
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Qiou Wei | -- | 1386 | 2022-10-14 17:31:39 | | | |
| 2 | Beatrix Zheng | + 1 word(s) | 1387 | 2022-10-17 04:12:25 | | |
Video Upload Options
Peroxiredoxin IV (Prx4) is a 2-Cysteine peroxidase with ubiquitous expression in human tissues. Prx4 scavenges hydrogen peroxide and participates in oxidative protein folding in the endoplasmic reticulum. In addition, Prx4 is secreted outside the cell. Prx4 is upregulated in several cancers and is a potential therapeutic target. Here, the resarchers have summarized the structure and function of Prx4. Oxidative stress is known to activate pro-inflammatory pathways. Chronic inflammation is a risk factor for cancer development. Hence, redox enzymes such as Prx4 are important players in the crosstalk between inflammation and cancer. Understanding molecular mechanisms of regulation of Prx4 expression and associated signaling pathways in normal physiological and disease conditions should reveal new therapeutic strategies. Although Prx4 is a promising therapeutic target for inflammatory diseases and cancer, further research needs to be conducted to bridge the gap to clinical application.
1. Structure
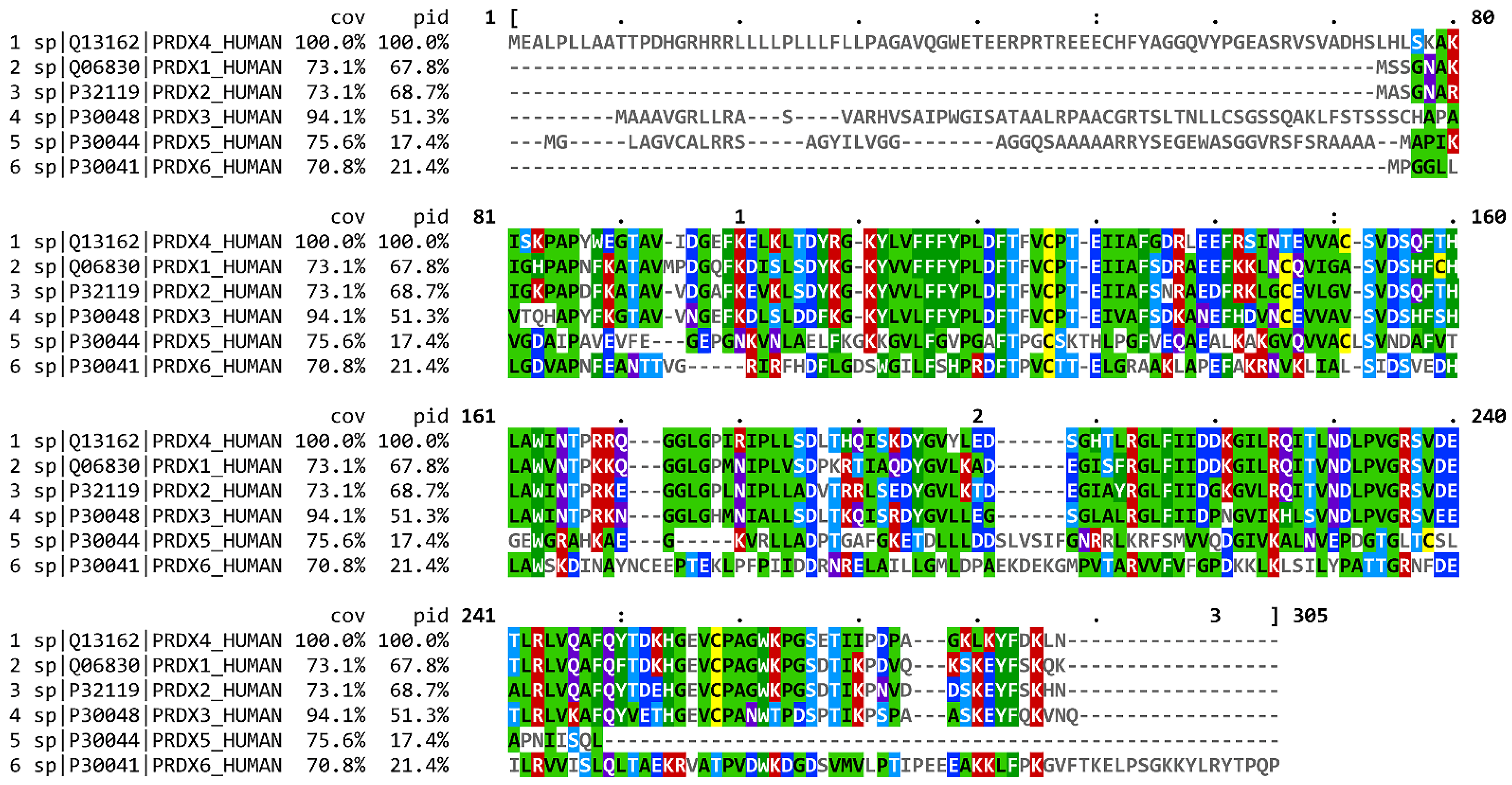
| Species | Gene Symbol | % Sequence Similarity |
|
|---|---|---|---|
| Protein | DNA | ||
| H. Sapiens | PRDX4 | ||
