Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Yingjie Li | -- | 2859 | 2022-10-15 04:33:25 | | | |
| 2 | Jessie Wu | Meta information modification | 2859 | 2022-10-17 04:35:40 | | | | |
| 3 | Jessie Wu | Meta information modification | 2859 | 2022-10-17 04:38:57 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Lei, Y.; Zhang, E.; Bai, L.; Li, Y. Autophagy in Tumor Immune System. Encyclopedia. Available online: https://encyclopedia.pub/entry/29447 (accessed on 07 March 2026).
Lei Y, Zhang E, Bai L, Li Y. Autophagy in Tumor Immune System. Encyclopedia. Available at: https://encyclopedia.pub/entry/29447. Accessed March 07, 2026.
Lei, Yuhe, Enxin Zhang, Liangliang Bai, Yingjie Li. "Autophagy in Tumor Immune System" Encyclopedia, https://encyclopedia.pub/entry/29447 (accessed March 07, 2026).
Lei, Y., Zhang, E., Bai, L., & Li, Y. (2022, October 15). Autophagy in Tumor Immune System. In Encyclopedia. https://encyclopedia.pub/entry/29447
Lei, Yuhe, et al. "Autophagy in Tumor Immune System." Encyclopedia. Web. 15 October, 2022.
Copy Citation
Autophagy is a stress-induced process that eliminates damaged organelles and dysfunctional cargos in cytoplasm, including unfolded proteins. Autophagy is involved in constructing the immunosuppressive microenvironment during tumor initiation and progression. It appears to be one of the most common processes involved in cancer immunotherapy, playing bidirectional roles in immunotherapy. Accumulating evidence suggests that inducing or inhibiting autophagy contributes to immunotherapy efficacy. Exploring autophagy targets and their modifiers to control autophagy in the tumor microenvironment is an emerging strategy to facilitate cancer immunotherapy.
autophagy
cancer
immunotherapy
1. Introduction
Autophagy in cancer cells often occurs in response to cytokines and damage-associated molecular patterns (DAMPs) derived from the tumor microenvironment (TME). Several pattern recognition receptors have been verified as autophagy inducers when they receive extracellular DAMPs, including ATP, DNA complexes, and high-mobility group box 1 protein (HMGB1). [1]. For example, polyinosinic:polycytidylic acid and lipopolysaccharide (LPS) activate Toll-like receptor 3 (TLR3) and TLR4, thus triggering autophagy in non-small cell lung cancer (NSCLC) cells [2]. Another study demonstrated that advanced glycosylation end product-specific receptor (AGER) is crucial for HMGB1-induced autophagy in pancreatic and colon cancer cells [3]. As for cytokines, they participate in autophagy initiation in a context-dependent way. Accumulated evidence suggests that transforming growth factor-β (TGF-β) and interferons (type I and II) induce autophagy in various kinds of cancer cells [4]. For instance, TGF-β activates autophagy in human hepatocellular carcinoma cells by upregulating the levels of Beclin 1, Atg5 and Atg7 [5]. In addition, tumor necrosis factor (TNF) and interleukin-6 (IL-6) were found to be autophagy inducers that are associated with carcinogenesis and tumor progression [6]. As a major mechanism of autophagy-associated tumor progression, the immunosuppressive microenvironment shaped by autophagy is researcher's focus. The immune system is essential for preventing the development and metastasis of tumors and for shaping the tumor response to treatment. The immune system recognizes and eliminates tumor cells by immune surveillance. However, some tumor cells survive because of immune evasion, a process where the immunogenicity of tumor cells is reduced, and the immunosuppressive networks are formed [7]. In TME, changes in the autophagy pathway are observed in cancer cells and immune cells, which shape tumor immunity and affect immunotherapy [8].
The role of autophagy in immune system activation and immunosuppressive TME formation is complex. On the one hand, autophagy may participate in the process of antigen presentation by APCs through proteasome degradation and the vacuolar pathway [9]. Autophagic degradation can promote APC efficiency by accelerating antigen processing [10]. Autophagy deficiency in pancreatic ductal adenocarcinoma stimulates PD-L1 expression, which may aid in the formation of immunosuppressive TME [11]. Similarly, autophagy inhibition can upregulate PD-L1 expression by accumulating p62/SQSTM1 and activating NF-κB in gastric cancer [12] (Figure 1a). Autophagy contributes to immune evasion by suppressing the innate and adaptive immune response. The inducers of the innate immune response, such as damaged proteins, organelles, and bacteria, are cleared by autophagy. DAMPs and pathogen-associated molecular patterns (PAMPs) are both inducers of innate immunity, which can be captured and degraded in autolysosomes [13]. These facts indicate that autophagy seems to be a key negative regulator of innate immune responses when activated [14]. As a consequence, inhibition of autophagy facilitates the production and secretion of proinflammatory cytokines such as IFNs (type I, II) and TNF-α in vitro and in vivo [15]. Inhibition of autophagy can also trigger programmed cell death of cancer cells, producing DAMPs to activate the adaptive immune system [16]. Moreover, autophagy can suppress the adaptive immune response by weakening T cells’ ability to kill tumor cells [17]. It has been shown that antigenic tumors are recognized and eliminated by T cells when autophagy is inhibited in mice [18]. There are several mechanisms by which autophagy restrains the antitumor T cell response. Autophagy suppresses the functions of NK cells, CD4+ T cells, and CD8+ T cells to help tumor cells combat immunosurveillance, acting as a protective mechanism in tumors [19]. For example, hypoxia-induced autophagy can impair NK cell-mediated killing of breast cancer cells by degrading NK-derived granzyme B in autophagosomes [20] (Figure 1b). Hypoxia-induced autophagy is also reported to attenuate cancer cell sensitivity to CTL by regulating the STAT3 pathway in IGR-Heu lung carcinoma cells [21]. Hence, autophagy in tumors may be a potential target to block immune evasion in the TME. Meanwhile, the initiation of immune response often needs a major histocompatibility complex (MHC) to present antigens to T cells. MHC includes class I and II, that are recognized by CD4+ and CD8+ T cells, respectively [22]. MHC-I presents peptides that prime and activate CD8+ CTLs. Then, CTLs clear the targeted tumor cells by detecting a matching antigen by the MHC-I [23]. The expression of MHC-I is downregulated in pancreatic cancer, resulting in defective antigen presentation, which limits the ability of tumor killing by T cells and immunotherapy. Mechanistically, MHC-I is recognized by NBR1 and degraded in lysosomes through autophagy [18] (Figure 1c). Additionally, in dendritic cells (DCs), autophagy has been shown to accelerate the internalization and degradation of MHC-I. DCs with autophagy inhibition enhance the presentation of viral antigens to CD8+ T cells [24][25]. Recently, hepatic autophagy immune tolerance (HAIT) has been studies and is regarded as another immune evasion mechanism. A study by Laura et al. revealed that autophagy in the liver induces tumor immune tolerance by promoting Treg function and inhibiting T-cell response and interferon-γ production, resulting in the growth of tumors with high tumor mutational burden (TMB). Therefore, autophagy may be a potential target to overcome HAIT [15].
