
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Spyros D MENTZELOPOULOS | -- | 2563 | 2022-10-12 12:09:46 | | | |
| 2 | Rita Xu | Meta information modification | 2563 | 2022-10-13 03:22:00 | | | | |
| 3 | Rita Xu | -13 word(s) | 2550 | 2022-10-14 08:59:32 | | |
Video Upload Options
Lower respiratory tract invasion by severe acute respiratory syndrome coronavirus 2 (SARS-COV-2) results in widespread damage of type II alveolar and pulmonary capillary endothelial cells, disruption of the alveolar-capillary barrier, activation of coagulation, and diffuse thrombogenesis amplified by recruited monocytes and neutrophils and concurrent hypofibrinolysis. These pathobiological mechanisms are associated with a distinct form of acute respiratory distress syndrome (ARDS), characterized by relatively high lung compliance, progressively worsening hypoxemia, low potential for lung recruitment, hyperperfusion of nonaerated lung tissue, and diffuse small-vessel thrombosis.
1. Introduction
Acute respiratory distress syndrome (ARDS) is characterized by high-permeability pulmonary edema, widespread compressive atelectasis, and inflammatory disruption of the alveolar-capillary barrier [1]. Neutralization of pulmonary surfactant by proteinaceous exudate [2] and increased intraparenchymal hydrostatic pressures due to accumulation of extravascular lung water promote collapse of dependent lung units. This results in hypoxemia and hypercapnia secondary to intrapulmonary shunt and constriction of the functional surface area of the respiratory membrane [3][4][5]. At lung unit level, there is “diffuse alveolar damage,” comprising disruption of the alveolar and endothelial cell lining, hyaline membranes, edema, and inflammatory cell infiltration [6]. The current definition of ARDS [7] is presented in Table 1.
Table 1. Berlin Definition of Acute Respiratory Distress Syndrome.
|
Timing |
Within 1 week of known clinical insult or new or worsening respiratory symptoms |
|
|
Chest imaging |
Bilateral opacities on CXR or CT not fully explained by effusions, lobar/lung collapse, or nodules |
|
|
Origin of edema |
Respiratory failure not fully explained by cardiac failure or fluid overload |
|
|
Oxygenation |
Mild |
200 mm Hg < PaO2/FiO2 ≤ 300 mm Hg with PEEP or CPAP ≥ 5 cm H2O |
|
Moderate |
100 mm Hg < PaO2/FiO2 ≤ 200 mm Hg with PEEP ≤ 5 cm H2O |
|
|
Severe |
PaO2/FiO2 ≤ 100 mm Hg with PEEP ≥ 5 cm H2O |
|
CXR, chest x-ray; CT computed tomography; PaO2/FiO2 partial pressure of arterial oxygen to fraction of inspired oxygen ratio; PEEP positive end-expiratory pressure; CPAP continuous positive airway pressure.
Reproduced in concordance with the Creative Commons Attribution License (CC-BY) from Selickman J, Vrettou CS, Mentzelopoulos SD, Marini JJ. COVID-19-Related ARDS: Key Mechanistic Features and Treatments. J Clin Med. 2022;11(16):4896. doi: 10.3390/jcm11164896.
In ″typical ″ [i.e. pre-coronavirus disease (COVID)-19] ARDS, shunt fraction and physiological dead space rise proportionally to the loss of aerated and perfused (i.e. functional) lung tissue. The ARDS lung is heterogenous with functional units interspersed with non-aerated units. The location and distribution of atelectatic lung units is determined by gravity and the presence of external compression by overlying edematous tissue [8][9][10].
2. Pathobiology of COVID-19 related ARDS (C-ARDS)
Following spread to the lower respiratory tract, SARS-COV-2 binds via its spike protein to angiotensin converting enzyme 2 (ACE-2) receptors of primarily type II alveolar cells [11][12]. The spike protein is then cleaved by a transmembrane serine protease, thereby facilitating the release of the viral ribonucleoprotein into the cytoplasm [13]. Subsequently, viral replicases promote viral RNA transcription using the host cell’s endoplasmic reticulum [14][15].
Intracellular viral RNA and damage associated molecular patterns trigger the release of interferons and pro-inflammatory cytokines, which mediate leukocyte recruitment [16][17]. The diffuse alveolar epithelial injury may promote a state of disequilibrium between coagulation and fibrinolysis and intra-alveolar hyaline membrane formation hindering gas exchange [14][18][19]. Diffuse alveolar injury is tightly associated with a concurrent, small-vessel, endothelial cell activation and damage due to hypoxia, cytokines, leukocyte infiltration and direct viral infection [14][20][21]. An inflammatory, pulmonary and extrapulmonary endothelial cell injury and microcirculatory dysfunction may ensue and contribute to the development of severe C-ARDS and multi-organ failure [14][20].
Severe COVID-19 / C-ARDS is characterized by widespread, immune cell-mediated, small-vessel thrombosis, termed immunothombosis [14][22]. Underlying mechanisms include: interleukin (IL)-6 release by alveolar epithelial cells [14]; coagulation activation by exposed extracellular matrix [14]; tissue factor expression and release of cytokines and neutrophil extracellular traps (NETs) by activated monocytes and neutrophils [14][23][24][25]; SARS-COV-2 complement activation, augmenting tissue factor expression by neutrophils and differentiation of CD-16+ cytotoxic T-cells [14][25][26]; propagation of the immunothrombotic process through platelet recruitment and activation [22][27]; neutralization of anticoagulants by neutrophil elastase and myeloperoxidase [22]; and increased expression of plasminogen activator inhibitor [14][28].
3. C-ARDS vs. ″typical″ ARDS
In contrast to ″typical″ ARDS, C-ARDS is characterized by extensive micro vascular thrombosis, which may involve more than 25% of the lung parenchyma in more than 50% of the patients with severe disease [29][30][31][32][33]. Additional pathological features of C-ARDS include severe endothelial injury, engorged pulmonary capillaries, and more frequent large-vessel pulmonary emboli compared to ″typical″ ARDS [20][21][35][36][37]. C-ARDS imaging studies also indicate the presence of dilated pulmonary vessels within opacified (i.e. poorly aerated or non-aerated) lung areas [37][38], as well as widespread perfusion defects in areas of normal radiographic density [39][40]. The regional vasodilation-induced overperfusion of gasless tissue has been associated with a shunt fraction-to-gasless tissue ratio of >2 [41][42], and more frequent severe hypoxemia (i.e. PaO2/FiO2 ≤ 100 mm Hg) at higher levels of tidal compliance (e.g. 50 mL/cmH2O) compared to ″typical″ ARDS [43]. Early-C-ARDS hypoxemia is mainly due to hyperperfusion of non-ventilated lung units, rather than non-cardiogenic pulmonary edema [36][44][45][46][47]. Nevertheless, following disease progression, late-phase C-ARDS becomes similar to late-phase ″typical″ ARDS, with respect to low lung compliance and capacity, high physiological dead space, and low potential for recruitment [48]. Table 2 displays a summary comparison of the major characteristics of ″typical″ ARDS and C-ARDS.
Table 2. Comparative presentation of major characteristic features of classical ARDS and C-ARDS.
|
|
Classical ARDS |
C-ARDS |
|
Etiology |
Diverse, pulmonary or extrapulmonary (e.g. bacterial or viral pneumonia, severe trauma, aspiration, sepsis, etc.) |
SARS-COV-2 infection of alveolar type 2 cells (primarily) |
|
Hypoxemia (PaO2/FiO2 ≤300 mmHg at a PEEP level of ≥5 cmH2O) |
Acute onset (e.g. within <48 hours after the clinical insult), or progressive onset (i.e. within 7 days after the clinical insult) |
Progressive onset (i.e. within 7 or more days after the onset of COVID-19 symptoms)* |
|
Lung compliance at hypoxemia onset |
Usually low (e.g. <40 cmH2O/L) |
Usually high (e.g. >40 cmH2O/L) |
|
Recruitment potential |
Low or high, depending on the extent / nature of lung unit involvement and associated atelectasis |
Initially low – may increase with disease progression and development of edema and atelectasis |
|
Functional-to-anatomical shunt ratio / hyperperfusion of gasless tissue * |
Usually 0.5-2.0 / No |
Usually > 2.0 / Yes |
|
Alveolar capillary microthrombosis / new vessel growth |
Present / present |
Diffuse (~9 times more prevalent) / marked (2.7 times higher) |
ARDS, acute respiratory distress syndrome; C-ARDS, coronavirus disease (COVID) 19-related ARDS; SARS-COV-2, severe acute, respiratory syndrome coronavirus 2; PaO2/FiO2, oxygen arterial partial pressure-to-fraction of inspired oxygen fraction ratio; PEEP, positive end-expiratory pressure.
