Peroxiredoxin IV (Prx4) is a 2-Cysteine peroxidase with ubiquitous expression in human tissues. Prx4 scavenges hydrogen peroxide and participates in oxidative protein folding in the endoplasmic reticulum. In addition, Prx4 is secreted outside the cell. Prx4 is upregulated in several cancers and is a potential therapeutic target. Here, we have summarized the structure and function of Prx4. Oxidative stress is known to activate pro-inflammatory pathways. Chronic inflammation is a risk factor for cancer development. Hence, redox enzymes such as Prx4 are important players in the crosstalk between inflammation and cancer. Understanding molecular mechanisms of regulation of Prx4 expression and associated signaling pathways in normal physiological and disease conditions should reveal new therapeutic strategies. Although Prx4 is a promising therapeutic target for inflammatory diseases and cancer, further research needs to be conducted to bridge the gap to clinical application.
1. Structure
Comparison of mammalian Prx4 protein sequence shows that human Prx4 has a highly similar amino acid sequence as commonly used animal models such as mice, rat and zebrafish (
Table 1) [
14]. In addition, Prx4 has approximately 68% homology with human Prx1 and Prx2, and 52% homology with Prx3 (
Figure 1) [
15,
16,
17]. Prx4 is found on the X chromosome (Xp22.11) and the longest transcript contains seven exons. Somatic Prx4 contains conventional exon 1 and exons 2–7 [
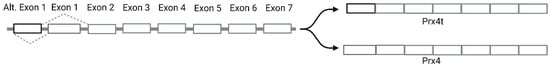
18]. However, alternative splicing of Prx4 can occur in the testes (
Figure 2). Prx4t in sexually mature testes contains an alternative exon 1 along with exons 2–7 [
19].
Figure 1. Multiple sequence alignment of human Prxs colored by consensus generated using MView. Coverage and percentage identity values are indicated by cov and pid, respectively. Cysteines are highlighted with yellow color. For other residues, Red = positively charged, blue = negatively charged, purple = polar, and green=hydrophobic.
Figure 2. Schematic representation of alternative splicing of Prx4. Systemic Prx4 contains exon 1, whereas Prx4t expressed in mature testes contains alternative exon 1.
Table 1. Summary of human Prx4 homologues and conservation relative to human Prx4 generated using Homologene.
| Species |
Gene Symbol |
% Sequence
Similarity |
| |
|
Protein |
DNA |
| H. Sapiens |
PRDX4 |
|
|
| M. mulatta (Rhesus macaque) |
PRDX4 |
98.5 |
98.4 |
| C. lupus (Wolf) |
PRDX4 |
93 |
89.2 |
| B. taurus (Cattle) |
PRDX4 |
93.8 |
90.8 |
| M. musculus (House mouse) |
Prdx4 |
95 |
89.1 |
| R. norvegicus (Brown rat) |
Prdx4 |
94.5 |
90.3 |
| G. gallus (Red junglefowl) |
PRDX4 |
91.9 |
81.6 |
| X. tropicalis (Western clawed frog) |
prdx4 |
93.6 |
81.1 |
| D. rerio (Zebrafish) |
prdx4 |
88.7 |
74.8 |
| D. melanogaster (Common fruit fly) |
Jafrac2 |
71 |
64.4 |
Prx4 is localized mainly in the endoplasmic reticulum [
20,
21]. It is also secreted into the extracellular matrix [
20,
22,
23]. The unique extended N-terminal region in Prx4 allows for translocation of Prx4 across the ER membrane into the luminal space [
21]. Since Prx4t lacks the N-terminal signal peptide, it is found in the cytosol. ER localization of Prx4 despite lacking the canonical ER retention ‘KDEL’ signal is due its interaction with PDI and ERp44. In HeLa cells, secretion due to overexpression of Prx4 could be suppressed by overexpression of ER proteins ERp44 and PDI [
24]. Knockdown of ERGIC-53 in HeLa cells or treatment with 4-phenylbutyrate induces secretion of Prx4, further confirming the importance of ERp44 in its ER localization [
25].
Human Prx4 has been crystallized in both oxidized and reduced states [
26,
27]. In both states, Prx4 was crystallized as a decamer, composed of five dimers. Similar to other Prxs, each subunit contains the thioredoxin fold (ββαβαβαβαββαα). A dimer acts as a catalytic subunit. In the dimer, β1- and β8- strands of partner subunits interact with each other whereas a central twisted β-sheet is surrounded by α-helices. Cp is located in α2 helix in a pocket surrounded by β4- and β5- strands and α3- and α5- helices. Cr is located in a flexible loop between α5- and α6- helices. In the reduced state, there is a distance of 13 Å between the Cp of one subunit and Cr of another. Upon oxidation, there is local unfolding in α2- helix, facilitating repositioning of Cp for the formation of disulfide bond with Cr of partner subunit. Cao et al. suggest that the higher stability of Prx4 decamer compared to other Prxs could be attributed to Phe-122 displacing Pro-260 to maintain the hydrophobic interaction between subunits [
27]. In addition, compared to other 2-Cys Prxs, Prx4 has a unique N-terminal sequence that is approximately 40 aa long. Wang et al. found that deletion of these N-terminal residues resulted in decreased stability of Prx4 decamers upon oxidation by H
2O
2 [
26].
