
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Francisco Javier Alvarez-Martinez | + 3376 word(s) | 3376 | 2020-10-14 08:17:31 | | | |
| 2 | Nicole Yin | Meta information modification | 3376 | 2020-11-04 03:20:58 | | |
Video Upload Options
Drug-resistant bacteria pose a serious threat to human health worldwide. Current antibiotics are losing efficacy and new antimicrobial agents are urgently needed. Living organisms are an invaluable source of antimicrobial compounds. The antimicrobial activity of the most representative natural products of animal, bacterial, fungal and plant origin are reviewed in this article. Their activity against drug-resistant bacteria, their mechanisms of action, the possible development of resistance against them, their role in current medicine and their future perspectives are discussed. Natural compounds of heterogeneous origins have been shown to possess antimicrobial capabilities, including against antibiotic-resistant bacteria. The most commonly found mechanisms of antimicrobial action are related to protein biosynthesis and alteration of cell walls and membranes. Various natural compounds, especially phytochemicals, have shown synergistic capacity with antibiotics. There is little literature on the development of specific resistance mechanisms against natural antimicrobial compounds. New technologies such as -omics, network pharmacology and informatics have the potential to identify and characterize new natural antimicrobial compounds in the future. This knowledge may be useful for the development of future therapeutic strategies.
1. Introduction
Antibiotic resistance is an example of the enormous capacity for natural evolution and adaptation of bacteria to different environments[1][2]. Although this process seems inevitable, humans have accelerated it through various anthropogenic activities[3][4]. The causes behind the increase in the number of antimicrobial-resistant bacteria in recent years include the misuse of antibiotics in humans and animals, inadequate[3][4][5] control of infections in hospitals and clinics or poor hygiene and sanitation[3][4][5]. In addition to the causes mentioned, the problem worsens as there is a drought in the discovery of new antibiotics. The increase in resistance rates in bacteria leads to a decrease in the effectiveness of existing antibiotics, making research in this field unattractive to companies that decide to invest in other types of fields with greater chances of success and benefits[6][7]. This concerning trend can be observed in Figure 1.
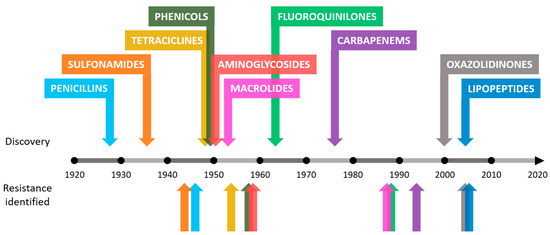
Figure 1. Approximate dates of discovery of new classes of antibiotics and identification of bacterial resistance.
In view of this scenario, research on alternative or complementary therapies to traditional antibiotics has emerged strongly. Antimicrobial products of natural origin have been positioned as compounds of great scientific interest due to their enormous chemical variety and intrinsic properties that have promoted their study as a possible therapeutic tool in recent years.
2. Main Classes of Natural Antimicrobial Products
NPs are extremely diverse in terms of their chemical structures, properties and mechanisms of action. These agents can be classified according to their original source: animal, bacterial, fungal or vegetal.
2.1. Animal origin
Animals have colonized virtually the entire planet Earth. For thousands of years, they have lived closely with different kinds of bacteria and have faced not a few pathogenic microorganisms. Evolution has shaped animal defense systems to deal with these microscopic threats. In recent years, attention has been focused on identifying which molecules confer resistance and allow certain animals to live in hostile environments with high pollution and pathogenic load, as is the case with certain insects such as cockroaches.
Currently, animals, and especially insects, are one of the main sources of antimicrobial proteins or peptides (AMPs). Since the discovery of AMPs in 1974, more than 150 new AMPs have been isolated or identified, the majority being cationic peptides between 20 and 50 residues in length. These molecules mainly have antimicrobial capacity mediated by disruption of the bacterial plasma membrane, most probably by forming pores or ion channels[8]. Some AMPs also have shown antifungal, antiparasitic or antiviral properties[9]. These AMPs can be divided into four subfamilies with different structures and sequences: the α-helical peptides, such as cecropin, which has a broad spectrum of antimicrobial activity against bacteria of both Gram-positive and Gram-negative bacteria; cysteine-rich peptides, such as insect defensins, which are mainly active against Gram-positive bacteria; proline-rich peptides, such as lebocins, which are active against both Gram-positive and Gram-negative bacteria and some fungi; and finally glycine-rich peptides or proteins, such as attacin, which are effective against Gram-negative bacteria and especially against Escherichia coli. These AMPs present a promising basis for the development of medical therapies, however, additional work must be developed to make them more powerful and stable[10]. Moreover, the intrinsic antimicrobial capacity of AMPs can be enhanced by a fusion of peptides to create more potent hybrid ones, such as in the case of attacin from Spodoptera exigua and a coleoptericin-like protein from Protaetia brevitarsis seulensis, which, when fused, exhibited a greater antimicrobial capacity than its two original peptides[11].