*, May predispose to early, profound hypoxemia and the conceptual risk of pre-intubation, patient self-inflicted lung injury. Reproduced in part, in concordance with the Creative Commons Attribution License (CC-BY) from Selickman J, Vrettou CS, Mentzelopoulos SD, Marini JJ. COVID-19-Related ARDS: Key Mechanistic Features and Treatments. J Clin Med. 2022;11(16):4896. doi: 10.3390/jcm11164896.
4. Ventilatory Settings and Maneuvers in C-ARDS
Mechanical ventilation of the ARDS lungs is invariably linked to increased parenchymal mechanical stress and strain, and the potential for ventilator-induced lung injury (VILI) [49][50]. Mechanistically, stress reflects forces tending to cause and oppose lung parenchymal expansion from the resting state, whereas strain reflects the amount of parenchymal mechanical deformation relative to the resting state [51]. Regarding clinical practice, stress is corresponded to end-inspiratory plateau airway pressure and its subcomponents, i.e. driving pressure and positive end-expiratory pressure (PEEP) [49][50][51]; in addition, strain has been defined as the tidal volume-to-end-expiratory lung volume ratio [49][50]. Both factors, along with flow and respiratory rate, constitute subcomponents of the mechanical power of ventilation [51][52]. In ARDS non-survivors, the calculated, specific mechanical power (i.e. the power per ventilated lung unit [51]) is approximately tenfold higher relative to healthy subjects [51][52]. Furthermore, mechanical power normalized to respiratory compliance and well-aerated lung tissue is associated with intensive care mortality [53].
Low-stress (i.e. plateau pressure target of <30 cmH2O and driving pressure target of <15 cmH2O) and strain (i.e. tidal volume of 4-8 mL/kg predicted body weight) ventilation has been the mainstay of the management of ARDS over the past 2 decades [54][55][56][57]. A PEEP level of >10 cmH2O is also suggested to prevent atelectrauma (i.e. small airway epithelial injury) due to tidal recruitment and derecruitment [56][57][58][59]. Furthermore, in cases of hypoxemia despite ventilation optimization, "non-staircase" recruitment maneuvers may also be considered [56][57].
Notably, despite the presence of an extensive evidence-base as regards "typical" ARDS, recently gained knowledge about C-ARDS pathophysiology may point toward the need for further, patient-level individualization of the ventilatory management. For example, patients with the "type L" ARDS phenotype (i.e. relatively high lung compliance, low ventilation-to-perfusion ratio, low lung weight by computerized tomography, and low lung recruitability [60][61]) may tolerate tidal volumes of 7-8 mL/kg-predicted and relatively low PEEP levels (e.g. 8-10 cmH2O), without appreciable risk of strain-related, or expiratory derecruitment-related VILI [51][59][63]. In such patients, a higher PEEP level (e.g. ≥14 cmH2O) may reduce lung compliance, and increase lung stress and physiological dead space [51][64][65].
The regional net effect of PEEP depends on the balance between 1) recruitment of previously collapsed and/or poorly aerated lung units (representing increase in functional parenchyma); and 2) overdistention of previously normally aerated lung units (representing potential loss of functional lung tissue and increase in dead space ventilation). Regional overdistention predisposes to hypercapnia and a decreased cardiac output. The latter may be associated with reduced shunt flow and increased PaO2/FiO2; in such cases, the changes in oxygenation do not reflect changes in lung recruitment [66][67].
The predominantly vascular pathophysiology of C-ARDS may explain recent computerized tomographic findings of low recruitability of functional lung units at higher PEEP levels [68][69]. Higher PEEP levels in C-ARDS have also been associated with worsening of gas exchange and respiratory mechanics, suggesting that PEEP-induced overdistention may prevail over PEEP-associated recruitment [64][68][70][71][72]. These data indicate that PEEP titration should be patient-specific. PEEP level selection could be guided by measurements of respiratory compliance and PaCO2, as their concurrent improvement suggests recruitment of functional lung units. Additional useful bedside "markers"/tests may include the ventilatory ratio [65] and the recruitment to inflation ratio [73].
In "typical," severe ARDS, prone positioning may improve gas exchange, and reduce lung stress / strain, physiological dead space and 28-day / 90-day mortality [74][75]. Relative to the supine position, pronation results in improved shape-matching between the lungs and the chest wall [76][77] (Figure 1). Prone position facilitates recruitment of the "massive" dorsal lung through relief of the superimposed hydrostatic pressure and external compression by the heart and abdominal contents [78][79]. Prone position is also associated with more homogenous distribution of pleural pressure, regional distending forces, and regional lung volumes and gas / tissue ratios [78][80][81][82][83][84][85][86]. Furthermore, as the pattern of pulmonary perfusion is minimally affected by body posture, the increased homogeneity of ventilation following pronation is frequently associated with improved ventilation-perfusion matching [85][87][88][89].
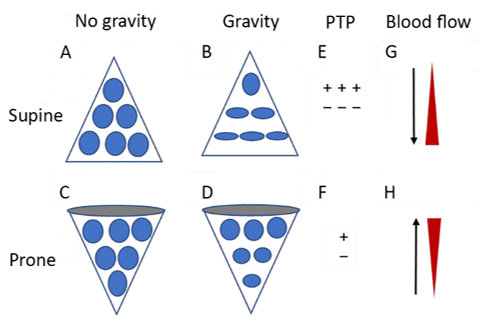
Figure 1. Diagrammatic presentation of physiological mechanisms associated with pronation in the acute respiratory distress syndrome (ARDS). (A) and (C) show the shape of lung units (i.e. alveoli) without the effect of gravity. (B) In the supine position, the volume of dorsal lung units is significantly smaller than the volume of ventral lung units, as a result of gravity and pleural pressure; thus, ventral lung units are more prone to overdistention and dorsal lung units are more prone to compression atelectasis. (D) In the prone position, gravity and pleural pressure result in a decrease in the volume of the ventral lung units and an increase in the volume of the dorsal lung units. (E) In the supine position, the ventral transpulmonary pressure (PTP) may substantially exceed the dorsal PTP. (F) Prone positioning reduces the ventral-to-dorsal PTP gradient, thereby augmenting the homogeneity of ventilation. (G) The reopening, dorsal lung units continue to receive most of the blood flow. (H) The ventral lung units may exhibit a greater tendency to collapse, but are still relatively underperfused. Reproduced in concordance with the Creative Commons Attribution License (CC-BY) from reference 77.
Prone positioning has been reportedly used in approximately 80% of patients with severe C-ARDS [90]. In a recent physiological study of moderate-to-severe C-ARDS (PaO2/FiO2 ≤ 150 mm Hg) [7][91], pronation was associated with a global net recruitment of 6%, a lower concavity index of the time-impedance curve (implying less risk for atelectrauma), and a lower dead space-to shunt ratio (suggesting improved ventilation perfusion-matching). In a multicenter cohort study of C-ARDS, pronation within 48 hours of intensive care unit (ICU) admission was associated with a 16% reduction in the adjusted risk of mortality [92].
Awake pronation relieves dorsal lung compression by the abdomen and mediastinal structures, improves secretion clearance and regional diaphragm movement, and increases functional residual capacity and regional ventilation of dependent lung units [93][94]. In a large meta-trial of severe COVID-19 requiring support with high-flow nasal cannula, awake pronation resulted in a 25% reduction in the risk of tracheal intubation [95]; survival without intubation was higher in patients who tolerated awake pronation for >8 h / day compared to those who did not [96]. Awake pronation may attenuate the risk of patient self-inflicted lung injury by decreasing the inspiratory effort [94][97][98][99].
