The COVID-19 pandemic has taken healthcare providers by storm, particularly regarding critically ill patients who are hospitalized in intensive care units (ICU). During the earlier epidemic of severe acute respiratory syndrome (SARS), caused by the closely related SARS-CoV-1 virus, few studies evaluated some of this disease’s endocrine aspects. The unprecedented scale of the current SARS-CoV-2/COVID-19 pandemic has also led to an extensive—yet fragmented—assessment of its endocrine repercussions; in many reports, the endocrine aspects of COVID-19 are lumped together in ICU and non-ICU patients.
2. Non-COVID-19 Critical Illness
The central nervous system, the endocrine system, and the immune system work together to mount the body’s response to stress and harmful stimuli. Briefly, the endocrine changes of the acute phase of the response to stress involve the secretion of catecholamines, changes in the pulsatility of growth hormone (GH) secretion, an increase in cortisol, changes in thyroid hormones, the reduction in the concentration of sulfate dehydroepiandrosterone, the lowering of luteinizing hormone, an increase in prolactin, and increased resistance to insulin.
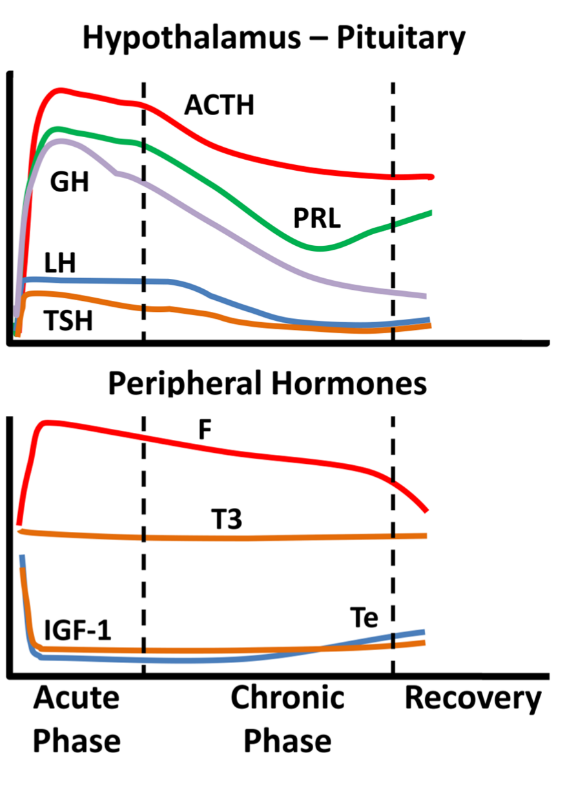
Critical illness entails considerable stress to patients; the body’s reaction includes an immediate increase in pituitary stress hormones, adrenocorticotropin (ACTH), GH, and prolactin (Figure 1).
Figure 1. Schematic endocrine changes over time in critically ill patients; ACTH: adrenocortocotropin, GH: growth hormone, LH: luteinizing hormone, TSH: thyrotropin, PRL: prolactin, F: cortisol, T3: triiodothyronine, IGF-1: insulin-like growth factor 1, Te: testosterone.
In the acute phase of critical illness (days 1 to 7) mainly free triiodothyronine (FT3) decreases while in the chronic phase reduction in both thyrotropin (TSH) and free thyroxine (FT4) are observed. In the acute phase of critical illness, a dramatic increase in GH with a simultaneous decrease in insulin-like growth factor 1 (IGF-1) levels are noted. In the chronic phase of critical illness (after 7 days), central suppression of the growth axis is also observed, with a relative decrease in GH in addition to IGF-1. Testosterone is dramatically reduced and relatively elevated estradiol levels have been associated with a worse prognosis. In the chronic phase, a central suppression of the hypothalamus-pituitary-gonadal axis (HPG) is also observed with a decrease in LH. Prolactin increases in the acute phase and decreases in the chronic phase. In the acute phase, a significant increase in ACTH and cortisol is observed. In the chronic phase, a relative decrease in ACTH levels is observed whereas cortisol levels stabilize
[1]. The acute reduction in binding proteins (TBG, CBG, SHBG, IGFPs) in critically ill patients affects the measurement of total hormones (cortisol, T3, T4, IGF-1, sex steroids) but also the action and clearance of their free moieties.
Of particular interest in critically ill patients is the hypothalamic-pituitary-adrenal axis (HPA). Accumulated evidence indicates that the noted increase in cortisol in such patients is in fact the result of low cortisol along with reduced cortisol breakdown, in lieu of increased cortisol production. In critically ill patients, the expression of glucocorticoid receptors is diminished over time. Pro-opiomelanocortin (the precursor of ACTH) is increased and may “whip” the adrenal cortex to produce some cortisol. In the chronic phase of critical illness, low ACTH, often due—in part—to the administration of corticosteroids, is associated with poor prognosis. A caveat regarding the assessment of adrenocortical reserve in ICU patients is that, despite earlier encouraging results, the cosyntropyn test may not be as reliable as previously thought in assessing adrenal reserve, due to the increased distribution volume of cortisol.
One-quarter of critically ill patients without prior history of diabetes have stress hyperglycemia
[2]; the latter is attributed to activation of the HPA axis, increased insulin resistance, and release of proinflammatory cytokines.
