint inhibitor; CEA, carcinoembryonic antigen; CTLA-4, CTL antigen-4; GITR, glucocorticoid-induced tumor necrosis factor; HPV, human papilloma virus; IRE, irreversible electroporation; LV101, Salmonella typhimurium AroC strain; mAbs, monoclonal antibodies; mSTEAP, mouse six-transmembrane epithelial antigen of the prostate; OVA. Ovalbumin; PR, partial response; PSA, prostate-specific antigen; PSMA, prostate-specific membrane antigen; rec IL-12, recombinant IL-12; SD, stable disease; SFV, Semliki Forest virus; SIN, Sindbis virus; TRP-2, tyrosine-related protein-2; VEE, Venezuelan equine encephalitis virus; VEGFR-2, vascular endothelial growth factor receptor-2; VV, vaccinia virus.