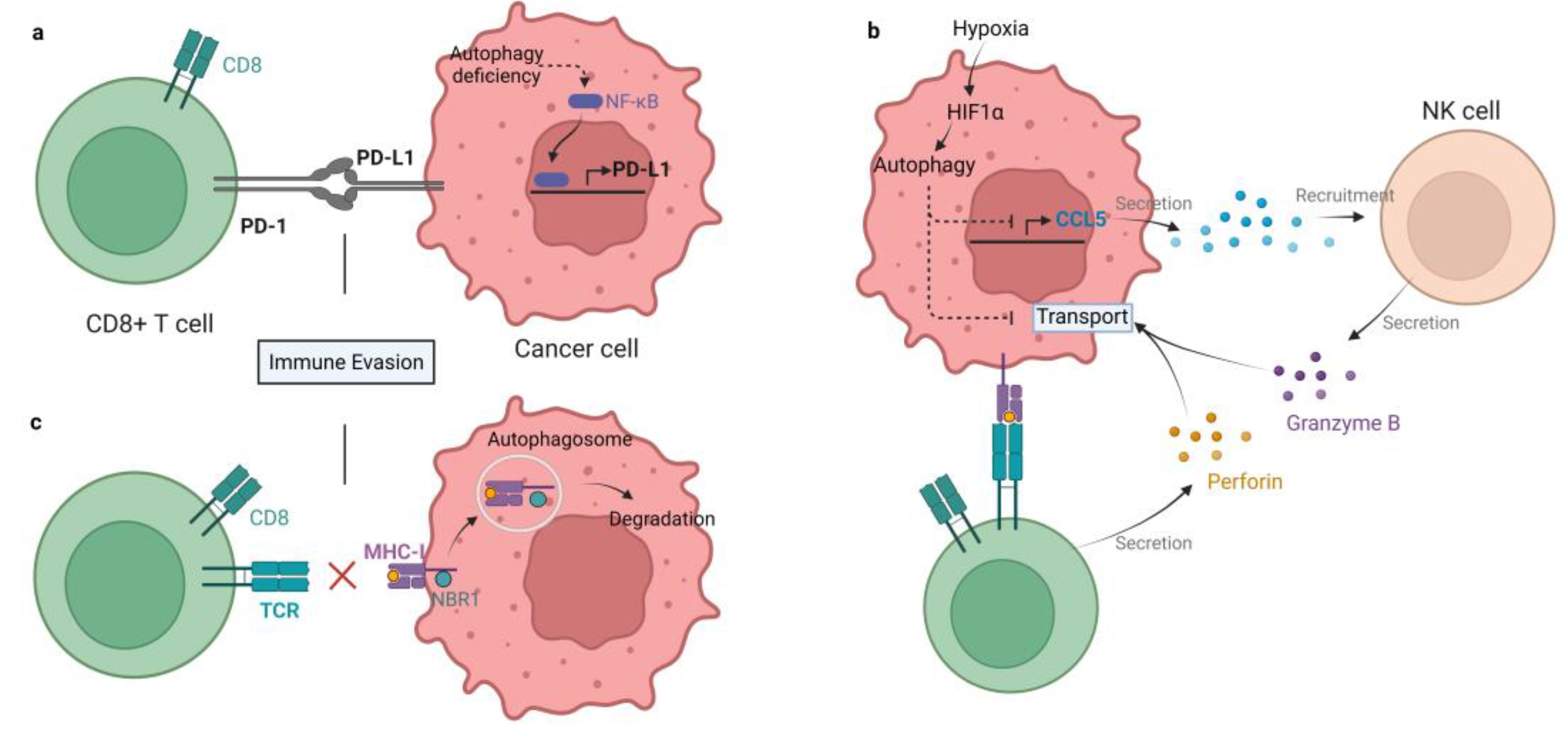
Figure 1. Mechanisms of autophagy-mediated immune evasion. (a) Autophagy deficiency in gastric cancer enhances PD-L1 expression by activating NF-κB, therefore promoting PD1/PD-L1-induced immune evasion. (b) Hypoxia-induced autophagy impairs killing of breast cancer by degrading NK and CD8+ T cell-derived granzyme B in autophagosomes. In addition, autophagy inhibits chemokine CCL5 production. As a result, the recruitment of NK cells to the TME is blocked. (c) MHC-I is recognized by NBR1 and degraded in lysosomes by autophagy in pancreatic cancer cells, which impairs antigen presentation and tumor killing by T cells.
2. Autophagy in Immune Cells
Tumor development and therapeutic response are influenced by tumor-infiltrating immune cells in TME [26]. A growing number of studies have revealed that autophagy is involved in the differentiation, homeostasis, and development of immune cells [27]. Hence, the alterations of autophagy pathway in immune cells may shape their phenotypes and functions in the TME [28].
2.1. Autophagy in T cells
Autophagy plays a vital role in the survival and proliferation of T cells. In TME of tumor-bearing mice and cancer patients, T cells undergo apoptosis. Xia et al. found that tumor-infiltrating T cells exhibit defective autophagy and a decreased level of FIP200, which is necessary for autophagosome formation. This process is mediated by tumor-derived lactate, which lowers FIP200 expression and causes T cell death by disrupting the balance between pro- and anti-apoptotic factors in the Bcl-2 family to boost immune evasion of cancer [29] (Figure 2). The interactions of T cell receptor (TCR) with stromal cells and IL-7 signaling are essential for naive T cells to survive in the periphery, and it appears that Atg3-dependent autophagy is intrinsically required for these processes [30]. Atg7-deficient T cells have poor proliferative capacity and cannot enter the S phase when TCR is activated. Mechanistically, this is attributed to the accumulation of CDKN1B, which cannot be degraded in autophagy-deficient naive T cells, and negatively regulates cell cycle [31]. Defective autophagy can also disturb T cell activation, differentiation, and stemness. It has been reported that the differentiation from T cells to invariant natural killer T (iNKT) and Treg is facilitated by autophagy [32]. Yang et al. found that deletion of autophagy-related protein PIK3C3/VPS34 reduced the activity of mitochondria upon T cell activation, so that CD4+ T cells failed to differentiate into T helper 1 cells [33]. Similarly, the quality of mitochondria declines when the mitochondrial components cannot be degraded adequately, resulting in ROS accumulation and T cell damage [34]. Depolarized mitochondria were also detected in IL-15-induced resident memory CD8+ T cells with autophagy inhibited by MRT68921 dihydrochloride and 3-methyladenine (3MA), causing T cells exhaustion [35]. CD4+ Treg cells in TME confer another primary mechanism of immune evasion and immunotherapy resistance. Autophagy was shown to participate in maintaining Treg cell function [36]. Inhibition of autophagy by Atg5, Atg7 and AMBRA1 deletion induces Treg cell apoptosis and dysfunction in vivo [37][38][39] (Figure 2).