The study of antimicrobial molecules existent in cockroaches (Periplaneta americana) has revealed that extracts derived from its brain have a great antimicrobial capacity against MRSA and neuropathogenic E. coli K1. Although not all the components of the extract could be accurately identified, a great variety of molecules with known biological activity were found, such as isoquinolines, flavanones, sulfonamides and imidazone among others. A hypothesis about the production of this antimicrobial cocktail in the cockroach brain suggests that there could be a constitutive expression of these antimicrobials to protect the animal’s neural system, since it is the central axis of its survival and a key piece to protect when it is lived in an environment of high pollution and exposure to pathogens and even superbugs[12]. Another example of insect producing antimicrobial molecules against resistant bacteria is Lucilia cuprina blowfly maggots. The extract obtained from excretions and secretions from maggots showed mild bacterial growth inhibition. However, using subinhibitory concentrations of this extract in combination with the antibiotic ciprofloxacin enhanced its activity, further delaying the appearance of bacteria resistant to it. The properties of this extract, including the presence of defensins and phenylacetaldehyde, make maggot debridement therapy a promising tool in the treatment of MRSA-infected wounds acquired in hospital[13].
2.2. Bacterial origin
Bacteria are the most prolific source of NPs with antimicrobial activity found so far, especially those of the actinomycetes class. Their great diversity, competitiveness and colonization capacity have led them to the development of secondary metabolites capable of giving them great advantages over other bacterial species. As described in previous sections, the detection and isolation of these bacterial antimicrobial NPs propelled medical science vertiginously in the middle of the last century. Some of the most relevant are described below.
Some of the most important antimicrobial molecules produced by bacteria of the actinomyces class are: vancomycin, baulamycin, fasamycin A and orthoformimycin. Vancomycin is a naturally occurring tricyclic glycopeptide extracted from Streptococcus orientalis that has reaped great success as an antibiotic against Gram-positive bacteria, especially against threats that are resistant to other treatments such as MRSA and penicillin-resistant pneumococci among others[14]. Vancomycin forms hydrogen bonds with the terminal dipeptide of the nascent peptidoglycan chain during biosynthesis of the bacterial cell wall. This union prevents the action of penicillin-binding proteins (PBPs), interrupting further wall formation and finally activating autolysin-triggered cell rupture and cell death[15]. Another important bacterial NP is produced by actinomyces is baulamycin, which is an isolated molecule of the marine bacterium Streptomyces tempisquensis that can inhibit the biosynthesis of iron-chelating siderophores in S. aureus (targeting staphylopherrin B) and Bacillus anthracis (targeting petrobactin), helping to treat MRSA and anthrax infections, respectively. In addition, it was also able to inhibit the growth of Gram-negative bacteria such as S. flexneri and E. coli, turning baulamycin and its derivatives into potential broad-spectrum antibiotics[16]. Fasamycin A is a polyketide isolated from Streptomyces albus that shows specific antimicrobial activity against Gram-positive bacteria such as vancomycin-resistant Enterococci (VRE) and MRSA with MIC values of 0.8 and 3.1 µg/mL, respectively. This molecule targets FabF in the initial condensation step of the elongation cycle from the lipidic biosynthetic bacterial metabolism[17]. Orthoformimycin is a molecule produced by S. griseus which can inhibit bacterial translation by more than 80% in the case of E. coli. Although the mechanism of action is not clear now, one hypothesis is the decoupling of mRNA and aminoacyl-tRNA in the bacterial ribosome[18].