| M. mulatta (Rhesus macaque) | PRDX4 | 98.5 | 98.4 |
| C. lupus (Wolf) | PRDX4 | 93 | 89.2 |
| B. taurus (Cattle) | PRDX4 | 93.8 | 90.8 |
| M. musculus (House mouse) | Prdx4 | 95 | 89.1 |
| R. norvegicus (Brown rat) | Prdx4 | 94.5 | 90.3 |
| G. gallus (Red junglefowl) | PRDX4 | 91.9 | 81.6 |
| X. tropicalis (Western clawed frog) | prdx4 | 93.6 | 81.1 |
| D. rerio (Zebrafish) | prdx4 | 88.7 | 74.8 |
| D. melanogaster (Common fruit fly) | Jafrac2 | 71 | 64.4 |
2. Biochemical Function
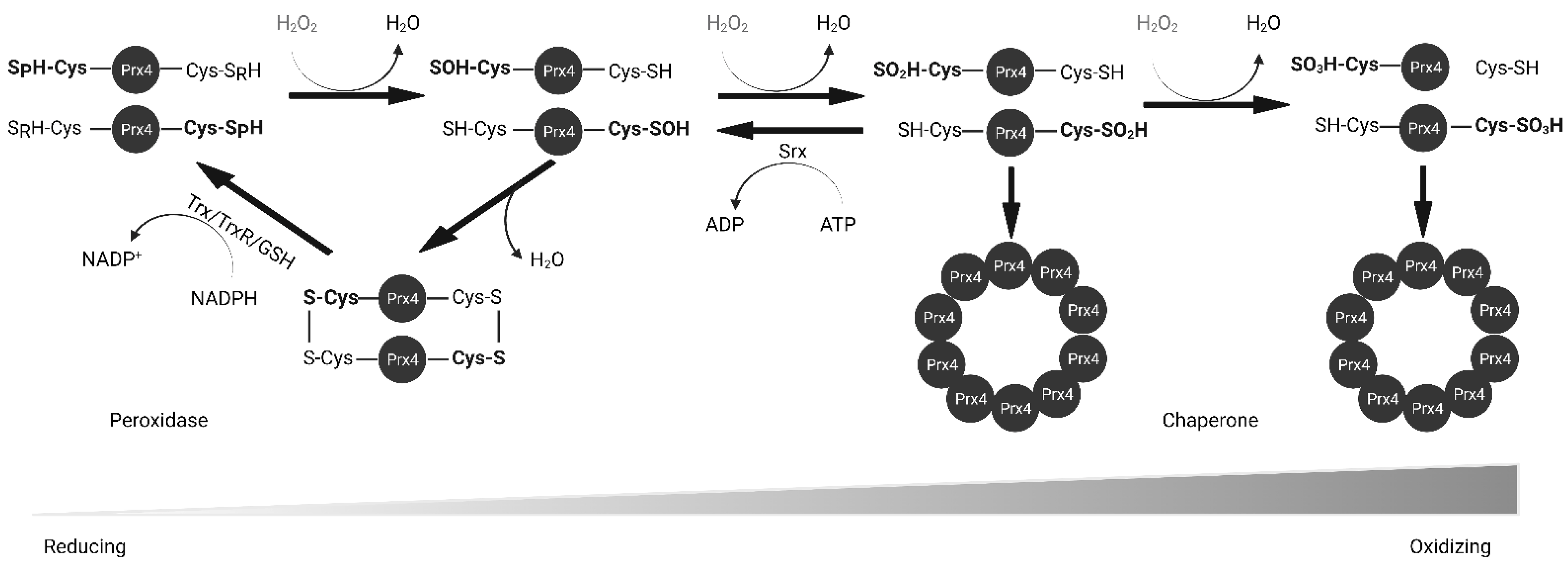
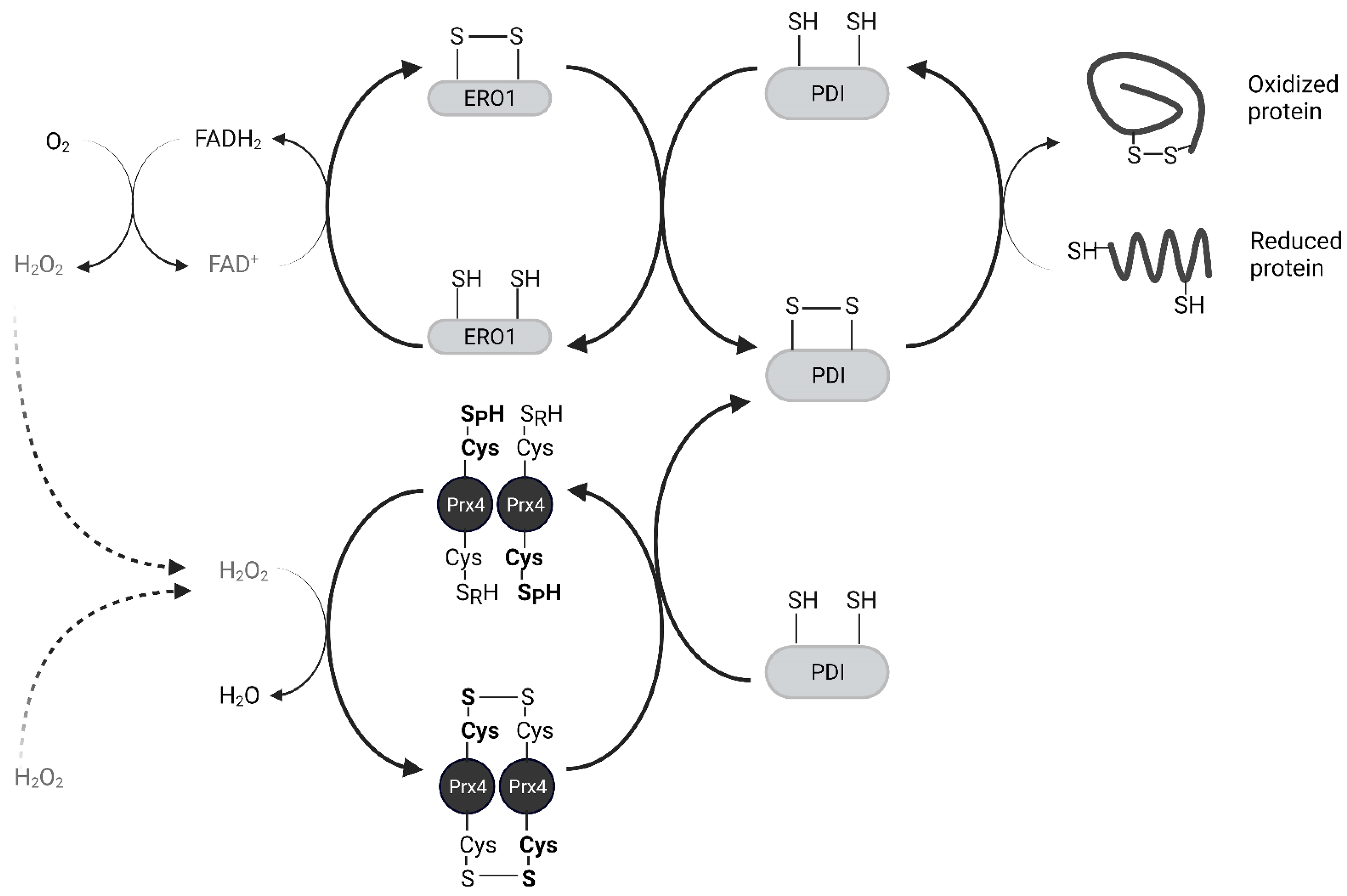
References
- NCBI Resource Coordinators. Database resources of the National Center for Biotechnology Information. Nucleic Acids Res. 2018, 46, D8–D13.