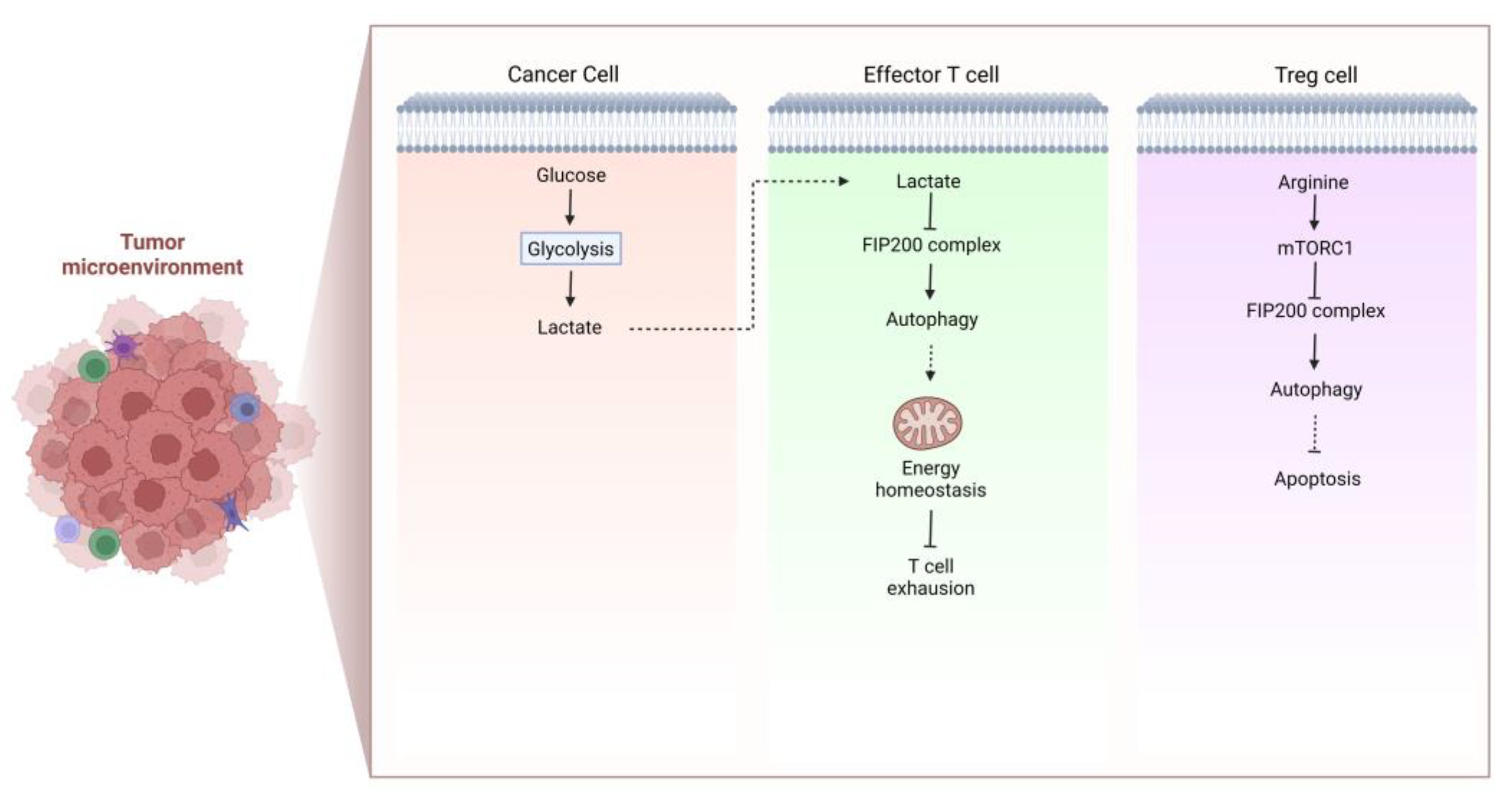
Figure 2. The formation of immunosuppressive TME. Cancer cell-derived lactate lowers FIP200 expression and attenuates autophagy, which is pivotal for energy homeostasis of effector T cells, thus facilitating T cell exhaustion and formation of immunosuppressive TME. In Treg cells, decreased intracellular arginine suppresses mTOR, leading to autophagy activation that is responsible for Treg cell survival. As a consequence, the function of Treg cell is improved to form the immunosuppressive TME.
Autophagy also serves a crucial function in other types of immune cells that interact with T cells. Lysosomal proteolysis of autophagy is necessary for the antigen presentation in dendritic cells [40]. Deleting PIK3C3 (a key player in autophagy) in dendritic cells contributes to reduced CD8α+ dendritic cells and B16 melanoma-specific CTL activity [25]. Myeloid-derived suppressor cells (MDSCs) dampen antitumor immune responses and treatment effectiveness by directly suppressing T cell activation in TME [41]. An elevated level of autophagy has been detected in MDSCs [42]. Blocking autophagy in MDSCs leads to increased MHC-II expression and impaired tumor growth in vivo [43].
2.2. Autophagy in B cells
The survival and development of B cells depends on autophagy. For instance, the development of B cells depends on Atg5 [44]. After ligand LPS activation, basal levels of autophagy are required to preserve the survival of a normal number of peripheral B cells [45]. Intrinsic autophagy in B cells is necessary to sustain the normal function of alloreactive B memory cells. Atg7 deletion in B cell blocks B cell autophagy, inhibiting secondary alloantibody responses and decreasing the frequencies of alloreactive B memory cells [46].
2.3. Autophagy in Natural Killer (NK) Cells
The survival of iNKT cells requires intrinsic autophagy. Facing viral infection, autophagy is activated with the aid of phosphorylated FoxO1 and Atg7, which promotes the development and function of NKT cells against viral infection [47]. Autophagy-deficient iNKT cells undergo apoptosis in the mitochondrial pathway. Absence of autophagy not only hinders the maturation of iNKT cells by reducing NKT cell proliferation, but also limits the transition of iNKT cells to a quiescent state [48]. Moreover, the secretion of iNKT can be affected by autophagy. Autophagy-deficient iNKT cells release low levels of IL-4 and IFN-γ compared with normal iNKT cells [49].
2.4. Autophagy in Dendritic Cells (DCs)
Autophagy is indispensable for cytokine secretion and antigen presentation in dendritic cells (DCs). It has been reported that Atg5 is essential for DCs to activate CD4+ T cells through antigen presentation [47][48]. Atg5 deficiency in DCs reduces the secretion of IL-2 and IFN-γ by CD4+ T cells, but the levels of IL-12, IL-6, and TNF-α are not affected [50]. Moreover, antigen presentation was hampered by Atg5 deficiency through the MHC-II pathway, which may be resulted from the delay of lysosome-phagosome fusion [47][48][49][50][51][52].