2.3. Fungal origin
Fungi are eukaryotic-type living things, such as mushrooms, yeasts, and molds. Currently, the existence of some 120,000 species of fungi has been accepted, however, it is estimated that the number of different species of fungi present on earth could be between 2.2 and 3.8 million[19]. This relatively unexplored kingdom is a source of antimicrobial NPs and has great potential to be studied in the future as new species are discovered and identified.
Aspergillomarasmine A is a polyaminoacid naturally produced by Aspergillus versicolor capable of inhibiting antibiotic resistance enzymes in Gram-negative pathogenic bacteria, such as Enterobacteriaceae, Acinetobacter spp., Pseudomonas spp. and Klebsiella pneumoniae. This compound has been used successfully to reverse resistance in mice infected with meropenem-resistant K. pneumoniae thanks to the NDM-I protein, making the bacterium sensitive to the antibiotic and ending the infection[20].
Mirandamycin is a quinol of fungal origin capable of inhibiting the growth of both Gram-negative and Gram-positive bacteria, being more effective against the latter group, including antibiotic-resistant strains such as MRSA or carbapenemase-producing K. pneumoniae. Its mechanism of action consists in the inhibition of the bacterial metabolism of sugars, interfering with their fermentation and transport[21].
There is evidence of the antibacterial capacity of various fungal species against Gram-positive bacteria. Extracts of Ganoderma lucidum, Ganoderma applanatum, Meripilus giganteus, Laetiporus sulphureus, Flammulina velutipes, Coriolus versicolor, Pleurotus ostreatus and Panus tigrinus demonstrated antimicrobial activity in Kirby–Bauer assays against Gram-positive bacteria, such as S. auerus and B. luteus[22].
2.4. Plant origin
Plants are a great source of biomolecules with various interesting properties for humans thanks to their enormous diversity and proven safety for human health[23]. Being sessile organisms, evolution has shaped its metabolism to produce certain molecules to cope with external aggressions and infections, since they cannot flee or defend themselves[24]. The Dictionary of Natural Products lists approximately 200,000 secondary plant metabolites, of which 170,000 have unique chemical structures[25]. Some of the families of molecules with antimicrobial capacity produced by plants are alkaloids, terpenoids, and polyphenols[26].
Plants that have been used in traditional medicine in various countries of the world for thousands of years. They are currently being studied at the molecular and functional level, rediscovering their properties and explaining their mechanisms of action.
Alkaloids have been shown to possess antimicrobial capacity against various bacterial species. Although studies of the antimicrobial capacity of pure alkaloids are limited, there are several studies on the antimicrobial activity of plant extracts that contain alkaloids as their main components. Different extracts rich in alkaloids obtained from Papaver rhoeas have shown activity against S. aureus, Staphylococcus epidermidis and K. pneumoniae, the main active component being roemerine[27]. Raw alkaloid-rich extracts of Annona squamosa seeds and Annona muricata root have also shown moderate antimicrobial capacity against E. coli and S. aureus[28].
Terpenoids, along with other families of compounds, are part of plant essential oils, many of which possess antimicrobial activity. Various in vitro studies affirm that terpenoids do not possess significant antimicrobial activity per se[29]. However, they can contribute to the antimicrobial activity of complete essential oils thanks to their hydrophobic nature and a low molecular weight that allow them to disrupt the cell wall and facilitate the action of the rest of the active components[30].
Polyphenols are molecules present in plants with a function of defense against stress and have one or more phenolic groups in their chemical structure as a common feature. There is abundant literature on the antimicrobial capacity of polyphenols and extracts of plants rich in them that have bactericidal and bacteriostatic capacity against many pathogens, both Gram-positive and Gram-negative. The potential use of polyphenols as antimicrobials is widely studied to be applied in different areas such as agriculture[31], food preservation[32] and medicine[33].
There are several subfamilies within the group of polyphenols according to their differentiated chemical structures: flavonoids, hydrolyzable tannins, lignans, phenolic acids and stilbenes. In turn, the flavonoid group can be subdivided into other subfamilies: anthocyanidins, flavanones, flavones, flavonols and isoflavones[34]. Examples of flavonoids with antimicrobial activity are quercetin[35], kaempferol[36], morin[37], myricetin[38] epigallocatechin gallate[39] or galangin[40] among many others[34][41]. Other known polyphenols with good antimicrobial activity are punicalagin, which exerts both antibacterial and antibiofilm effect against S. aureus[38][42], and resveratrol, which has antimicrobial activity against a wide range of bacteria[33].