5. Non-Ventilatory Therapeutic Interventions in C-ARDS
Several pharmaceutical interventions, including the antiviral remdesivir [100][101], and anti-inflammatory agents such as dexamethasone [102][103], tocilizumab [104], baricitinib [105][106][107], and anakinra [108] have been associated with improved outcomes of patients with severe COVID-19 / C-ARDS. Extracorporeal membrane oxygenation (ECMO) alone [109] or combined with prone positioning [110][111][112] may also be considered in patients with severe C-ARDS. Nevertheless, additional high-quality evidence is required to clearly establish the clinical benefit of ECMO in severe C-ARDS. A summary presentation of evidence-based C-ARDS treatment options is provided in Table 3.
Table 3. Evidence-based treatments for coronavirus disease-19 (COVID-19) related acute respiratory distress syndrome (ARDS).
|
Intervention |
Mechanism of Action |
Evidence for Efficacy |
|
Remdesivir day 1: 200 mg IV days 2-10: 100 mg IV |
Inhibition of the viral RNA-dependent, RNA polymerase |
Shortens the time to recovery in hospitalized COVID-19 patients |
|
Dexamethasone days 1-10*: 6 mg IV |
Anti-inflammatory - linked to the activation of the glucocorticoid receptor |
Reduces the probability of in-hospital death in critically ill COVID-19 patients |
|
Tocilizumab single dose: 8 mg/kg IV (max. 800 mg) |
Interleukin-6 antagonism |
Reduces the probability of in-hospital death in critically ill COVID-19 patients |
|
Baracitinib days 1-14*: 4mg† oral or enteral |
Janus kinase inhibition |
Reduces the probability of in-hospital death in critically ill COVID-19 patients |
|
Anakinra days 1-10*: 100 mg subcutaneously |
Interleukin 1 alpha/beta antagonism |
Reduces the probability of in-hospital death in critically ill COVID-19 patients |
|
Prone positioning for at least 16 hours per day until PaO2/FiO2 ≥150 mmHg at PEEP ≤10 cmH2O and FiO2 ≤0.6 |
Attenuation of lung stress and strain Reversal of compression atelectasis Increased homogeneity of ventilation Improved ventilation/perfusion matching |
Reduces the probability of in-hospital death in moderate to severe ARDS |
|
Extracorporeal membrane oxygenation |
Minimization of lung stress and strain ("lung rest") with very low tidal volumes and ventilation pressures |
Possible mortality benefit in severe ARDS |
PaO2/FiO2, oxygen arterial partial pressure-to-inspired oxygen fraction ratio; PEEP, positive end-expiratory pressure.
Reproduced in concordance with the Creative Commons Attribution License (CC-BY) from Selickman J, Vrettou CS, Mentzelopoulos SD, Marini JJ. COVID-19-Related ARDS: Key Mechanistic Features and Treatments. J Clin Med. 2022;11(16):4896. doi: 10.3390/jcm11164896.
6. Conclusions
Key characteristics of early C-ARDS comprise widespread pulmonary (and extrapulmonary) endothelitis and microcirculatory immunothrombosis, in conjunction with hyperperfusion of gasless lung tissue, initial relative preservation of lung compliance with concurrent frequently severe hypoxemia, and low potential for recruitment of functional lung units. Late-phase C-ARDS is associated with low lung compliance, high physiological deadspace and low lung recruitability. Such characteristics may necessitate tailoring of the ventilatory settings to the individual patient, while still avoiding any potentially injurious lung stress and strain. In this context, early pronation and use of relatively low PEEP levels (e.g. 8-10 cmH2O) may be considered. Treatments of proven efficacy should be used, whereas future research should further elucidate the role of ECMO with or without concurrent pronation.
References
- Thompson, B.T.; Chambers, R.C.; Liu, K.D. Acute Respiratory Distress Syndrome. N Engl J Med 2017, 377, 1904 1905, doi:10.1056/NEJMc1711824.
- Holm, B.A.; Matalon, S. Role of pulmonary surfactant in the development and treatment of adult respiratory distress syndrome. Anesth Analg 1989, 69, 805-818.
- Niklason, L.; Eckerstrom, J.; Jonson, B. The influence of venous admixture on alveolar dead space and carbon dioxide exchange in acute respiratory distress syndrome: computer modelling. Crit Care 2008, 12, R53, doi:10.1186/cc6872.
- Radermacher, P.; Maggiore, S.M.; Mercat, A. Fifty Years of Research in ARDS. Gas Exchange in Acute Respiratory Distress Syndrome. Am J Respir Crit Care Med 2017, 196, 964-984, doi:10.1164/rccm.201610-2156SO.
- Robertson, H.T.; Swenson, E.R. What do dead-space measurements tell us about the lung with acute respiratory distress syndrome? Respir Care 2004, 49, 1006-1007.
- Katzenstein, A.L.; Bloor, C.M.; Leibow, A.A. Diffuse alveolar damage--the role of oxygen, shock, and related factors. A review. Am J Pathol 1976, 85, 209-228.
- Force, A.D.T.; Ranieri, V.M.; Rubenfeld, G.D.; Thompson, B.T.; Ferguson, N.D.; Caldwell, E.; Fan, E.; Camporota, L.; Slutsky, A.S. Acute respiratory distress syndrome: the Berlin Definition. JAMA 2012, 307, 2526-2533, doi:10.1001/jama.2012.5669.
- Gattinoni, L.; Pesenti, A.; Bombino, M.; Baglioni, S.; Rivolta, M.; Rossi, F.; Rossi, G.; Fumagalli, R.; Marcolin, R.; Mascheroni, D., et al. Relationships between lung computed tomographic density, gas exchange, and PEEP in acute respiratory failure. Anesthesiology 1988, 69, 824-832, doi:10.1097/00000542-198812000-00005.
- Gattinoni, L.; Mascheroni, D.; Torresin, A.; Marcolin, R.; Fumagalli, R.; Vesconi, S.; Rossi, G.P.; Rossi, F.; Baglioni, S.; Bassi, F., et al. Morphological response to positive end expiratory pressure in acute respiratory failure. Computerized tomography study. Intensive Care Med 1986, 12, 137-142, doi:10.1007/BF00254928.
- Gattinoni, L.; Pelosi, P.; Vitale, G.; Pesenti, A.; D'Andrea, L.; Mascheroni, D. Body position changes redistribute lung computed-tomographic density in patients with acute respiratory failure. Anesthesiology 1991, 74, 15-23, doi:10.1097/00000542-199101000-00004.
- Wiersinga, W.J.; Rhodes, A.; Cheng, A.C.; Peacock, S.J.; Prescott, H.C. Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review. JAMA 2020, 324, 782-793, doi:10.1001/jama.2020.12839.
- Zhou, P.; Yang, X.L.; Wang, X.G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.R.; Zhu, Y.; Li, B.; Huang, C.L., et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270-273, doi:10.1038/s41586-020-2012-7.
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Kruger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A., et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271-280 e278, doi:10.1016/j.cell.2020.02.052.
- Lamers, M.M.; Haagmans, B.L. SARS-CoV-2 pathogenesis. Nat Rev Microbiol 2022, 20, 270-284, doi:10.1038/s41579-022-00713-0.
- Ogando, N.S.; Dalebout, T.J.; Zevenhoven-Dobbe, J.C.; Limpens, R.; van der Meer, Y.; Caly, L.; Druce, J.; de Vries, J.J.C.; Kikkert, M.; Barcena, M., et al. SARS-coronavirus-2 replication in Vero E6 cells: replication kinetics, rapid adaptation and cytopathology. J Gen Virol 2020, 101, 925-940, doi:10.1099/jgv.0.001453.
- Carty, M.; Guy, C.; Bowie, A.G. Detection of Viral Infections by Innate Immunity. Biochem Pharmacol 2021, 183, 114316, doi:10.1016/j.bcp.2020.114316.
- Land, W.G. Role of DAMPs in respiratory virus-induced acute respiratory distress syndrome-with a preliminary reference to SARS-CoV-2 pneumonia. Genes Immun 2021, 22, 141-160, doi:10.1038/s41435-021-00140-w.
- Sebag, S.C.; Bastarache, J.A.; Ware, L.B. Therapeutic modulation of coagulation and fibrinolysis in acute lung injury and the acute respiratory distress syndrome. Curr Pharm Biotechnol 2011, 12, 1481-1496, doi:10.2174/138920111798281171.