- Sievers, F.; Wilm, A.; Dineen, D.; Gibson, T.J.; Karplus, K.; Li, W.; Lopez, R.; McWilliam, H.; Remmert, M.; Söding, J.; et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 2011, 7, 539.
- Goujon, M.; McWilliam, H.; Li, W.; Valentin, F.; Squizzato, S.; Paern, J.; Lopez, R. A new bioinformatics analysis tools framework at EMBL-EBI. Nucleic Acids Res. 2010, 38, W695–W699.
- Madeira, F.; Park, Y.M.; Lee, J.; Buso, N.; Gur, T.; Madhusoodanan, N.; Basutkar, P.; Tivey, A.R.N.; Potter, S.C.; Finn, R.D.; et al. The EMBL-EBI search and sequence analysis tools APIs in 2019. Nucleic Acids Res. 2019, 47, W636–W641.
- Fujii, J.; Ikeda, Y.; Kurahashi, T.; Homma, T. Physiological and pathological views of peroxiredoxin 4. Free Radic. Biol. Med. 2015, 83, 373–379.
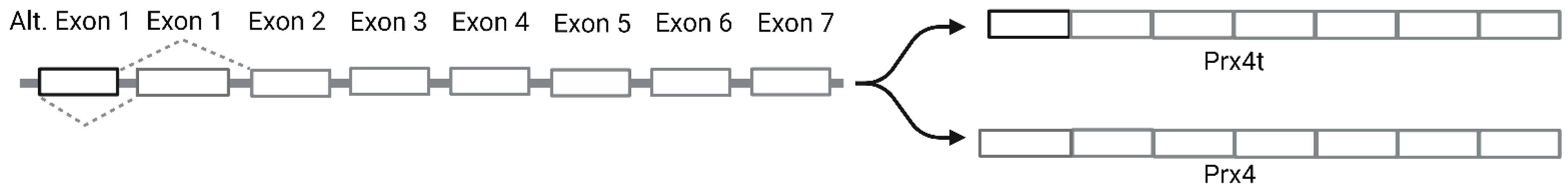
- Yim, S.H.; Kim, Y.J.; Oh, S.Y.; Fujii, J.; Zhang, Y.; Gladyshev, V.N.; Rhee, S.G. Identification and characterization of alternatively transcribed form of peroxiredoxin IV gene that is specifically expressed in spermatids of postpubertal mouse testis. J. Biol. Chem. 2011, 286, 39002–39012.
- Matsumoto, A.; Okado, A.; Fujii, T.; Fujii, J.; Egashira, M.; Niikawa, N.; Taniguchi, N. Cloning of the peroxiredoxin gene family in rats and characterization of the fourth member. FEBS Lett. 1999, 443, 246–250.
- Tavender, T.J.; Sheppard, A.M.; Bulleid, N.J. Peroxiredoxin IV is an endoplasmic reticulum-localized enzyme forming oligomeric complexes in human cells. Biochem. J. 2008, 411, 191–199.
- Haridas, V.; Ni, J.; Meager, A.; Su, J.; Yu, G.L.; Zhai, Y.; Kyaw, H.; Akama, K.T.; Hu, J.; Van Eldik, L.J.; et al. TRANK, a novel cytokine that activates NF-kappa B and c-Jun N-terminal kinase. J. Immunol. 1998, 161, 1–6.
- Okado-Matsumoto, A.; Matsumoto, A.; Fujii, J.; Taniguchi, N. Peroxiredoxin IV is a secretable protein with heparin-binding properties under reduced conditions. J. Biochem. 2000, 127, 493–501.