In addition to synergy with antibiotics, there are also studies that point to the synergy between the polyphenols themselves, such as that between EGCg and quercetin against MRSA, attributed to a co-permeabilization process that would facilitate the activity of the compounds inside of the cell[43]. Synergic activity has also been found between the polyphenols quercetin-3-glucoside, punicalagin, ellagic acid and myricetin in different proportions and combinations against S. aureus CECT 59[38].
Apart from the antimicrobial use of concrete molecules of plant origin, the use of complex extracts made from different parts of plants is common and effective. Plant extracts have a great diversity in their composition, since even from the same plant multiple completely different extracts can be obtained varying the extraction conditions. Time, temperature, solvents, pressure and other parameters such as the use of ultrasound or microwave have a huge impact on the final extract composition[44]. There is numerous evidence of the antimicrobial activity of plant extracts[34][45] and the synergistic effect that exists between different phytochemicals[38] when acting against different bacteria. An example of a plant extract with potent activity against AMR bacteria are extracts from Lantana camara leaves against clinical isolates of MRSA, Streptococcus pyogenes, VRE, Acinetobacter baumannii, Citrobacter freundii, Proteus mirabilis, Proteus vulgaris and P. aeruginosa[46]. The ethanolic extracts of Anthocephalus cadamba, Pterocarpus santalinus and Butea monosperma Lam. they have also demonstrated antimicrobial activity against MDR clinical isolates of 10 different microbial species: S. aureus, Acinetobacter sp., C. freundii, Chromobacterium violeceum, E. coli, Klebsiella sp., Proteus sp., P. aeruginosa, Salmonella typhi and Vibrio cholerae[47][48]. In the case of B. monosperma Lam., antimicrobial activity was also found in the extract made with hot water from leaf.
2.5. Summary
As a summary, Table 1 contains all the NPs mentioned above together with their producing organism, type, target bacteria, mechanism of action, main use and references. Figure 2 shows the main molecular targets of the most relevant antimicrobial NPs.
Table 1. Alphabetically ordered natural products (NPs) with their properties and capabilities. Asterisk (*) means no antimicrobial activity alone.
|
Natural Product |
Productor Organism |
Type of Organism |
Activity Against |
Mechanism of Action |
Main Use |
Reference |
|
Actinorhodin |
Streptomyces coelicolor |
Actinomycete |
Gram-positive, including multidrug-resistant S. aureus |
ROS production inside bacterial cells |
Research |
[49] |
|
Albomycin |
Streptomyces sp. ATCC 700974 |
Actinomycete |
Gram-negative and Gram-positive, including MRSA |
Seryl t-RNA synthetase inhibition |
Medicine |
|
|
Amphomycin |
Streptomyces canus |
Actinomycete |
Gram-positive, including MRSA, VRE and MDR S. pneumoniae |
Inhibition of peptidoglycan and wall teichoic acid biosyntheses |
Medicine |
[52] |
|
Apramycin |
Streptoalloteicus hindustanus |
Actinomycete |
Gram-negative, including MDR A. baumannii and P. areuginosa |
Inhibition of protein synthesis |
Veterinary |
[53] |
|
Arlomycins |
Streptomyces sp. Tü 6075 |
Actinomycete |
Gram-positive and Gram-negative |
Inhibition of type I bacterial signal peptidase |
In research for medical use |
[54] |
|
Aspergillomarasmine A* |
A. versicolor |
Fungus |
Sensitivizes carbapenem-resistant bacteria |
Inhibition of bacterial metallo-β-lactamases |