- Iba, T.; Levy, J.H.; Levi, M.; Thachil, J. Coagulopathy in COVID-19. J Thromb Haemost 2020, 18, 2103-2109, doi:10.1111/jth.14975.
- Varga, Z.; Flammer, A.J.; Steiger, P.; Haberecker, M.; Andermatt, R.; Zinkernagel, A.S.; Mehra, M.R.; Schuepbach, R.A.; Ruschitzka, F.; Moch, H. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020, 395, 1417-1418, doi:10.1016/S0140-6736(20)30937-5.
- Ackermann, M.; Verleden, S.E.; Kuehnel, M.; Haverich, A.; Welte, T.; Laenger, F.; Vanstapel, A.; Werlein, C.; Stark, H.; Tzankov, A., et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in Covid-19. N Engl J Med 2020, 383, 120-128, doi:10.1056/NEJMoa2015432.
- Bonaventura, A.; Vecchie, A.; Dagna, L.; Martinod, K.; Dixon, D.L.; Van Tassell, B.W.; Dentali, F.; Montecucco, F.; Massberg, S.; Levi, M., et al. Endothelial dysfunction and immunothrombosis as key pathogenic mechanisms in COVID-19. Nat Rev Immunol 2021, 21, 319-329, doi:10.1038/s41577-021-00536-9.
- Osuchowski, M.F.; Winkler, M.S.; Skirecki, T.; Cajander, S.; Shankar-Hari, M.; Lachmann, G.; Monneret, G.; Venet, F.; Bauer, M.; Brunkhorst, F.M., et al. The COVID-19 puzzle: deciphering pathophysiology and phenotypes of a new disease entity. Lancet Respir Med 2021, 9, 622-642, doi:10.1016/S2213-2600(21)00218-6.
- Rodrigues, T.S.; de Sa, K.S.G.; Ishimoto, A.Y.; Becerra, A.; Oliveira, S.; Almeida, L.; Goncalves, A.V.; Perucello, D.B.; Andrade, W.A.; Castro, R., et al. Inflammasomes are activated in response to SARS-CoV-2 infection and are associated with COVID-19 severity in patients. J Exp Med 2021, 218, doi:10.1084/jem.20201707.
- Kambas, K.; Markiewski, M.M.; Pneumatikos, I.A.; Rafail, S.S.; Theodorou, V.; Konstantonis, D.; Kourtzelis, I.; Doumas, M.N.; Magotti, P.; Deangelis, R.A., et al. C5a and TNF-alpha up-regulate the expression of tissue factor in intra-alveolar neutrophils of patients with the acute respiratory distress syndrome. J Immunol 2008, 180, 7368-7375, doi:10.4049/jimmunol.180.11.7368.
- Georg, P.; Astaburuaga-Garcia, R.; Bonaguro, L.; Brumhard, S.; Michalick, L.; Lippert, L.J.; Kostevc, T.; Gabel, C.; Schneider, M.; Streitz, M., et al. Complement activation induces excessive T cell cytotoxicity in severe COVID-19. Cell 2022, 185, 493-512 e425, doi:10.1016/j.cell.2021.12.040.
- Ebeyer-Masotta, M.; Eichhorn, T.; Weiss, R.; Laukova, L.; Weber, V. Activated Platelets and Platelet-Derived Extracellular Vesicles Mediate COVID-19-Associated Immunothrombosis. Front Cell Dev Biol 2022, 10, 914891, doi:10.3389/fcell.2022.914891.
- Mackman, N.; Antoniak, S.; Wolberg, A.S.; Kasthuri, R.; Key, N.S. Coagulation Abnormalities and Thrombosis in Patients Infected With SARS-CoV-2 and Other Pandemic Viruses. Arterioscler Thromb Vasc Biol 2020, 40, 2033-2044, doi:10.1161/ATVBAHA.120.314514.
- Tomashefski, J.F., Jr.; Davies, P.; Boggis, C.; Greene, R.; Zapol, W.M.; Reid, L.M. The pulmonary vascular lesions of the adult respiratory distress syndrome. Am J Pathol 1983, 112, 112-126.
- Hariri, L.P.; North, C.M.; Shih, A.R.; Israel, R.A.; Maley, J.H.; Villalba, J.A.; Vinarsky, V.; Rubin, J.; Okin, D.A.; Sclafani, A., et al. Lung Histopathology in Coronavirus Disease 2019 as Compared With Severe Acute Respiratory Sydrome and H1N1 Influenza: A Systematic Review. Chest 2021, 159, 73-84, doi:10.1016/j.chest.2020.09.259.
- Milross, L.; Majo, J.; Cooper, N.; Kaye, P.M.; Bayraktar, O.; Filby, A.; Fisher, A.J. Post-mortem lung tissue: the fossil record of the pathophysiology and immunopathology of severe COVID-19. Lancet Respir Med 2022, 10, 95-106, doi:10.1016/S2213-2600(21)00408-2.
- Carsana, L.; Sonzogni, A.; Nasr, A.; Rossi, R.S.; Pellegrinelli, A.; Zerbi, P.; Rech, R.; Colombo, R.; Antinori, S.; Corbellino, M., et al. Pulmonary post-mortem findings in a series of COVID-19 cases from northern Italy: a two-centre descriptive study. Lancet Infect Dis 2020, 20, 1135-1140, doi:10.1016/S1473-3099(20)30434-5.
- Poissy, J.; Goutay, J.; Caplan, M.; Parmentier, E.; Duburcq, T.; Lassalle, F.; Jeanpierre, E.; Rauch, A.; Labreuche, J.; Susen, S., et al. Pulmonary Embolism in Patients With COVID-19: Awareness of an Increased Prevalence. Circulation 2020, 142, 184-186, doi:10.1161/CIRCULATIONAHA.120.047430.
- Helms, J.; Tacquard, C.; Severac, F.; Leonard-Lorant, I.; Ohana, M.; Delabranche, X.; Merdji, H.; Clere-Jehl, R.; Schenck, M.; Fagot Gandet, F., et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med 2020, 46, 1089-1098, doi:10.1007/s00134-020-06062-x.
- Villalba, J.A.; Hilburn, C.F.; Garlin, M.A.; Elliott, G.A.; Li, Y.; Kunitoki, K.; Poli, S.; Alba, G.A.; Madrigal, E.; Taso, M., et al. Vasculopathy and Increased Vascular Congestion in Fatal COVID-19 and ARDS. Am J Respir Crit Care Med 2022, 10.1164/rccm.202109-2150OC, doi:10.1164/rccm.202109-2150OC.
- Li, Q.; Huang, X.T.; Li, C.H.; Liu, D.; Lv, F.J. CT features of coronavirus disease 2019 (COVID-19) with an emphasis on the vascular enlargement pattern. Eur J Radiol 2021, 134, 109442, doi:10.1016/j.ejrad.2020.109442.
- Poschenrieder, F.; Meiler, S.; Lubnow, M.; Zeman, F.; Rennert, J.; Scharf, G.; Schaible, J.; Stroszczynski, C.; Pfeifer, M.; Hamer, O.W. Severe COVID-19 pneumonia: Perfusion analysis in correlation with pulmonary embolism and vessel enlargement using dual-energy CT data. PLoS One 2021, 16, e0252478, doi:10.1371/journal.pone.0252478.
- Santamarina, M.G.; Boisier Riscal, D.; Beddings, I.; Contreras, R.; Baque, M.; Volpacchio, M.; Martinez Lomakin, F. COVID-19: What Iodine Maps From Perfusion CT can reveal-A Prospective Cohort Study. Crit Care 2020, 24, 619, doi:10.1186/s13054-020-03333-3.
- Patel, B.V.; Arachchillage, D.J.; Ridge, C.A.; Bianchi, P.; Doyle, J.F.; Garfield, B.; Ledot, S.; Morgan, C.; Passariello, M.; Price, S., et al. Pulmonary Angiopathy in Severe COVID-19: Physiologic, Imaging, and Hematologic Observations. Am J Respir Crit Care Med 2020, 202, 690-699, doi:10.1164/rccm.202004-1412OC.
- Gattinoni, L.; Coppola, S.; Cressoni, M.; Busana, M.; Rossi, S.; Chiumello, D. COVID-19 Does Not Lead to a "Typical" Acute Respiratory Distress Syndrome. Am J Respir Crit Care Med 2020, 201, 1299-1300, doi:10.1164/rccm.202003-0817LE.