2.5. Autophagy in Macrophages
The generation of macrophages requires autophagy at different stages. It is well accepted that tumor-associated macrophages (TAMs) originate from monocytes. The recruitment of monocytes to tumors requires chemokines and cytokines derived from tumor cells and stromal cells. One of the chemokines, [C-C motif] ligand 2 (CCL2), protects monocytes from apoptosis and induces a high level of autophagy in TAMs [53]. The transition from monocytes into macrophages requires colony-stimulating factor 1 (CSF1), which upregulates and activates ULK1 to trigger autophagy [54]. CSF2 also facilitates the differentiation of monocytes. Autophagy in monocytes maintains a high-level during differentiation, since JNK signaling is activated to release Beclin 1 and inhibits Atg5 cleavage. Autophagy inhibition restrains the differentiation process [55]. In addition, autophagy is involved in macrophage polarization. Downregulated autophagy promotes M1 polarization and upregulated autophagy promotes M2 polarization in macrophages [56]. M1 macrophages stimulate immune responses, whereas M2 macrophages play an immunosuppressive role, indicating that autophagy is a potential target to modulate macrophage polarization toward the M1 phenotype [57].
3. Autophagy in Regulating Immune Checkpoint Molecules
Immune checkpoint molecules, including PD-1 and CTLA-4, play crucial roles in tumor immune tolerance, which is the main reason for the poor clinical outcomes of immunotherapy. These immune checkpoint molecules regulate tumor immune tolerance through autophagy. For example, activation of PD-1 by PD-L1 promotes immunologic tolerance, suppresses effector T cells and maintains tumor Tregs, boosting tumor survival [58]. Hence, elucidating the regulation of immune checkpoints by autophagy is of great significance in immunotherapy.
As a T cell checkpoint molecule, PD-1 inhibits T cell proliferation and impedes the recognition of tumor cells once activated by PD-L1. Clark et al. found that tumor cells initiate autophagy in response to anti-PD1 or anti-PD-L1 antibody treatment. Engagement of PD-1 to PD-L1 induces autophagy in nearby T cells [59]. A study indicated that inhibition of autophagy by the Sigma1 inhibitor leads to degradation of PD-L1 and impaired PD-1/PD-L1 interaction, suggesting the Sigma1 inhibitor as a promising tool to block PD-1/PD-L1 [60]. Therefore, combining antibodies with autophagy inhibitors may be an attractive therapeutic strategy [61].
CTLA-4 was confirmed to be another immune checkpoint molecule that mediates tumor immune tolerance. Shukla et al. identified a subcluster of MAGE-A cancer-germline antigens that confer resistance to the CTLA-4 blockade. Autophagy activation combats this resistance by decreasing MAGE-A protein levels in human melanomas [62]. However, another study reported that mTORC1 inhibitor-induced autophagy restores CTLA-4 expression and corrects Treg cell function in systemic lupus erythematosus (SLE) [63]. CTLA4 engagement inhibits autophagy by activating PI3K/Akt/mTOR signaling pathway [64].
IDO promotes immunologic tolerance by suppressing responses of CTLs and maturation of DCs, magnifying tolerogenic APCs, and inducing Tregs generation. Autophagy can prevent inflammation-induced IDO synthesis by reducing inflammation [65]. IDO triggers the activation of general control nonderepressible 2 (GCN2) and inhibition of eukaryotic translation initiation factor 2α (eIF2α), which participate in inflammatory carcinogenesis [66]. Autophagy induced by IDO1-GCN2 plays a protective role against fatal inflammation disease [67]. Another mechanism of IDO-induced autophagy has been reported. The inhibition of tryptophan sufficiency signaling by IDO causes mTOR inactivation, thus triggering autophagy in an LC3-dependent way [66].
4. Autophagy in Immune Cytokines
Autophagy can increase or inhibit cytokine production and affect tumor progression. Autophagy and cytokines can regulate each other. Numerous cytokines have been demonstrated to govern or be governed by autophagy.
4.1. Interleukins
IL-1 is a class of pro-inflammatory cytokines that promotes cancer progression by inhibiting COX-1, IkB, and JNK signaling pathways. Inhibition of IL-1 in tumor cells restrains tumor development [68]. IL-1β is negatively regulated by autophagy in most situations, whereas IL-1α is only negatively regulated by autophagy [69]. However, both IL-1α and IL-1β triggers autophagy, indicating that autophagy is a negative feedback mechanism in regulating IL-1 [70]. Autophagy activation in Atg5-deficient macrophages causes decreased IL-1β secretion, suppressing T cell activation and cytokine production [50]. Conversely, IL-1 level is elevated in autophagy-deficient macrophages to promote tumorigenesis through ROS-NF-κB signaling pathway [71]. IL-2 induces autophagy in an ATG5, HMGB1, and Beclin1-dependent way [72]. High-dose interleukin-2 (HDIL-2) inhibits the growth of metastatic liver tumors in vivo, accompanied by elevated levels of HMGB1, IFN-γ, IL-6, and IL-18 in serum. HMGB1 is translocated from the nucleus to the cytosol to trigger autophagy, and suppression of autophagy by CQ may enhance the proliferation and infiltration of immune cells in the liver and spleen [73]. IL-6 negatively regulates autophagy by activating STAT3 and inhibiting LC3-II and Beclin 1 expression in vitro [74]. However, IL-6 trans-signaling stimulates autophagy when IL-6 interacts with soluble IL-6R in vivo, which is mediated by AMPK and AKT activation [75]. In addition, IL-10 is also an autophagy suppressor by activating the JAK/STAT3 and PI3K/Akt/mTORC1 pathway [76]. As Th2 cytokines, IL-4, IL-13, and IL-10 can activate the PI3K/mTORC1 pathway to inhibit autophagy in most environments [77]. In turn, autophagy may exert dual effects on the production of IL-10, which needs further investigation [78].
4.2. Interferons
Autophagy is needed in the synthesis of IFN-γ. IFN can be categorized into type I, II, and III. Type I IFN directly induces autophagy by activating JAK/STAT pathway [79]. IFN-α is a class of type I IFN, and the effect of IFN-α varies on different cell types [80]. As a type II IFN, IFN-γ induces autophagy in various types of immune cells and tumor cells, which is mediated by the acceleration of autophagosome formation and maturation [81]. In addition, IFN-γ can facilitate the MHCI expression and induce autophagy [82]. It has been reported that effector CD4+ T cells lacking Atg7 express low amounts of IFN-γ in the absence of autophagy [83].
4.3. Transforming Growth Factor Beta (TGF-β)
TGF-β contributes to immune evasion by directly suppressing effector cells and indirectly facilitating the differentiation of Treg cells. TGF-β can also inhibit NK cells and IFN-γ to form immunosuppressive TME [84]. Inhibition of autophagy upregulates TGF-β expression by impairing its degradation [85]. In turn, TGF-β is responsible for autophagy induction in cancer cells [80]. For example, TGF-β induces autophagy in HCC and breast cancer cells by increasing the levels of ATGs, including Beclin1, Atg5, and Atg7. Autophagy promotes the expression of proapoptotic genes such as Bim and Bmf in the Bcl-2 family to mediate apoptosis, suggesting the correlations between autophagy and apoptosis [86][87].