Regarding prognosis, some endocrine parameters were associated with critical illness assessment tools’ scores, although there are caveats to be considered, especially as far as the HPA axis in general, and cortisol in particular, are concerned (please see above). Most studies have honed in on cortisol or thyroid hormones. Cortisol has been associated with the APACHE II tool score and the Glasgow Coma Scale score; the latter has also been associated with levels of thyroid hormones. The prognostic value of adrenal androgens, prolactin, or gonadotropins has been explored to a limited extent. Prolactin has been correlated with the Glasgow Coma Scale, luteinizing hormone (LH) but not follicle-stimulating hormone (FSH) was associated with APACHE II, whereas adrenal androgens could be prognostic in septic shock.
3. Endocrine Aspects of SARS-CoV-1 Infection
Scant data in a few published reports have been put forth regarding endocrine aspects of the serious infection caused by SARS-CoV-1. Low TSH, T3, cortisol, and estradiol as well as elevated prolactin (PRL), FSH, and LH blood levels have been reported. The thyroid was found to show extensive damage by the virus (with increased apoptosis and disturbed microarchitecture in follicular cells and absence of parafollicular cells), whereas in the pituitary glands of five patients, scant positive cells and diminished staining intensity of immunoreactivity for GH, TSH, and ACTH were noted; the inverse was noted for PRL, FSH, and LH. The relationship between SARS-CoV-1 and the adrenal glands is well-proven (the virus was found in the adrenal glands during autopsy studies). After recovery from infection, low ACTH and cortisol were noted in some patients. Nevertheless, this finding could not be ruled out as being an after-effect of glucocorticoid administration during active SARS-CoV-1 disease. Glucocorticoid administration could also be implicated in cases of patients with femoral neck osteonecrosis.
Acute onset diabetes/hyperglycemia was observed in half of the patients in a small series with SARS-CoV-1 infection; the ACE2-virus-attaching protein has been found in pancreatic islets. Apparently, pancreatic islet cell function was compromised in the studied patients; in most of them normal glycemia was noted after three years of follow-up .
The endocrine aspects of patients with SARS are summarized in Table 1.
Table 1. Summary of endocrine aspects of patients with SARS.
TSH: Thyroid Stimulating Hormone; T3: Triiodothyronine; F: Cortisol; ACTH: Adrenocorticotropic Hormone; E2: Estradiol; PRL: Prolactin; FSH: Follicle Stimulating Hormone; LH: Luteinizing Hormone; GH: Growth Hormone.
4. Endocrine Aspects of SARS-CoV-2 Infection
The thyroid gland and its function are among the most researched of endocrine aspects of Covid-19. A high serum cortisol level (above 31 μg/dL; 855 nmol/L) upon ICU admission was reported to be the harbinger of worse prognosis for Covid-19 patients. This is in contrast to a study, where cortisol was low in six of nine critically ill patients with Covid-19. The researchers' team has found that the expression of glucocorticoid receptor alpha is increased in ICU hospitalized Covid-19 patients compared to non-Covid-19 cases. Male sex lends susceptibility to more severe Covid-19; this is attributed to direct gender-hormonal status and indirectly to gender-associated behaviors (for instance women are more prone to seek medical help in case of illness compared to men). Few studies have honed on the growth axis in critically ill Covid-19 patients. Lower IGF-1 levels were found in ICU patients compared to mildly ill patients; the levels of IGF-1 were negatively associated with interleukin-6 (IL6) levels (which served as a measure of disease severity) and IGF-1 was lower in critically ill patients who did not survive compared to survivors. A field of intense study vis-à-vis Covid-19 is (OH)25-vitamin-D3 (VD3). Maintenance of VD3 within normal limits is advised, in case of known nutritional deficiencies. Patients with Covid-19 and pre-existing diabetes, compared to those without diabetes, are more prone to be hospitalized, be admitted to ICU/be mechanically ventilated or even die. Additionally, transient hyperglycemia has been noted in critically ill patients with Covid-19, even in the absence of prior diagnosis of diabetes.
The endocrine aspects of ICU-hospitalized patients with COVID-19 are summarized in Table 2.
Table 2. Summary of endocrine aspects of ICU-hospitalized patients with COVID-19.
|
Affected Axis
|
|
|
Hypothalamic–pituitary–thyroid
|
Low T3 or free T3, low free T4 and low TSH;
NTIS or Thyrotoxicosis
|
|
Hypothalamic–pituitary–growth
|
Low IGF-1;
Low IGF-1 and low GH associated with worse prognosis
|
|
Hypothalamic–pituitary–adrenal
|
High or low F
|
|
Hypothalamic–pituitary–gonadal
|
In women with high Te: worse prognosis;
In men: high total Te or lower Te than control patients; low free Te and bioavailable Te, high E2
|
|
Glucose-insulin
|
Acute onset transient diabetes/hyperglycemia;
Impaired beta cell function
|
|
Vitamin D
|
Low (OH)25-vitamin-D3
|
TSH: Thyroid Stimulating Hormone; T4: Thyroxine; T3: Triiodothyronine; NTIS: Non-thyroidal illness syndrome; F: Cortisol; E2: Estradiol; FSH: Follicle Stimulating Hormone; LH: Luteinizing Hormone; GH: Growth Hormone; IGF-1: Insulin like Growth factor-1; Te: Testosterone.