- Cressoni, M.; Caironi, P.; Polli, F.; Carlesso, E.; Chiumello, D.; Cadringher, P.; Quintel, M.; Ranieri, V.M.; Bugedo, G.; Gattinoni, L. Anatomical and functional intrapulmonary shunt in acute respiratory distress syndrome. Crit Care Med 2008, 36, 669-675, doi:10.1097/01.CCM.0000300276.12074.E1.
- Chiumello, D.; Busana, M.; Coppola, S.; Romitti, F.; Formenti, P.; Bonifazi, M.; Pozzi, T.; Palumbo, M.M.; Cressoni, M.; Herrmann, P., et al. Physiological and quantitative CT-scan characterization of COVID-19 and typical ARDS: a matched cohort study. Intensive Care Med 2020, 46, 2187-2196, doi:10.1007/s00134-020-06281-2.
- Barbeta, E.; Motos, A.; Torres, A.; Ceccato, A.; Ferrer, M.; Cilloniz, C.; Bueno, L.; Badia, J.R.; Castro, P.; Ferrando, C., et al. SARS-CoV-2-induced Acute Respiratory Distress Syndrome: Pulmonary Mechanics and Gas-Exchange Abnormalities. Ann Am Thorac Soc 2020, 17, 1164-1168, doi:10.1513/AnnalsATS.202005-462RL.
- Vasques, F.; Sanderson, B.; Formenti, F.; Shankar-Hari, M.; Camporota, L. Physiological dead space ventilation, disease severity and outcome in ventilated patients with hypoxaemic respiratory failure due to coronavirus disease 2019. Intensive Care Med 2020, 46, 2092-2093, doi:10.1007/s00134-020-06197-x.
- Camporota, L.; Sanderson, B.; Chiumello, D.; Terzi, N.; Argaud, L.; Rimmele, T.; Metuor, R.; Verstraete, A.; Cour, M.; Bohe, J., et al. Prone Position in COVID-19 and -COVID-19 Acute Respiratory Distress Syndrome: An International Multicenter Observational Comparative Study. Crit Care Med 2022, 50, 633-643, doi:10.1097/CCM.0000000000005354.
- Grieco, D.L.; Bongiovanni, F.; Chen, L.; Menga, L.S.; Cutuli, S.L.; Pintaudi, G.; Carelli, S.; Michi, T.; Torrini, F.; Lombardi, G., et al. Respiratory physiology of COVID-19-induced respiratory failure compared to ARDS of other etiologies. Crit Care 2020, 24, 529, doi:10.1186/s13054-020-03253-2.
- Kummer, R.L.; Shapiro, R.S.; Marini, J.J.; Huelster, J.S.; Leatherman, J.W. Paradoxically Improved Respiratory Compliance With Abdominal Compression in COVID-19 ARDS. Chest 2021, 160, 1739-1742, doi:10.1016/j.chest.2021.05.012.
- Gattinoni L, Carlesso E, Cadringher P, Valenza F, Vagginelli F, Chiumello D. Physical and biological triggers of ventilator-induced lung injury and its prevention. Eur Respir J Suppl. 2003;47:15s-25s. doi: 10.1183/09031936.03.00021303. PMID: 14621113.
- Mentzelopoulos, S.D.; Roussos, C.; Zakynthinos, S.G. Prone position reduces lung stress and strain in severe acute respiratory distress syndrome. Eur Respir J 2005, 25, 534-544, doi:10.1183/09031936.05.00105804.
- Marini JJ, Rocco PRM, Gattinoni L. Static and Dynamic Contributors to Ventilator-induced Lung Injury in Clinical Practice. Pressure, Energy, and Power. Am J Respir Crit Care Med. 2020;201(7):767-774.
- Gattinoni L, Tonetti T, Cressoni M, Cadringher P, Herrmann P, Moerer O, Protti A, Gotti M, Chiurazzi C, Carlesso E, Chiumello D, Quintel M. Ventilator-related causes of lung injury: the mechanical power. Intensive Care Med. 2016;42(10):1567-1575. doi: 10.1007/s00134-016-4505-2.
- Coppola S, Caccioppola A, Froio S, Formenti P, De Giorgis V, Galanti V, Consonni D, Chiumello D. Effect of mechanical power on intensive care mortality in ARDS patients. Crit Care. 2020;24(1):246. doi: 10.1186/s13054-020-02963-x.
- Acute Respiratory Distress Syndrome, N.; Brower, R.G.; Matthay, M.A.; Morris, A.; Schoenfeld, D.; Thompson, B.T.; Wheeler, A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 2000, 342, 1301-1308, doi:10.1056/NEJM200005043421801.
- Amato MB, Meade MO, Slutsky AS, Brochard L, Costa EL, Schoenfeld DA, Stewart TE, Briel M, Talmor D, Mercat A, Richard JC, Carvalho CR, Brower RG. Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med. 2015 Feb 19;372(8):747-55. doi: 10.1056/NEJMsa1410639. PMID: 25693014.
- Fan, E.; Del Sorbo, L.; Goligher, E.C.; Hodgson, C.L.; Munshi, L.; Walkey, A.J.; Adhikari, N.K.J.; Amato, M.B.P.; Branson, R.; Brower, R.G., et al. An Official American Thoracic Society/European Society of Intensive Care Medicine/Society of Critical Care Medicine Clinical Practice Guideline: Mechanical Ventilation in Adult Patients with Acute Respiratory Distress Syndrome. Am J Respir Crit Care Med 2017, 195, 1253-1263, doi:10.1164/rccm.201703-0548ST.
- Alhazzani, W.; Moller, M.H.; Arabi, Y.M.; Loeb, M.; Gong, M.N.; Fan, E.; Oczkowski, S.; Levy, M.M.; Derde, L.; Dzierba, A., et al. Surviving Sepsis Campaign: Guidelines on the Management of Critically Ill Adults with Coronavirus Disease 2019 (COVID-19). Crit Care Med 2020, 48, e440-e469, doi:10.1097/CCM.0000000000004363.
- Brochard L, Roudot-Thoraval F, Roupie E, Delclaux C, Chastre J, Fernandez-Mondéjar E, Clémenti E, Mancebo J, Factor P, Matamis D, Ranieri M, Blanch L, Rodi G, Mentec H, Dreyfuss D, Ferrer M, Brun-Buisson C, Tobin M, Lemaire F. Tidal volume reduction for prevention of ventilator-induced lung injury in acute respiratory distress syndrome. The Multicenter Trail Group on Tidal Volume reduction in ARDS. Am J Respir Crit Care Med. 1998;158(6):1831-8. doi: 10.1164/ajrccm.158.6.9801044.
- Yalcin HC, Perry SF, Ghadiali SN. Influence of airway diameter and cell confluence on epithelial cell injury in an in vitro model of airway reopening. J Appl Physiol (1985). 2007 Nov;103(5):1796-807. doi: 10.1152/japplphysiol.00164.2007.
- Gattinoni L, Chiumello D, Caironi P, Busana M, Romitti F, Brazzi L, Camporota L. COVID-19 pneumonia: different respiratory treatments for different phenotypes? Intensive Care Med. 2020;46(6):1099-1102. doi: 10.1007/s00134-020-06033-2.
- Marini JJ, Gattinoni L. Management of COVID-19 Respiratory Distress. JAMA. 2020 ;323(22):2329-2330. doi: 10.1001/jama.2020.6825.
- Gattinoni L, Caironi P, Cressoni M, Chiumello D, Ranieri VM, Quintel M, Russo S, Patroniti N, Cornejo R, Bugedo G. Lung recruitment in patients with the acute respiratory distress syndrome. N Engl J Med. 2006;354(17):1775-86.
- Grasselli, G.; Zangrillo, A.; Zanella, A.; Antonelli, M.; Cabrini, L.; Castelli, A.; Cereda, D.; Coluccello, A.; Foti, G.; Fumagalli, R., et al. Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA 2020, 323, 1574-1581, doi:10.1001/jama.2020.5394.
- Chiumello D, Bonifazi M, Pozzi T, Formenti P, Papa GFS, Zuanetti G, Coppola S. Positive end-expiratory pressure in COVID-19 acute respiratory distress syndrome: the heterogeneous effects. Crit Care. 2021;25(1):431. doi: 10.1186/s13054-021-03839-4.