4.4. Tumor Necrosis Factor Alpha (TNF-α)
TNF-α is an apoptosis and necrosis inducer in multiple cells and can impair autophagy by decreasing lysosomal acidification [88]. Inhibition of autophagy by Bafilomycin A1 contributes to TNF-α-induced cell death by increasing oxidative stress and toxicity, indicating that autophagy is a negative regulator of TNF-α [89]. A mechanism study revealed that autophagy inhibits TNF-α expression through p38MAPK dephosphorylation and TRAF6 downregulation [90].
References
- Ma, Y.; Galluzzi, L.; Zitvogel, L.; Kroemer, G. Autophagy and cellular immune responses. Immunity 2013, 39, 211–227.
- Zhan, Z.; Xie, X.; Cao, H.; Zhou, X.; Zhang, X.D.; Fan, H.; Liu, Z. Autophagy facilitates TLR4- and TLR3-triggered migration and invasion of lung cancer cells through the promotion of TRAF6 ubiquitination. Autophagy 2014, 10, 257–268.
- Tang, D.; Kang, R.; Cheh, C.W.; Livesey, K.M.; Liang, X.; Schapiro, N.E.; Benschop, R.; Sparvero, L.J.; Amoscato, A.A.; Tracey, K.J.; et al. HMGB1 release and redox regulates autophagy and apoptosis in cancer cells. Oncogene 2010, 29, 5299–5310.
- Schmeisser, H.; Fey, S.B.; Horowitz, J.; Fischer, E.R.; Balinsky, C.A.; Miyake, K.; Bekisz, J.; Snow, A.L.; Zoon, K.C. Type I interferons induce autophagy in certain human cancer cell lines. Autophagy 2013, 9, 683–696.
- Kiyono, K.; Suzuki, H.I.; Matsuyama, H.; Morishita, Y.; Komuro, A.; Kano, M.R.; Sugimoto, K.; Miyazono, K. Autophagy is activated by TGF-beta and potentiates TGF-beta-mediated growth inhibition in human hepatocellular carcinoma cells. Cancer Res. 2009, 69, 8844–8852.
- Katheder, N.S.; Khezri, R.; O’Farrell, F.; Schultz, S.W.; Jain, A.; Rahman, M.M.; Schink, K.O.; Theodossiou, T.A.; Johansen, T.; Juhasz, G.; et al. Microenvironmental autophagy promotes tumour growth. Nature 2017, 541, 417–420.
- Zou, W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat. Rev. Cancer 2005, 5, 263–274.
- Xia, H.; Green, D.R.; Zou, W. Autophagy in tumour immunity and therapy. Nat. Rev. Cancer 2021, 21, 281–297.
- Semmling, V.; Lukacs-Kornek, V.; Thaiss, C.A.; Quast, T.; Hochheiser, K.; Panzer, U.; Rossjohn, J.; Perlmutter, P.; Cao, J.; Godfrey, D.I.; et al. Alternative cross-priming through CCL17-CCR4-mediated attraction of CTLs toward NKT cell-licensed DCs. Nat. Immunol. 2010, 11, 313–320.
- Li, H.; Li, Y.; Jiao, J.; Hu, H.M. Alpha-alumina nanoparticles induce efficient autophagy-dependent cross-presentation and potent antitumour response. Nat. Nanotechnol. 2011, 6, 645–650.
- Yang, S.; Imamura, Y.; Jenkins, R.W.; Canadas, I.; Kitajima, S.; Aref, A.; Brannon, A.; Oki, E.; Castoreno, A.; Zhu, Z.; et al. Autophagy Inhibition Dysregulates TBK1 Signaling and Promotes Pancreatic Inflammation. Cancer Immunol. Res. 2016, 4, 520–530.
- Wang, X.; Wu, W.K.K.; Gao, J.; Li, Z.; Dong, B.; Lin, X.; Li, Y.; Li, Y.; Gong, J.; Qi, C.; et al. Autophagy inhibition enhances PD-L1 expression in gastric cancer. J. Exp. Clin. Cancer Res. 2019, 38, 140.
- Deretic, V.; Levine, B. Autophagy balances inflammation in innate immunity. Autophagy 2018, 14, 243–251.
- Mathew, R.; Khor, S.; Hackett, S.R.; Rabinowitz, J.D.; Perlman, D.H.; White, E. Functional role of autophagy-mediated proteome remodeling in cell survival signaling and innate immunity. Mol. Cell 2014, 55, 916–930.
- Poillet-Perez, L.; Sharp, D.W.; Yang, Y.; Laddha, S.V.; Ibrahim, M.; Bommareddy, P.K.; Hu, Z.S.; Vieth, J.; Haas, M.; Bosenberg, M.W.; et al. Autophagy promotes growth of tumors with high mutational burden by inhibiting a T-cell immune response. Nat. Cancer 2020, 1, 923–934.
- Yatim, N.; Cullen, S.; Albert, M.L. Dying cells actively regulate adaptive immune responses. Nat. Rev. Immunol. 2017, 17, 262–275.
- DeVorkin, L.; Pavey, N.; Carleton, G.; Comber, A.; Ho, C.; Lim, J.; McNamara, E.; Huang, H.; Kim, P.; Zacharias, L.G.; et al. Autophagy Regulation of Metabolism Is Required for CD8(+) T Cell Anti-tumor Immunity. Cell Rep. 2019, 27, 502–513.e5.
- Yamamoto, K.; Venida, A.; Yano, J.; Biancur, D.E.; Kakiuchi, M.; Gupta, S.; Sohn, A.S.W.; Mukhopadhyay, S.; Lin, E.Y.; Parker, S.J.; et al. Autophagy promotes immune evasion of pancreatic cancer by degrading MHC-I. Nature 2020, 581, 100–105.
- Garg, A.D.; Dudek, A.M.; Agostinis, P. Autophagy-dependent suppression of cancer immunogenicity and effector mechanisms of innate and adaptive immunity. Oncoimmunology 2013, 2, e26260.
- Baginska, J.; Viry, E.; Berchem, G.; Poli, A.; Noman, M.Z.; van Moer, K.; Medves, S.; Zimmer, J.; Oudin, A.; Niclou, S.P.; et al. Granzyme B degradation by autophagy decreases tumor cell susceptibility to natural killer-mediated lysis under hypoxia. Proc. Natl. Acad. Sci. USA 2013, 110, 17450–17455.
- Noman, M.Z.; Janji, B.; Kaminska, B.; Van Moer, K.; Pierson, S.; Przanowski, P.; Buart, S.; Berchem, G.; Romero, P.; Mami-Chouaib, F.; et al. Blocking hypoxia-induced autophagy in tumors restores cytotoxic T-cell activity and promotes regression. Cancer Res. 2011, 71, 5976–5986.