- Kakihana, T.; Araki, K.; Vavassori, S.; Iemura, S.; Cortini, M.; Fagioli, C.; Natsume, T.; Sitia, R.; Nagata, K. Dynamic regulation of Ero1α and peroxiredoxin 4 localization in the secretory pathway. J. Biol. Chem. 2013, 288, 29586–29594.
- Tempio, T.; Orsi, A.; Sicari, D.; Valetti, C.; Yoboue, E.D.; Anelli, T.; Sitia, R. A virtuous cycle operated by ERp44 and ERGIC-53 guarantees proteostasis in the early secretory compartment. iScience 2021, 24, 102244.
- Wang, X.; Wang, L.; Wang, X.; Sun, F.; Wang, C.C. Structural insights into the peroxidase activity and inactivation of human peroxiredoxin 4. Biochem. J. 2012, 441, 113–118.
- Cao, Z.; Tavender, T.J.; Roszak, A.W.; Cogdell, R.J.; Bulleid, N.J. Crystal structure of reduced and of oxidized peroxiredoxin IV enzyme reveals a stable oxidized decamer and a non-disulfide-bonded intermediate in the catalytic cycle. J. Biol. Chem. 2011, 286, 42257–42266.
- Zeida, A.; Reyes, A.M.; Lebrero, M.C.; Radi, R.; Trujillo, M.; Estrin, D.A. The extraordinary catalytic ability of peroxiredoxins: A combined experimental and QM/MM study on the fast thiol oxidation step. Chem. Commun. 2014, 50, 10070–10073.
- Tavender, T.J.; Springate, J.J.; Bulleid, N.J. Recycling of peroxiredoxin IV provides a novel pathway for disulphide formation in the endoplasmic reticulum. EMBO J. 2010, 29, 4185–4197.
- Rabilloud, T.; Heller, M.; Gasnier, F.; Luche, S.; Rey, C.; Aebersold, R.; Benahmed, M.; Louisot, P.; Lunardi, J. Proteomics analysis of cellular response to oxidative stress. Evidence for in vivo overoxidation of peroxiredoxins at their active site. J. Biol. Chem. 2002, 277, 19396–19401.
- Roussel, X.; Béchade, G.; Kriznik, A.; Van Dorsselaer, A.; Sanglier-Cianferani, S.; Branlant, G.; Rahuel-Clermont, S. Evidence for the formation of a covalent thiosulfinate intermediate with peroxiredoxin in the catalytic mechanism of sulfiredoxin. J. Biol. Chem. 2008, 283, 22371–22382.
- Mishra, M.; Jiang, H.; Wu, L.; Chawsheen, H.A.; Wei, Q. The sulfiredoxin-peroxiredoxin (Srx-Prx) axis in cell signal transduction and cancer development. Cancer Lett. 2015, 366, 150–159.
- Elko, E.A.; Manuel, A.M.; White, S.; Zito, E.; van der Vliet, A.; Anathy, V.; Janssen-Heininger, Y.M.W. Oxidation of peroxiredoxin-4 induces oligomerization and promotes interaction with proteins governing protein folding and endoplasmic reticulum stress. J. Biol. Chem. 2021, 296, 100665.
- Bonnichsen, R.K.; Chance, B.; Theorell, H. Catalase Activity. Acta Chem. Scand. 1947, 1, 685–709.
- Winterbourn, C.C. The biological chemistry of hydrogen peroxide. Methods Enzymol. 2013, 528, 3–25.
- Winterbourn, C.C.; Metodiewa, D. Reactivity of biologically important thiol compounds with superoxide and hydrogen peroxide. Free Radic. Biol. Med. 1999, 27, 322–328.
- Nguyen, V.D.; Saaranen, M.J.; Karala, A.R.; Lappi, A.K.; Wang, L.; Raykhel, I.B.; Alanen, H.I.; Salo, K.E.; Wang, C.C.; Ruddock, L.W. Two endoplasmic reticulum PDI peroxidases increase the efficiency of the use of peroxide during disulfide bond formation. J. Mol. Biol. 2011, 406, 503–515.