- Sinha P, Calfee CS, Beitler JR, Soni N, Ho K, Matthay MA, Kallet RH. Physiologic Analysis and Clinical Performance of the Ventilatory Ratio in Acute Respiratory Distress Syndrome. Am J Respir Crit Care Med. 2019 Feb 1;199(3):333-341. doi: 10.1164/rccm.201804-0692OC.
- Gattinoni L, Marini JJ. In search of the Holy Grail: identifying the best PEEP in ventilated patients. Intensive Care Med. 2022;48(6):728-731. doi: 10.1007/s00134-022-06698-x.
- Barthélémy R, Beaucoté V, Bordier R, Collet M, Le Gall A, Hong A, de Roquetaillade C, Gayat E, Mebazaa A, Chousterman BG. Haemodynamic impact of positive end-expiratory pressure in SARS-CoV-2 acute respiratory distress syndrome: oxygenation versus oxygen delivery. Br J Anaesth. 2021;126(2):e70-e72. doi: 10.1016/j.bja.2020.10.026.
- Ball L, Robba C, Maiello L, Herrmann J, Gerard SE, Xin Y, Battaglini D, Brunetti I, Minetti G, Seitun S, Vena A, Giacobbe DR, Bassetti M, Rocco PRM, Cereda M, Castellan L, Patroniti N, Pelosi P; GECOVID (GEnoa COVID-19) group. Computed tomography assessment of PEEP-induced alveolar recruitment in patients with severe COVID-19 pneumonia. Crit Care. 2021;25(1):81. doi: 10.1186/s13054-021-03477-w.
- Protti A, Santini A, Pennati F, Chiurazzi C, Cressoni M, Ferrari M, Iapichino GE, Carenzo L, Lanza E, Picardo G, Caironi P, Aliverti A, Cecconi M. Lung Response to a Higher Positive End-Expiratory Pressure in Mechanically Ventilated Patients With COVID-19. Chest. 2022;161(4):979-988. doi: 10.1016/j.chest.2021.10.012.
- Roesthuis L, van den Berg M, van der Hoeven H. Advanced respiratory monitoring in COVID-19 patients: use less PEEP! Crit Care. 2020;24(1):230. doi: 10.1186/s13054-020-02953-z.
- Perier F, Tuffet S, Maraffi T, Alcala G, Victor M, Haudebourg AF, De Prost N, Amato M, Carteaux G, Mekontso Dessap A. Effect of Positive End-Expiratory Pressure and Proning on Ventilation and Perfusion in COVID-19 Acute Respiratory Distress Syndrome. Am J Respir Crit Care Med. 2020;202(12):1713-1717. doi: 10.1164/rccm.202008-3058LE.
- Mauri T, Spinelli E, Scotti E, Colussi G, Basile MC, Crotti S, Tubiolo D, Tagliabue P, Zanella A, Grasselli G, Pesenti A. Potential for Lung Recruitment and Ventilation-Perfusion Mismatch in Patients With the Acute Respiratory Distress Syndrome From Coronavirus Disease 2019. Crit Care Med. 2020;48(8):1129-1134. doi: 10.1097/CCM.0000000000004386.
- Chen L, Del Sorbo L, Grieco DL, Junhasavasdikul D, Rittayamai N, Soliman I, Sklar MC, Rauseo M, Ferguson ND, Fan E, Richard JM, Brochard L. Potential for Lung Recruitment Estimated by the Recruitment-to-Inflation Ratio in Acute Respiratory Distress Syndrome. A Clinical Trial. Am J Respir Crit Care Med. 2020;201(2):178-187. doi: 10.1164/rccm.201902-0334OC.
- Mentzelopoulos SD, Roussos C, Zakynthinos SG. Prone position reduces lung stress and strain in severe acute respiratory distress syndrome. Eur Respir J. 2005;25(3):534-44. doi: 10.1183/09031936.05.00105804.
- Guérin C, Reignier J, Richard JC, Beuret P, Gacouin A, Boulain T, Mercier E, Badet M, Mercat A, Baudin O, Clavel M, Chatellier D, Jaber S, Rosselli S, Mancebo J, Sirodot M, Hilbert G, Bengler C, Richecoeur J, Gainnier M, Bayle F, Bourdin G, Leray V, Girard R, Baboi L, Ayzac L; PROSEVA Study Group. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013;368(23):2159-68. doi: 10.1056/NEJMoa1214103.
- Gattinoni L, Taccone P, Carlesso E, Marini JJ. Prone position in acute respiratory distress syndrome. Rationale, indications, and limits. Am J Respir Crit Care Med. 2013;188(11):1286-93. doi: 10.1164/rccm.201308-1532CI.
- Chen L, Zhang Y, Li Y, Song C, Lin F, Pan P. The Application of Awake-Prone Positioning Among Non-intubated Patients With COVID-19-Related ARDS: A Narrative Review. Front Med (Lausanne). 2022;9:817689. doi: 10.3389/fmed.2022.817689.
- Guérin C, Albert RK, Beitler J, Gattinoni L, Jaber S, Marini JJ, Munshi L, Papazian L, Pesenti A, Vieillard-Baron A, Mancebo J. Prone position in ARDS patients: why, when, how and for whom. Intensive Care Med. 2020;46(12):2385-2396. doi: 10.1007/s00134-020-06306-w.
- Albert RK, Hubmayr RD. The prone position eliminates compression of the lungs by the heart. Am J Respir Crit Care Med. 2000;161(5):1660-5. doi: 10.1164/ajrccm.161.5.9901037.
- Tobin MJ. Mechanical ventilation. N Engl J Med. 1994;330(15):1056-61. doi: 10.1056/NEJM199404143301507.
- Guerin C, Baboi L, Richard JC. Mechanisms of the effects of prone positioning in acute respiratory distress syndrome. Intensive Care Med. 2014;40(11):1634-42. doi: 10.1007/s00134-014-3500-8.
- Galiatsou E, Kostanti E, Svarna E, Kitsakos A, Koulouras V, Efremidis SC, Nakos G. Prone position augments recruitment and prevents alveolar overinflation in acute lung injury. Am J Respir Crit Care Med. 2006 Jul 15;174(2):187-97. doi: 10.1164/rccm.200506-899OC.
- Mutoh T, Guest RJ, Lamm WJ, Albert RK. Prone position alters the effect of volume overload on regional pleural pressures and improves hypoxemia in pigs in vivo. Am Rev Respir Dis. 1992;146(2):300-6. doi: 10.1164/ajrccm/146.2.300.
- Mure M, Domino KB, Lindahl SG, Hlastala MP, Altemeier WA, Glenny RW. Regional ventilation-perfusion distribution is more uniform in the prone position. J Appl Physiol (1985). 2000;88(3):1076-83. doi: 10.1152/jappl.2000.88.3.1076.
- Musch G, Layfield JD, Harris RS, Melo MF, Winkler T, Callahan RJ, Fischman AJ, Venegas JG. Topographical distribution of pulmonary perfusion and ventilation, assessed by PET in supine and prone humans. J Appl Physiol (1985). 2002;93(5):1841-51. doi: 10.1152/japplphysiol.00223.2002.
- Henderson AC, Sá RC, Theilmann RJ, Buxton RB, Prisk GK, Hopkins SR. The gravitational distribution of ventilation-perfusion ratio is more uniform in prone than supine posture in the normal human lung. J Appl Physiol (1985). 2013;115(3):313-24. doi: 10.1152/japplphysiol.01531.2012.
- Lamm WJ, Graham MM, Albert RK. Mechanism by which the prone position improves oxygenation in acute lung injury. Am J Respir Crit Care Med. 1994;150(1):184-93. doi: 10.1164/ajrccm.150.1.8025748.
- Wiener CM, Kirk W, Albert RK. Prone position reverses gravitational distribution of perfusion in dog lungs with oleic acid-induced injury. J Appl Physiol (1985). 1990;68(4):1386-92. doi: 10.1152/jappl.1990.68.4.1386.
- Glenny RW, Lamm WJ, Albert RK, Robertson HT. Gravity is a minor determinant of pulmonary blood flow distribution. J Appl Physiol (1985). 1991;71(2):620-9. doi: 10.1152/jappl.1991.71.2.620.