- Zhong, Z.; Sanchez-Lopez, E.; Karin, M. Autophagy, Inflammation, and Immunity: A Troika Governing Cancer and Its Treatment. Cell 2016, 166, 288–298.
- Blum, J.S.; Wearsch, P.A.; Cresswell, P. Pathways of antigen processing. Annu. Rev. Immunol. 2013, 31, 443–473.
- Loi, M.; Muller, A.; Steinbach, K.; Niven, J.; Barreira da Silva, R.; Paul, P.; Ligeon, L.A.; Caruso, A.; Albrecht, R.A.; Becker, A.C.; et al. Macroautophagy Proteins Control MHC Class I Levels on Dendritic Cells and Shape Anti-viral CD8(+) T Cell Responses. Cell Rep. 2016, 15, 1076–1087.
- Parekh, V.V.; Pabbisetty, S.K.; Wu, L.; Sebzda, E.; Martinez, J.; Zhang, J.; Van Kaer, L. Autophagy-related protein Vps34 controls the homeostasis and function of antigen cross-presenting CD8alpha(+) dendritic cells. Proc. Natl. Acad. Sci. USA 2017, 114, E6371–E6380.
- Zou, W.; Wolchok, J.D.; Chen, L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: Mechanisms, response biomarkers, and combinations. Sci. Transl. Med. 2016, 8, 328rv324.
- Jang, Y.J.; Kim, J.H.; Byun, S. Modulation of Autophagy for Controlling Immunity. Cells 2019, 8, 138.
- Clarke, A.J.; Simon, A.K. Autophagy in the renewal, differentiation and homeostasis of immune cells. Nat. Rev. Immunol. 2019, 19, 170–183.
- Xia, H.; Wang, W.; Crespo, J.; Kryczek, I.; Li, W.; Wei, S.; Bian, Z.; Maj, T.; He, M.; Liu, R.J.; et al. Suppression of FIP200 and autophagy by tumor-derived lactate promotes naive T cell apoptosis and affects tumor immunity. Sci. Immunol. 2017, 2, eaan4631.
- Sena, L.A.; Li, S.; Jairaman, A.; Prakriya, M.; Ezponda, T.; Hildeman, D.A.; Wang, C.R.; Schumacker, P.T.; Licht, J.D.; Perlman, H.; et al. Mitochondria are required for antigen-specific T cell activation through reactive oxygen species signaling. Immunity 2013, 38, 225–236.
- Jia, W.; He, M.X.; McLeod, I.X.; Guo, J.; Ji, D.; He, Y.W. Autophagy regulates T lymphocyte proliferation through selective degradation of the cell-cycle inhibitor CDKN1B/p27Kip1. Autophagy 2015, 11, 2335–2345.
- Bronietzki, A.W.; Schuster, M.; Schmitz, I. Autophagy in T-cell development, activation and differentiation. Immunol. Cell Biol. 2015, 93, 25–34.
- Yang, G.; Song, W.; Postoak, J.L.; Chen, J.; Martinez, J.; Zhang, J.; Wu, L.; Van Kaer, L. Autophagy-related protein PIK3C3/VPS34 controls T cell metabolism and function. Autophagy 2021, 17, 1193–1204.
- Pua, H.H.; Guo, J.; Komatsu, M.; He, Y.W. Autophagy is essential for mitochondrial clearance in mature T lymphocytes. J. Immunol. 2009, 182, 4046–4055.
- Swadling, L.; Pallett, L.J.; Diniz, M.O.; Baker, J.M.; Amin, O.E.; Stegmann, K.A.; Burton, A.R.; Schmidt, N.M.; Jeffery-Smith, A.; Zakeri, N.; et al. Human Liver Memory CD8(+) T Cells Use Autophagy for Tissue Residence. Cell Rep. 2020, 30, 687–698.e686.
- Delgoffe, G.M.; Kole, T.P.; Zheng, Y.; Zarek, P.E.; Matthews, K.L.; Xiao, B.; Worley, P.F.; Kozma, S.C.; Powell, J.D. The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity 2009, 30, 832–844.
- Le Texier, L.; Lineburg, K.E.; Cao, B.; McDonald-Hyman, C.; Leveque-El Mouttie, L.; Nicholls, J.; Melino, M.; Nalkurthi, B.C.; Alexander, K.A.; Teal, B.; et al. Autophagy-dependent regulatory T cells are critical for the control of graft-versus-host disease. JCI Insight 2016, 1, e86850.
- Wei, J.; Long, L.; Yang, K.; Guy, C.; Shrestha, S.; Chen, Z.; Wu, C.; Vogel, P.; Neale, G.; Green, D.R.; et al. Autophagy enforces functional integrity of regulatory T cells by coupling environmental cues and metabolic homeostasis. Nat. Immunol. 2016, 17, 277–285.
- Becher, J.; Simula, L.; Volpe, E.; Procaccini, C.; La Rocca, C.; D’Acunzo, P.; Cianfanelli, V.; Strappazzon, F.; Caruana, I.; Nazio, F.; et al. AMBRA1 Controls Regulatory T-Cell Differentiation and Homeostasis Upstream of the FOXO3-FOXP3 Axis. Dev. Cell 2018, 47, 592–607.e596.
- Munz, C. Autophagy proteins in antigen processing for presentation on MHC molecules. Immunol. Rev. 2016, 272, 17–27.
- Li, W.; Tanikawa, T.; Kryczek, I.; Xia, H.; Li, G.; Wu, K.; Wei, S.; Zhao, L.; Vatan, L.; Wen, B.; et al. Aerobic Glycolysis Controls Myeloid-Derived Suppressor Cells and Tumor Immunity via a Specific CEBPB Isoform in Triple-Negative Breast Cancer. Cell Metab. 2018, 28, 87–103.e106.
- Parker, K.H.; Horn, L.A.; Ostrand-Rosenberg, S. High-mobility group box protein 1 promotes the survival of myeloid-derived suppressor cells by inducing autophagy. J. Leukoc. Biol. 2016, 100, 463–470.
- Alissafi, T.; Hatzioannou, A.; Mintzas, K.; Barouni, R.M.; Banos, A.; Sormendi, S.; Polyzos, A.; Xilouri, M.; Wielockx, B.; Gogas, H.; et al. Autophagy orchestrates the regulatory program of tumor-associated myeloid-derived suppressor cells. J. Clin. Investig. 2018, 128, 3840–3852.
- Pan, H.; Chen, L.; Xu, Y.; Han, W.; Lou, F.; Fei, W.; Liu, S.; Jing, Z.; Sui, X. Autophagy-associated immune responses and cancer immunotherapy. Oncotarget 2016, 7, 21235–21246.