- Hatahet, F.; Ruddock, L.W. Protein disulfide isomerase: A critical evaluation of its function in disulfide bond formation. Antioxid. Redox Signal. 2009, 11, 2807–2850.
- Tu, B.P.; Weissman, J.S. The FAD- and O(2)-dependent reaction cycle of Ero1-mediated oxidative protein folding in the endoplasmic reticulum. Mol. Cell 2002, 10, 983–994.
- Gross, E.; Sevier, C.S.; Heldman, N.; Vitu, E.; Bentzur, M.; Kaiser, C.A.; Thorpe, C.; Fass, D. Generating disulfides enzymatically: Reaction products and electron acceptors of the endoplasmic reticulum thiol oxidase Ero1p. Proc. Natl. Acad. Sci. USA 2006, 103, 299–304.
- Sato, Y.; Kojima, R.; Okumura, M.; Hagiwara, M.; Masui, S.; Maegawa, K.; Saiki, M.; Horibe, T.; Suzuki, M.; Inaba, K. Synergistic cooperation of PDI family members in peroxiredoxin 4-driven oxidative protein folding. Sci. Rep. 2013, 3, 2456.
- Zito, E.; Hansen, H.G.; Yeo, G.S.; Fujii, J.; Ron, D. Endoplasmic reticulum thiol oxidase deficiency leads to ascorbic acid depletion and noncanonical scurvy in mice. Mol. Cell 2012, 48, 39–51.
- Moon, J.C.; Kim, G.M.; Kim, E.K.; Lee, H.N.; Ha, B.; Lee, S.Y.; Jang, H.H. Reversal of 2-Cys peroxiredoxin oligomerization by sulfiredoxin. Biochem. Biophys. Res. Commun. 2013, 432, 291–295.
- Pattwell, D.; Ashton, T.; McArdle, A.; Griffiths, R.D.; Jackson, M.J. Ischemia and reperfusion of skeletal muscle lead to the appearance of a stable lipid free radical in the circulation. Am. J. Physiol. Heart Circ. Physiol. 2003, 284, H2400–H2404.
- Pattwell, D.M.; McArdle, A.; Morgan, J.E.; Patridge, T.A.; Jackson, M.J. Release of reactive oxygen and nitrogen species from contracting skeletal muscle cells. Free Radic. Biol. Med. 2004, 37, 1064–1072.
- Salzano, S.; Checconi, P.; Hanschmann, E.M.; Lillig, C.H.; Bowler, L.D.; Chan, P.; Vaudry, D.; Mengozzi, M.; Coppo, L.; Sacre, S.; et al. Linkage of inflammation and oxidative stress via release of glutathionylated peroxiredoxin-2, which acts as a danger signal. Proc. Natl. Acad. Sci. USA 2014, 111, 12157–12162.
- Manabe, Y.; Takagi, M.; Nakamura-Yamada, M.; Goto-Inoue, N.; Taoka, M.; Isobe, T.; Fujii, N.L. Redox proteins are constitutively secreted by skeletal muscle. J. Physiol. Sci. 2014, 64, 401–409.
- Wadley, A.J.; Keane, G.; Cullen, T.; James, L.; Vautrinot, J.; Davies, M.; Hussey, B.; Hunter, D.J.; Mastana, S.; Holliday, A.; et al. Characterization of extracellular redox enzyme concentrations in response to exercise in humans. J. Appl. Physiol. 2019, 127, 858–866.
- V Haridas; J Ni; A Meager; J Su; G L Yu; Y Zhai; H Kyaw; K T Akama; J Hu; L J Van Eldik; et al. TRANK, a novel cytokine that activates NF-kappa B and c-Jun N-terminal kinase.. The Journal of Immunology 1998, 161, 1-6.
- Li-Xue Zhao; Jun-Rong Du; Hong-Jing Zhou; Dong-Ling Liu; Man-Xia Gu; Fang-Yi Long; Differences in Proinflammatory Property of Six Subtypes of Peroxiredoxins and Anti-Inflammatory Effect of Ligustilide in Macrophages. PLOS ONE 2016, 11, e0164586-e0164586, 10.1371/journal.pone.0164586.