In research for medical use |
[20] |
|
Carbomycin |
Streptomyces halstedii |
Actinomycete |
Gram-positive and Mycoplasma |
Inhibition of protein synthesis |
Medicine |
[55] |
|
Cathelicidin-BF |
Bungarus fasciatus |
Reptile |
Mainly Gram-negative, including MDR strains |
Damage in microbial cytoplasmic membrane |
Research |
[56] |
|
CaTx-II |
C. adamanteus |
Reptile |
Gram-positive and Gram-negative |
Membrane pore formation and cell wall disintegration |
Research |
[57] |
|
Cecropin A |
Aedes aegypti |
Insect |
Gram-negative |
Disruption of the cytoplasmic membrane |
In research for medical use |
[58] |
|
Cephalosporin |
Cephalosporium acremonium |
Fungus |
Gram-positive and Gram-negative |
Inhibition of cell wall synthesis |
Medicine |
[59] |
|
Cephamycin C |
Streptomyces clavuligerus |
Actinomycete |
Gram-positive and Gram-negative |
Inhibition of cell wall synthesis |
Medicine and veterinary |
[60] |
|
Chloramphenicol |
Streptomyces venezuelae |
Actinomycete |
Gram-positive and Gram-negative |
Inhibition of protein synthesis |
Medicine and veterinary |
[61] |
|
Chloroeremomycin |
Amycolatopsis orientalis |
Actinomycete |
Gram-positive, including VRE |
Inhibition of bacterial cell wall formation |
Medicine |
[62] |
|
Clavulanic acid* |
S. clavuligerus |
Actinomycete |
Sensitivizes β-lactam-resistant bacteria |
β-lactamase inhibitor |
Medicine and veterinary |
[63] |
|
Clorobiocin |
Strteptomyces roseochromogenes |
Actinomycete |
Gram-positive |
Inhibitors of DNA gyrase |
Medicine |
[64] |
|
Coumermycin |
Streptomyces rishiriensis |
Actinomycete |
Mainly Gram-positive |
Inhibition of DNA gyrase |
Research |
|
|
Dalbavancin |
Nonomuraea sp. |
Actinomycete |
Gram-positive, including MRSA |
Inhibition of cell wall synthesis |
Medicine |
[67] |
|
Daptomycin |
Streptomyces roseosporus |
Actinomycete |
Gram-positive |
Inhibition of protein, DNA and RNA synthesis |
Medicine |
[68] |
|
Epigallocatechin gallate |
Abundant in Camellia sinensis |
Plant |
Gram-positive and Gram-negative |
Damage in microbial cytoplasmic membrane |
In research for medical use |
|
|
Erythromycin |
Saccharopolyspora erythraea |
Actinomycete |
Gram-positive |
Inhibition of protein synthesis |
Medicine |
[70] |
|
Fosfomycin |
Streptomyces wedmorensis |
Actinomycete |
Gram-positive and Gram-negative |
Inhibition of cell wall synthesis |
Medicine |
[71] |
|
Fusidic acid |
Fusidium coccineus |
Fungus |
Gram-positive, including MRSA |
Inhibition of protein synthesis |
Medicine |
[72] |
|
Gentamicin |
Micromonospora purpurea |
Actinomycete |
Gram-negative |
Inhibition of protein synthesis |
Medicine |
[73] |
|
Gramicidin S |
B. subtilis |
Bacillales |
Gram-positive and Gram-negative |
Delocalizes peripheral membrane proteins involved in cell division and cell envelope synthesis |
Medicine |
[74] |
|
Hc-CATH |
Hydrophis cyanocinctus |
Reptile |
Gram-positive and Gram-negative |
Damage in microbial cytoplasmic membrane |
Research |
[75] |
|
Hygromycin |
Streptomyces hygroscopicus |
Actinomycete |
Gram-positive |
Inhibition of protein synthesis |
Veterinary and research |
[76] |
|
Josamycin |
Streptomyces narbonensis |
Actinomycete |
Gram-positive, certain Gram-negative and mycoplasma |
Inhibition of protein synthesis |
Medicine |
[77] |
|
Kanamycin |
Streptomyces kanamyceticus |
Actinomycete |
Mainly Gram-negative and certain Gram-positive |
Inhibition of protein synthesis |
Medicine |
[78] |
|
Kirromycin |
Streptomyces collinus |
Actinomycete |
Anaerobes, neisseriae and streptococci |