- Arnold, J.; Murera, D.; Arbogast, F.; Fauny, J.D.; Muller, S.; Gros, F. Autophagy is dispensable for B-cell development but essential for humoral autoimmune responses. Cell Death Differ. 2016, 23, 853–864.
- Fribourg, M.; Ni, J.; Nina Papavasiliou, F.; Yue, Z.; Heeger, P.S.; Leventhal, J.S. Allospecific Memory B Cell Responses Are Dependent on Autophagy. Am. J. Transpl. 2018, 18, 102–112.
- Wang, S.; Xia, P.; Huang, G.; Zhu, P.; Liu, J.; Ye, B.; Du, Y.; Fan, Z. FoxO1-mediated autophagy is required for NK cell development and innate immunity. Nat. Commun. 2016, 7, 11023.
- Salio, M.; Puleston, D.J.; Mathan, T.S.; Shepherd, D.; Stranks, A.J.; Adamopoulou, E.; Veerapen, N.; Besra, G.S.; Hollander, G.A.; Simon, A.K.; et al. Essential role for autophagy during invariant NKT cell development. Proc. Natl. Acad. Sci. USA 2014, 111, E5678–E5687.
- Pei, B.; Zhao, M.; Miller, B.C.; Vela, J.L.; Bruinsma, M.W.; Virgin, H.W.; Kronenberg, M. Invariant NKT cells require autophagy to coordinate proliferation and survival signals during differentiation. J. Immunol. 2015, 194, 5872–5884.
- Liu, E.; Van Grol, J.; Subauste, C.S. Atg5 but not Atg7 in dendritic cells enhances IL-2 and IFN-gamma production by Toxoplasma gondii-reactive CD4+ T cells. Microbes Infect. 2015, 17, 275–284.
- Seto, S.; Tsujimura, K.; Horii, T.; Koide, Y. Autophagy adaptor protein p62/SQSTM1 and autophagy-related gene Atg5 mediate autophagosome formation in response to Mycobacterium tuberculosis infection in dendritic cells. PLoS ONE 2013, 8, e86017.
- Lee, H.K.; Mattei, L.M.; Steinberg, B.E.; Alberts, P.; Lee, Y.H.; Chervonsky, A.; Mizushima, N.; Grinstein, S.; Iwasaki, A. In vivo requirement for Atg5 in antigen presentation by dendritic cells. Immunity 2010, 32, 227–239.
- Chen, P.; Cescon, M.; Bonaldo, P. Autophagy-mediated regulation of macrophages and its applications for cancer. Autophagy 2014, 10, 192–200.
- Jacquel, A.; Obba, S.; Solary, E.; Auberger, P. Proper macrophagic differentiation requires both autophagy and caspase activation. Autophagy 2012, 8, 1141–1143.
- Zhang, Y.; Morgan, M.J.; Chen, K.; Choksi, S.; Liu, Z.G. Induction of autophagy is essential for monocyte-macrophage differentiation. Blood 2012, 119, 2895–2905.
- Liu, K.; Zhao, E.; Ilyas, G.; Lalazar, G.; Lin, Y.; Haseeb, M.; Tanaka, K.E.; Czaja, M.J. Impaired macrophage autophagy increases the immune response in obese mice by promoting proinflammatory macrophage polarization. Autophagy 2015, 11, 271–284.
- Li, N.; Qin, J.; Lan, L.; Zhang, H.; Liu, F.; Wu, Z.; Ni, H.; Wang, Y. PTEN inhibits macrophage polarization from M1 to M2 through CCL2 and VEGF-A reduction and NHERF-1 synergism. Cancer Biol. Ther. 2015, 16, 297–306.
- Khatoon, E.; Parama, D.; Kumar, A.; Alqahtani, M.S.; Abbas, M.; Girisa, S.; Sethi, G.; Kunnumakkara, A.B. Targeting PD-1/PD-L1 axis as new horizon for ovarian cancer therapy. Life Sci. 2022, 306, 120827.
- Robainas, M.; Otano, R.; Bueno, S.; Ait-Oudhia, S. Understanding the role of PD-L1/PD1 pathway blockade and autophagy in cancer therapy. OncoTargets Ther. 2017, 10, 1803–1807.
- Maher, C.M.; Thomas, J.D.; Haas, D.A.; Longen, C.G.; Oyer, H.M.; Tong, J.Y.; Kim, F.J. Small-Molecule Sigma1 Modulator Induces Autophagic Degradation of PD-L1. Mol. Cancer Res. 2018, 16, 243–255.
- Clark, C.A.; Gupta, H.B.; Curiel, T.J. Tumor cell-intrinsic CD274/PD-L1: A novel metabolic balancing act with clinical potential. Autophagy 2017, 13, 987–988.
- Shukla, S.A.; Bachireddy, P.; Schilling, B.; Galonska, C.; Zhan, Q.; Bango, C.; Langer, R.; Lee, P.C.; Gusenleitner, D.; Keskin, D.B.; et al. Cancer-Germline Antigen Expression Discriminates Clinical Outcome to CTLA-4 Blockade. Cell 2018, 173, 624–633.e628.
- Kato, H.; Perl, A. Blockade of Treg Cell Differentiation and Function by the Interleukin-21-Mechanistic Target of Rapamycin Axis Via Suppression of Autophagy in Patients With Systemic Lupus Erythematosus. Arthritis Rheumatol. 2018, 70, 427–438.
- Alissafi, T.; Banos, A.; Boon, L.; Sparwasser, T.; Ghigo, A.; Wing, K.; Vassilopoulos, D.; Boumpas, D.; Chavakis, T.; Cadwell, K.; et al. Tregs restrain dendritic cell autophagy to ameliorate autoimmunity. J. Clin. Investig. 2017, 127, 2789–2804.
- Folgiero, V.; Miele, E.; Carai, A.; Ferretti, E.; Alfano, V.; Po, A.; Bertaina, V.; Goffredo, B.M.; Benedetti, M.C.; Camassei, F.D.; et al. IDO1 involvement in mTOR pathway: A molecular mechanism of resistance to mTOR targeting in medulloblastoma. Oncotarget 2016, 7, 52900–52911.
- Metz, R.; Rust, S.; Duhadaway, J.B.; Mautino, M.R.; Munn, D.H.; Vahanian, N.N.; Link, C.J.; Prendergast, G.C. IDO inhibits a tryptophan sufficiency signal that stimulates mTOR: A novel IDO effector pathway targeted by D-1-methyl-tryptophan. Oncoimmunology 2012, 1, 1460–1468.
- McGaha, T.L. IDO-GCN2 and autophagy in inflammation. Oncotarget 2015, 6, 21771–21772.
- Schauer, I.G.; Zhang, J.; Xing, Z.; Guo, X.; Mercado-Uribe, I.; Sood, A.K.; Huang, P.; Liu, J. Interleukin-1beta promotes ovarian tumorigenesis through a p53/NF-kappaB-mediated inflammatory response in stromal fibroblasts. Neoplasia 2013, 15, 409–420.