- Langer T, Brioni M, Guzzardella A, Carlesso E, Cabrini L, Castelli G, Dalla Corte F, De Robertis E, Favarato M, Forastieri A, Forlini C, Girardis M, Grieco DL, Mirabella L, Noseda V, Previtali P, Protti A, Rona R, Tardini F, Tonetti T, Zannoni F, Antonelli M, Foti G, Ranieri M, Pesenti A, Fumagalli R, Grasselli G; PRONA-COVID Group. Prone position in intubated, mechanically ventilated patients with COVID-19: a multi-centric study of more than 1000 patients. Crit Care. 2021;25(1):128. doi: 10.1186/s13054-021-03552-2.
- Fossali T, Pavlovsky B, Ottolina D, Colombo R, Basile MC, Castelli A, Rech R, Borghi B, Ianniello A, Flor N, Spinelli E, Catena E, Mauri T. Effects of Prone Position on Lung Recruitment and Ventilation-Perfusion Matching in Patients With COVID-19 Acute Respiratory Distress Syndrome: A Combined CT Scan/Electrical Impedance Tomography Study. Crit Care Med. 2022;50(5):723-732. doi: 10.1097/CCM.0000000000005450.
- Mathews KS, Soh H, Shaefi S, Wang W, Bose S, Coca S, Gupta S, Hayek SS, Srivastava A, Brenner SK, Radbel J, Green A, Sutherland A, Leonberg-Yoo A, Shehata A, Schenck EJ, Short SAP, Hernán MA, Chan L, Leaf DE; STOP-COVID Investigators. Prone Positioning and Survival in Mechanically Ventilated Patients With Coronavirus Disease 2019-Related Respiratory Failure. Crit Care Med. 2021;49(7):1026-1037. doi: 10.1097/CCM.0000000000004938.
- Gattinoni L, Caironi P. Prone positioning: beyond physiology. Anesthesiology. 2010;113(6):1262-4. doi: 10.1097/ALN.0b013e3181fcd97e.
- McNicholas BA, Ehrmann S, Laffey JG. Awake prone positioning. Intensive Care Med. 2022. doi: 10.1007/s00134-022-06893-w. Epub ahead of print.
- Ehrmann S, Li J, Ibarra-Estrada M, Perez Y, Pavlov I, McNicholas B, Roca O, Mirza S, Vines D, Garcia-Salcido R, Aguirre-Avalos G, Trump MW, Nay MA, Dellamonica J, Nseir S, Mogri I, Cosgrave D, Jayaraman D, Masclans JR, Laffey JG, Tavernier E; Awake Prone Positioning Meta-Trial Group. Awake prone positioning for COVID-19 acute hypoxaemic respiratory failure: a randomised, controlled, multinational, open-label meta-trial. Lancet Respir Med. 2021;9(12):1387-1395. doi: 10.1016/S2213-2600(21)00356-8.
- Ibarra-Estrada M, Li J, Pavlov I, Perez Y, Roca O, Tavernier E, McNicholas B, Vines D, Marín-Rosales M, Vargas-Obieta A, García-Salcido R, Aguirre-Díaz SA, López-Pulgarín JA, Chávez-Peña Q, Mijangos-Méndez JC, Aguirre-Avalos G, Ehrmann S, Laffey JG. Factors for success of awake prone positioning in patients with COVID-19-induced acute hypoxemic respiratory failure: analysis of a randomized controlled trial. Crit Care. 2022;26(1):84. doi: 10.1186/s13054-022-03950-0.
- Cammarota G, Rossi E, Vitali L, Simonte R, Sannipoli T, Anniciello F, Vetrugno L, Bignami E, Becattini C, Tesoro S, Azzolina D, Giacomucci A, Navalesi P, De Robertis E. Effect of awake prone position on diaphragmatic thickening fraction in patients assisted by noninvasive ventilation for hypoxemic acute respiratory failure related to novel coronavirus disease. Crit Care. 2021 ;25(1):305. doi: 10.1186/s13054-021-03735-x.
- Ferrando C, Mellado-Artigas R, Gea A, Arruti E, Aldecoa C, Adalia R, Ramasco F, Monedero P, Maseda E, Tamayo G, Hernández-Sanz ML, Mercadal J, Martín-Grande A, Kacmarek RM, Villar J, Suárez-Sipmann F; COVID-19 Spanish ICU Network. Awake prone positioning does not reduce the risk of intubation in COVID-19 treated with high-flow nasal oxygen therapy: a multicenter, adjusted cohort study. Crit Care. 2020 Oct 6;24(1):597. doi: 10.1186/s13054-020-03314-6.
- Yoshida T, Tanaka A, Roldan R, Quispe R, Taenaka H, Uchiyama A, Fujino Y. Prone Position Reduces Spontaneous Inspiratory Effort in Patients with Acute Respiratory Distress Syndrome: A Bicenter Study. Am J Respir Crit Care Med. 2021;203(11):1437-1440. doi: 10.1164/rccm.202012-4509LE.
- Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, Hohmann E, Chu HY, Luetkemeyer A, Kline S, Lopez de Castilla D, Finberg RW, Dierberg K, Tapson V, Hsieh L, Patterson TF, Paredes R, Sweeney DA, Short WR, Touloumi G, Lye DC, Ohmagari N, Oh MD, Ruiz-Palacios GM, Benfield T, Fätkenheuer G, Kortepeter MG, Atmar RL, Creech CB, Lundgren J, Babiker AG, Pett S, Neaton JD, Burgess TH, Bonnett T, Green M, Makowski M, Osinusi A, Nayak S, Lane HC; ACTT-1 Study Group Members. Remdesivir for the Treatment of Covid-19 - Final Report. N Engl J Med. 2020;383(19):1813-1826. doi: 10.1056/NEJMoa2007764.
- Goldman JD, Lye DCB, Hui DS, Marks KM, Bruno R, Montejano R, Spinner CD, Galli M, Ahn MY, Nahass RG, Chen YS, SenGupta D, Hyland RH, Osinusi AO, Cao H, Blair C, Wei X, Gaggar A, Brainard DM, Towner WJ, Muñoz J, Mullane KM, Marty FM, Tashima KT, Diaz G, Subramanian A; GS-US-540-5773 Investigators. Remdesivir for 5 or 10 Days in Patients with Severe Covid-19. N Engl J Med. 2020;383(19):1827-1837. doi: 10.1056/NEJMoa2015301.
- RECOVERY Collaborative Group, Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, Staplin N, Brightling C, Ustianowski A, Elmahi E, Prudon B, Green C, Felton T, Chadwick D, Rege K, Fegan C, Chappell LC, Faust SN, Jaki T, Jeffery K, Montgomery A, Rowan K, Juszczak E, Baillie JK, Haynes R, Landray MJ. Dexamethasone in Hospitalized Patients with Covid-19. N Engl J Med. 2021;384(8):693-704. doi: 10.1056/NEJMoa2021436.
- WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group, Sterne JAC, Murthy S, Diaz JV, Slutsky AS, Villar J, Angus DC, Annane D, Azevedo LCP, Berwanger O, Cavalcanti AB, Dequin PF, Du B, Emberson J, Fisher D, Giraudeau B, Gordon AC, Granholm A, Green C, Haynes R, Heming N, Higgins JPT, Horby P, Jüni P, Landray MJ, Le Gouge A, Leclerc M, Lim WS, Machado FR, McArthur C, Meziani F, Møller MH, Perner A, Petersen MW, Savovic J, Tomazini B, Veiga VC, Webb S, Marshall JC. Association Between Administration of Systemic Corticosteroids and Mortality Among Critically Ill Patients With COVID-19: A Meta-analysis. JAMA. 2020;324(13):1330-1341. doi: 10.1001/jama.2020.17023.
- WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group, Shankar-Hari M, Vale CL, Godolphin PJ, Fisher D, Higgins JPT, Spiga F, Savovic J, Tierney J, Baron G, Benbenishty JS, Berry LR, Broman N, Cavalcanti AB, Colman R, De Buyser SL, Derde LPG, Domingo P, Omar SF, Fernandez-Cruz A, Feuth T, Garcia F, Garcia-Vicuna R, Gonzalez-Alvaro I, Gordon AC, Haynes R, Hermine O, Horby PW, Horick NK, Kumar K, Lambrecht BN, Landray MJ, Leal L, Lederer DJ, Lorenzi E, Mariette X, Merchante N, Misnan NA, Mohan SV, Nivens MC, Oksi J, Perez-Molina JA, Pizov R, Porcher R, Postma S, Rajasuriar R, Ramanan AV, Ravaud P, Reid PD, Rutgers A, Sancho-Lopez A, Seto TB, Sivapalasingam S, Soin AS, Staplin N, Stone JH, Strohbehn GW, Sunden-Cullberg J, Torre-Cisneros J, Tsai LW, van Hoogstraten H, van Meerten T, Veiga VC, Westerweel PE, Murthy S, Diaz JV, Marshall JC, Sterne JAC. Association Between Administration of IL-6 Antagonists and Mortality Among Patients Hospitalized for COVID-19: A Meta-analysis. JAMA. 2021;326(6):499-518. doi: 10.1001/jama.2021.11330.
- Kalil AC, Patterson TF, Mehta AK, Tomashek KM, Wolfe CR, Ghazaryan V, Marconi VC, Ruiz-Palacios GM, Hsieh L, Kline S, Tapson V, Iovine NM, Jain MK, Sweeney DA, El Sahly HM, Branche AR, Regalado Pineda J, Lye DC, Sandkovsky U, Luetkemeyer AF, Cohen SH, Finberg RW, Jackson PEH, Taiwo B, Paules CI, Arguinchona H, Erdmann N, Ahuja N, Frank M, Oh MD, Kim ES, Tan SY, Mularski RA, Nielsen H, Ponce PO, Taylor BS, Larson L, Rouphael NG, Saklawi Y, Cantos VD, Ko ER, Engemann JJ, Amin AN, Watanabe M, Billings J, Elie MC, Davey RT, Burgess TH, Ferreira J, Green M, Makowski M, Cardoso A, de Bono S, Bonnett T, Proschan M, Deye GA, Dempsey W, Nayak SU, Dodd LE, Beigel JH; ACTT-2 Study Group Members. Baricitinib plus Remdesivir for Hospitalized Adults with Covid-19. N Engl J Med. 2021;384(9):795-807. doi: 10.1056/NEJMoa2031994.
- Marconi VC, Ramanan AV, de Bono S, Kartman CE, Krishnan V, Liao R, Piruzeli MLB, Goldman JD, Alatorre-Alexander J, de Cassia Pellegrini R, Estrada V, Som M, Cardoso A, Chakladar S, Crowe B, Reis P, Zhang X, Adams DH, Ely EW; COV-BARRIER Study Group. Efficacy and safety of baricitinib for the treatment of hospitalised adults with COVID-19 (COV-BARRIER): a randomised, double-blind, parallel-group, placebo-controlled phase 3 trial. Lancet Respir Med. 2021;9(12):1407-1418. doi: 10.1016/S2213-2600(21)00331-3.. Erratum in: Lancet Respir Med. 2021 Oct;9(10):e102.
- Wolfe CR, Tomashek KM, Patterson TF, Gomez CA, Marconi VC, Jain MK, Yang OO, Paules CI, Palacios GMR, Grossberg R, Harkins MS, Mularski RA, Erdmann N, Sandkovsky U, Almasri E, Pineda JR, Dretler AW, de Castilla DL, Branche AR, Park PK, Mehta AK, Short WR, McLellan SLF, Kline S, Iovine NM, El Sahly HM, Doernberg SB, Oh MD, Huprikar N, Hohmann E, Kelley CF, Holodniy M, Kim ES, Sweeney DA, Finberg RW, Grimes KA, Maves RC, Ko ER, Engemann JJ, Taylor BS, Ponce PO, Larson L, Melendez DP, Seibert AM, Rouphael NG, Strebe J, Clark JL, Julian KG, de Leon AP, Cardoso A, de Bono S, Atmar RL, Ganesan A, Ferreira JL, Green M, Makowski M, Bonnett T, Beresnev T, Ghazaryan V, Dempsey W, Nayak SU, Dodd LE, Beigel JH, Kalil AC; ACTT-4 Study Group. Baricitinib versus dexamethasone for adults hospitalised with COVID-19 (ACTT-4): a randomised, double-blind, double placebo-controlled trial. Lancet Respir Med. 2022;10(9):888-899. doi: 10.1016/S2213-2600(22)00088-1.
- Kyriazopoulou E, Poulakou G, Milionis H, Metallidis S, Adamis G, Tsiakos K, Fragkou A, Rapti A, Damoulari C, Fantoni M, Kalomenidis I, Chrysos G, Angheben A, Kainis I, Alexiou Z, Castelli F, Serino FS, Tsilika M, Bakakos P, Nicastri E, Tzavara V, Kostis E, Dagna L, Koufargyris P, Dimakou K, Savvanis S, Tzatzagou G, Chini M, Cavalli G, Bassetti M, Katrini K, Kotsis V, Tsoukalas G, Selmi C, Bliziotis I, Samarkos M, Doumas M, Ktena S, Masgala A, Papanikolaou I, Kosmidou M, Myrodia DM, Argyraki A, Cardellino CS, Koliakou K, Katsigianni EI, Rapti V, Giannitsioti E, Cingolani A, Micha S, Akinosoglou K, Liatsis-Douvitsas O, Symbardi S, Gatselis N, Mouktaroudi M, Ippolito G, Florou E, Kotsaki A, Netea MG, Eugen-Olsen J, Kyprianou M, Panagopoulos P, Dalekos GN, Giamarellos-Bourboulis EJ. Early treatment of COVID-19 with anakinra guided by soluble urokinase plasminogen receptor plasma levels: a double-blind, randomized controlled phase 3 trial. Nat Med. 2021;27(10):1752-1760. doi: 10.1038/s41591-021-01499-z.. Erratum in: Nat Med. 2021 Oct 8;: PMID: 34480127; PMCID: PMC8516650.
- Ramanathan K, Shekar K, Ling RR, Barbaro RP, Wong SN, Tan CS, Rochwerg B, Fernando SM, Takeda S, MacLaren G, Fan E, Brodie D. Extracorporeal membrane oxygenation for COVID-19: a systematic review and meta-analysis. Crit Care. 2021;25(1):211. doi: 10.1186/s13054-021-03634-1. Erratum in: Crit Care. 2021 Oct 27;25(1):375.
- Giani M, Rezoagli E, Guervilly C, Rilinger J, Duburcq T, Petit M, Textoris L, Garcia B, Wengenmayer T, Grasselli G, Pesenti A, Combes A, Foti G, Schmidt M; EuroPronECMO Investigators. Prone positioning during venovenous extracorporeal membrane oxygenation for acute respiratory distress syndrome: a pooled individual patient data analysis. Crit Care. 2022;26(1):8. doi: 10.1186/s13054-021-03879-w.
- Poon WH, Ramanathan K, Ling RR, Yang IX, Tan CS, Schmidt M, Shekar K. Prone positioning during venovenous extracorporeal membrane oxygenation for acute respiratory distress syndrome: a systematic review and meta-analysis. Crit Care. 2021;25(1):292. doi: 10.1186/s13054-021-03723-1.
- Laghlam D, Charpentier J, Hamou ZA, Nguyen LS, Pene F, Cariou A, Mira JP, Jozwiak M. Effects of Prone Positioning on Respiratory Mechanics and Oxygenation in Critically Ill Patients With COVID-19 Requiring Venovenous Extracorporeal Membrane Oxygenation. Front Med (Lausanne). 2022;8:810393. doi: 10.3389/fmed.2021.810393.
- Giani M, Martucci G, Madotto F, Belliato M, Fanelli V, Garofalo E, Forlini C, Lucchini A, Panarello G, Bottino N, Zanella A, Fossi F, Lissoni A, Peroni N, Brazzi L, Bellani G, Navalesi P, Arcadipane A, Pesenti A, Foti G, Grasselli G. Prone Positioning during Venovenous Extracorporeal Membrane Oxygenation in Acute Respiratory Distress Syndrome. A Multicenter Cohort Study and Propensity-matched Analysis. Ann Am Thorac Soc. 2021;18(3):495-501. doi: 10.1513/AnnalsATS.202006-625OC.