Inhibition of protein synthesis |
Research |
|
|
Lincomycin |
Streptomyces lincolnensis |
Actinomycete |
Gram-positive |
Inhibition of protein synthesis |
Medicine |
[81] |
|
Lipiaramycin |
Dactosporangium aurantiacum |
Actinomycete |
Gram-positive and Mycobacterium, including MDR strains |
Inhibition of early transcription |
Medicine |
[82] |
|
Melittin |
A. mellifera |
Insect |
Gram-positive and Gram-negative, including MDR strains |
Damage in microbial cytoplasmic membrane |
Medicine |
[83] |
|
Mirandamycin |
Endophytic fungus isolated from the twig of Neomirandea angularis |
Fungus |
Gram-negative and Gram-positive, including MRSA |
Inhibition of bacterial quinol oxidase/ROS production |
In research for medical use |
[21] |
|
Moenomycin |
Streptomyces ghanaensis |
Actinomycete |
Gram-positive |
Inhibition of cell wall synthesis |
Veterinary |
[84] |
|
Morin |
Moraceae family |
Plant |
Gram-positive and Gram-negative |
Inhibition of adhesion to host tissue and DNA helicase |
Food technology |
[37] |
|
Mucroporin |
Lychas mucronatus |
Arachnid |
Gram-positive and Gram-negative, including MDR strains |
Damage in microbial cytoplasmic membrane |
Research |
[85] |
|
Neomycin |
S. fradiae |
Actinomycete |
Gram-positive and Gram-negative |
Inhibition of ribonuclease P |
Medicine |
[86] |
|
Orthoformimycin |
S. griseus |
Actinomycete |
Gram-positive and Gram-negative |
Inhibition of protein synthesis |
In research for medical use |
[18] |
|
Oxytetracycline |
Streptomyces rimosus |
Actinomycete |
Gram-positive and Gram-negative |
Inhibition of protein synthesis |
Aquaculture |
[87] |
|
Penicillins |
Penicillium crysogenum |
Fungus |
Gram-positive and Gram-negative |
Inhibition of cell wall synthesis and activation of the endogenous autolytic system |
Medicine |
[88] |
|
Pleuromutalin |
Clitopilus scyphoides |
Fungus |
Gram-positive, Gram-negative and Mycoplasma |
Inhibition of translation |
Veterinary |
[89] |
|
Polymyxin |
Paenibacillus polymyxa |
Bacillales |
Mainly Gram-negative (including MDR) and certain Gram-positive |
Disruption of the cytoplasmic membrane |
Medicine |
[90] |
|
Pristinamycin |
Streptomyces pristinaespiralis |
Actinomycete |
Gram-positive, including MRSA |
Inhibition of protein synthesis |
Medicine |
[91] |
|
Punicalagin |
Abundant in Punica granatum |
Plant |
Gram-positive and Gram-negative |
Damage in microbial cytoplasmic membrane |
Food technology |
|
|
Quercetin |
Ubiquitous in plants |
Plant |
Gram-positive and Gram-negative |
Damage in the structure of the bacterial cell wall and cell membrane |
In research for medical use |
[92] |
|
Ramoplanin |
Actinoplanes sp. ATCC 33076 |
Actinomycete |
Gram-positive, including MDR strains |
Inhibition of cell wall synthesis |
Medicine |
[93] |
|
Resveratrol |
Abundant in grapes, berries and legumes |
Plant |
Gram-positive and Gram-negative, including MDR strains |
Inhibition of motility, adhesion, quorum sensing, biofilm formation, flagellar gene expression and hemolytic activity |
Medicine |
[33] |
|
Rifamycin |
Amycolatopsis mediterranei |
Actinomycete |
Gram-positive and certain Gram-negative |
Inhibition of DNA-dependent RNA synthesis |
Medicine |
[94] |
|
Ristocetin A |
A. orientalis |
Actinomycete |
Gram-positive, including MRSA |
Inhibition of cell wall synthesis |
Medicine |
[95] |
|
Royalisin |
Apis melifera |
Insect |
Mainly gram-positive |
Damage in the structure of the bacterial cell wall and cell membrane |
Research |
[96] |
|
Skyllamycins |
Streptomyces sp. KY 11784 |
Actinomycete |