2. Biochemical Function
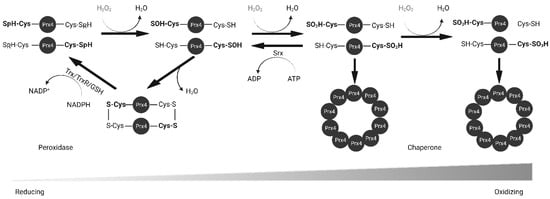
Prxs are thiol-based peroxidases and cysteine residues that are utilized for redox purposes. Prx1–4 have two cysteine residues that participate in catalysis: peroxidatic Cys (Cp) and resolving Cys (Cr). The N-terminal peroxidatic cysteine has a pKa of 5–6, much lower than the normal Cys pKa of 8–9 because it is stabilized by neighboring arginine and threonine residues [
28]. The lower pKa makes it more reactive to H
2O
2. As shown in
Figure 3, upon contact with peroxide, the thiol group of Cp in Prx4 is oxidized to sulfenic acid (Cys-SOH). The oxidized Prx4 forms a disulfide bond with Cr of another polypeptide resulting in a stable homodimer whereas H
2O
2 is reduced to water. This disulfide can be reduced by Trx, GSH, PDI or other ER oxidoreductases [
22,
23,
29]. However, at higher concentrations of H
2O
2, Prx4 is further oxidized into sulfinic acid (Cys-SOOH) and sulfonic acid (Cys-SOOOH) leading to loss of enzyme activity [
30]. Sulfinic acids can be reduced by Sulfiredoxin (Srx) in an ATP-dependent manner [
31,
32]. Unlike other 2-Cys Prxs, over-oxidized Prx4 can maintain stable decamers through hydrophobic interactions between subunits and disulfide bond between non-catalytic N-terminal cysteine residue [
26,
27]. Later studies revealed that mutation of Cp and Cr alone or in combination prevents the formation of decamers [
33]. The rate constant for H
2O
2 reduction of Prx4 is 2.2 × 10
7 M
–1 S
–1, which is comparable to that of catalase and several orders of magnitude higher than that for reduction by GSH (0.87 M
–1 S
–1) or Trx (1.05 M
–1 S
–1) [
26,
34,
35,
36]. This rate is also significantly higher than that of another ER antioxidant, GPx8 (95 M
–1 S
–1) [
37].
Figure 3. Reduction of H2O2 by Prx4. Peroxidatic cysteine (Cys-SpH) is oxidized to sulfenic acid and either resolved and recycled with the help of Trx or GSH, or further oxidized into sulfinic and sulfonic acid forms. Srx reduces sulfinic acid. Prx4 loses peroxidase activity and gains chaperone activity with increasing oxidizing environment. Trx, thioredoxin; TrxR, thioredoxin reductase; GSH, glutathione; Srx, sulfiredoxin.
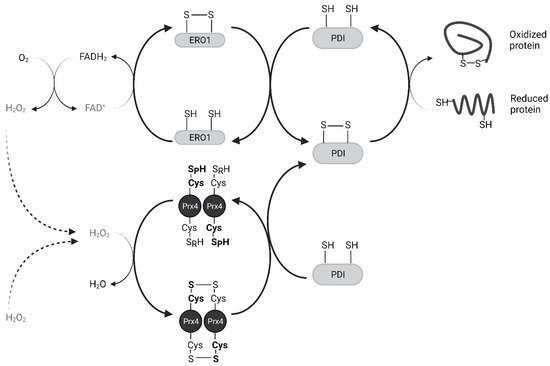
Oxidized Protein Disulfide Isomerase (PDI) family proteins introduce disulfide bonds in nascent proteins in the ER [
38]. ER oxidoreductin 1 (Ero1) re-oxidizes PDI using its cofactor Flavin Adenine Dinucleotide (FAD) and releases H
2O
2 as a byproduct [
39]. For every disulfide bond introduced into nascent proteins, one molecule of H
2O
2 is produced [
40]. Prx4 scavenges these H
2O
2 molecules and prevents their accumulation. Prx4 also contributes to the folding of plasma membrane proteins and secreted proteins, acting upstream of PDIs. The interaction of Prx4 with PDIs and other ER-associated proteins increases with its oxidation, likely through its recognition of Trx-domain within PDIs [
33,
41]. Oxidized Prx4 engages in thiol-disulfide exchange with reduced PDIs, which results in restoration of activity for both (
Figure 4) [
29]. Loss of an Ero1 gene or Prx4 alone has no apparent phenotype in mice, but loss of both interferes with collagen synthesis and compromises the extracellular matrix [
42]. Thus, Prx4 neutralizes peroxides in its reduced state and promotes protein folding in its oxidized state. H
2O
2 and Srx act as an on-off switch in regulating these activities [
43].
Figure 4. Prx4 mediates nascent peptide folding in the endoplasmic reticulum. Prx4 neutralizes H2O2 including those produced by Ero1. Oxidized Prx4 transfers disulfides to protein disulfide isomerases which catalyze the formation of disulfide bonds in nascent proteins. Only the catalytic dimer of Prx4 is shown for simplicity. Ero1, ER oxidoreductin 1; PDI, protein disulfide isomerase.
The mechanism of Prx4 secretion and the function of secreted Prx4 are not well understood. Okado-Matsumoto et al. have previously shown that Prx4 can be secreted in both reduced and oxidized forms [
23]. Since the reduced form of extracellular Prx4 binds to heparin and human umbilical vein endothelial cells in a manner similar to another extracellular antioxidant SOD3, the authors suggest that extracellular Prx4 also protects tissues against antioxidant injury. Additionally, acute exercise is known to affect redox balance in skeletal muscle [
44,
45] whereas muscle cells and immune cells have been shown to secrete redox proteins such as Trx and Glutaredoxin in response to increased H
2O
2 [
46,
47]. Wadley et al. reported a significant increase in plasma Prx4 levels after high intensity exercise [
48]. These studies suggest a role for Prx4 in scavenging H
2O
2 in the extracellular space. Furthermore, secreted Prx4 also likely plays a role in regulating inflammation via NF-κB signaling
[1][2].
This entry is adapted from the peer-reviewed paper 10.3390/molecules27196513