- Jiang, S.; Dupont, N.; Castillo, E.F.; Deretic, V. Secretory versus degradative autophagy: Unconventional secretion of inflammatory mediators. J. Innate Immun. 2013, 5, 471–479.
- Peral de Castro, C.; Jones, S.A.; Ni Cheallaigh, C.; Hearnden, C.A.; Williams, L.; Winter, J.; Lavelle, E.C.; Mills, K.H.; Harris, J. Autophagy regulates IL-23 secretion and innate T cell responses through effects on IL-1 secretion. J. Immunol. 2012, 189, 4144–4153.
- Sun, K.; Xu, L.; Jing, Y.; Han, Z.; Chen, X.; Cai, C.; Zhao, P.; Zhao, X.; Yang, L.; Wei, L. Autophagy-deficient Kupffer cells promote tumorigenesis by enhancing mtROS-NF-kappaB-IL1alpha/beta-dependent inflammation and fibrosis during the preneoplastic stage of hepatocarcinogenesis. Cancer Lett. 2017, 388, 198–207.
- Kang, R.; Tang, D.; Lotze, M.T.; Zeh Iii, H.J. Autophagy is required for IL-2-mediated fibroblast growth. Exp. Cell Res. 2013, 319, 556–565.
- Liang, X.; De Vera, M.E.; Buchser, W.J.; Romo de Vivar Chavez, A.; Loughran, P.; Beer Stolz, D.; Basse, P.; Wang, T.; Van Houten, B.; Zeh, H.J., 3rd; et al. Inhibiting systemic autophagy during interleukin 2 immunotherapy promotes long-term tumor regression. Cancer Res. 2012, 72, 2791–2801.
- Qin, B.; Zhou, Z.; He, J.; Yan, C.; Ding, S. IL-6 Inhibits Starvation-induced Autophagy via the STAT3/Bcl-2 Signaling Pathway. Sci. Rep. 2015, 5, 15701.
- Linnemann, A.K.; Blumer, J.; Marasco, M.R.; Battiola, T.J.; Umhoefer, H.M.; Han, J.Y.; Lamming, D.W.; Davis, D.B. Interleukin 6 protects pancreatic beta cells from apoptosis by stimulation of autophagy. FASEB J. 2017, 31, 4140–4152.
- Santarelli, R.; Gonnella, R.; Di Giovenale, G.; Cuomo, L.; Capobianchi, A.; Granato, M.; Gentile, G.; Faggioni, A.; Cirone, M. STAT3 activation by KSHV correlates with IL-10, IL-6 and IL-23 release and an autophagic block in dendritic cells. Sci. Rep. 2014, 4, 4241.
- Cho, S.H.; Oh, S.Y.; Lane, A.P.; Lee, J.; Oh, M.H.; Lee, S.; Zheng, T.; Zhu, Z. Regulation of nasal airway homeostasis and inflammation in mice by SHP-1 and Th2/Th1 signaling pathways. PLoS ONE 2014, 9, e103685.
- Qi, G.M.; Jia, L.X.; Li, Y.L.; Li, H.H.; Du, J. Adiponectin suppresses angiotensin II-induced inflammation and cardiac fibrosis through activation of macrophage autophagy. Endocrinology 2014, 155, 2254–2265.
- Schmeisser, H.; Bekisz, J.; Zoon, K.C. New function of type I IFN: Induction of autophagy. J. Interf. Cytokine Res. 2014, 34, 71–78.
- Buchser, W.J.; Laskow, T.C.; Pavlik, P.J.; Lin, H.M.; Lotze, M.T. Cell-mediated autophagy promotes cancer cell survival. Cancer Res. 2012, 72, 2970–2979.
- Matsuzawa, T.; Kim, B.H.; Shenoy, A.R.; Kamitani, S.; Miyake, M.; Macmicking, J.D. IFN-gamma elicits macrophage autophagy via the p38 MAPK signaling pathway. J. Immunol. 2012, 189, 813–818.
- Tu, S.P.; Quante, M.; Bhagat, G.; Takaishi, S.; Cui, G.; Yang, X.D.; Muthuplani, S.; Shibata, W.; Fox, J.G.; Pritchard, D.M.; et al. IFN-gamma inhibits gastric carcinogenesis by inducing epithelial cell autophagy and T-cell apoptosis. Cancer Res. 2011, 71, 4247–4259.
- Hubbard, V.M.; Valdor, R.; Patel, B.; Singh, R.; Cuervo, A.M.; Macian, F. Macroautophagy regulates energy metabolism during effector T cell activation. J. Immunol. 2010, 185, 7349–7357.
- Wilson, E.B.; El-Jawhari, J.J.; Neilson, A.L.; Hall, G.D.; Melcher, A.A.; Meade, J.L.; Cook, G.P. Human tumour immune evasion via TGF-beta blocks NK cell activation but not survival allowing therapeutic restoration of anti-tumour activity. PLoS ONE 2011, 6, e22842.
- Ding, Y.; Kim, S.; Lee, S.Y.; Koo, J.K.; Wang, Z.; Choi, M.E. Autophagy regulates TGF-beta expression and suppresses kidney fibrosis induced by unilateral ureteral obstruction. J. Am. Soc. Nephrol. 2014, 25, 2835–2846.
- Zhang, C.; Zhang, X.; Xu, R.; Huang, B.; Chen, A.J.; Li, C.; Wang, J.; Li, X.G. TGF-beta2 initiates autophagy via Smad and non-Smad pathway to promote glioma cells’ invasion. J. Exp. Clin. Cancer Res. 2017, 36, 162.
- Suzuki, H.I.; Kiyono, K.; Miyazono, K. Regulation of autophagy by transforming growth factor-beta (TGF-beta) signaling. Autophagy 2010, 6, 645–647.
- Wang, M.X.; Cheng, X.Y.; Jin, M.; Cao, Y.L.; Yang, Y.P.; Wang, J.D.; Li, Q.; Wang, F.; Hu, L.F.; Liu, C.F. TNF compromises lysosome acidification and reduces alpha-synuclein degradation via autophagy in dopaminergic cells. Exp. Neurol. 2015, 271, 112–121.
- Ullio, C.; Brunk, U.T.; Urani, C.; Melchioretto, P.; Bonelli, G.; Baccino, F.M.; Autelli, R. Autophagy of metallothioneins prevents TNF-induced oxidative stress and toxicity in hepatoma cells. Autophagy 2015, 11, 2184–2198.
- Pun, N.T.; Subedi, A.; Kim, M.J.; Park, P.H. Globular Adiponectin Causes Tolerance to LPS-Induced TNF-alpha Expression via Autophagy Induction in RAW 264.7 Macrophages: Involvement of SIRT1/FoxO3A Axis. PLoS ONE 2015, 10, e0124636.
More
Information
Subjects:
Oncology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
881
Revisions:
3 times
(View History)
Update Date:
17 Oct 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