Gram-positive |
Inhibition of biofilm formation |
In research for medical use |
[97] |
|
SlLebocin1 |
Spodoptera litura |
Insect |
Gram-positive and Gram-negative |
Damage in microbial cytoplasmic membrane or cell division inhibition |
Research |
[98] |
|
Spectinomycin |
Streptomyces spectabilis |
Actinomycete |
Gram-positive and Gram-negative |
Inhibition of protein synthesis |
Medicine |
[99] |
|
Spiramycin |
Streptomyces ambofaciens |
Actinomycete |
Gram-positive and Gram-negative |
Inhibition of protein synthesis |
Medicine |
[100] |
|
Streptothricin |
Streptomyces (multiple species) |
Actinomycete |
Gram-positive and Gram-negative |
Inhibition of protein synthesis |
Veterinary and plant production |
[101] |
|
Teicoplanin |
Actinoplanes teichomyceticus |
Actinomycete |
Gram-positive, including MRSA |
Inhibition of bacterial cell wall synthesis |
Medicine |
[102] |
|
Teixobactin |
Eleftheria terrae |
Betaproteobacteria |
Gram-positive, including MRSA |
Causes digestion of the cell wall by autolysins |
Medicine |
[103] |
|
Tetracycline |
Streptomyces rimosus |
Actinomycete |
Gram-positive and Gram-negative |
Inhibition of protein synthesis |
Medicine |
[104] |
|
Thienamycin |
Streptomyces cattleya |
Actinomycete |
Gram-positive and Gram-negative |
Inhibition of bacterial cell wall synthesis |
Derivates used in medicine |
[105] |
|
Thiostrepton |
Streptomyces azureus |
Actinomycete |
Gram-positive and Gram-negative |
Inhibition of protein synthesis |
Veterinary and research |
[106] |
|
Tobramycin |
Streptoalloteicus hindustanus |
Actinomycete |
Gram-negative |
Inhibition of protein synthesis and membrane destabilization |
Medicine |
[107] |
|
Tunicamycin |
Streptomyces chartreusis |
Actinomycete |
Gram-positive |
Inhibition of peptidoglycan and lipopolysaccharide synthesis |
Research |
[108] |
|
Tylosin |
S. fradiae |
Actinomycete |
Gram-positive and Mycoplasma |
Inhibition of protein synthesis |
Veterinary |
[109] |
|
Vancomycin |
S. orientalis |
Actinomycete |
Gram-positive, including MRSA |
Inhibition of bacterial cell wall synthesis |
Medicine |
[14] |
|
Vejovine |
V. mexicanus |
Arachnid |
Gram-negative, including MDR |
Damage in microbial cytoplasmic membrane |
Research |
[110] |
|
Viomycin |
Streptomyces sp. 11861 |
Actinomycete |
MDR Mycobacterium |
Inhibition of protein synthesis |
Medicine |
[111] |
|
Virginiamycin |
Streptomyces virginiae |
Actinomycete |
Gram-positive |
Inhibition of protein synthesis |
Agriculture and industry |
[112] |
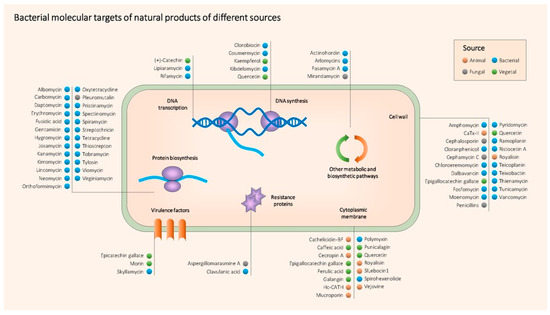
Figure 2. Main known molecular targets of antimicrobial NPs described in this entry.
References
- Clarke, L.; Pelin, A.; Phan, M.; Wong, A. The effect of environmental heterogeneity on the fitness of antibiotic resistance mutations in Escherichia coli. Evol. Ecol. 2020, 34, 379–390.
- Davies, J.; Davies, D. Origins and evolution of antibiotic resistance. Microbiol. Mol. Biol. Rev. 2010, 74, 417–433.
- Hiltunen, T.; Virta, M.; Laine, A.L. Antibiotic resistance in the wild: An eco-evolutionary perspective. Philos. Trans. R. Soc. B Biol. Sci. 2017, 372, 20160039.
- Wong, A. Unknown risk on the farm: Does agricultural use of ionophores contribute to the burden of antimicrobial resistance? mSphere 2019, 4, e00433-19.
- Machowska, A.; Stalsby Lundborg, C. Drivers of irrational use of antibiotics in Europe. Int. J. Environ. Res. Public Health 2018, 16, 27.
- Towse, A.; Hoyle, C.K.; Goodall, J.; Hirsch, M.; Mestre-Ferrandiz, J.; Rex, J.H. Time for a change in how new antibiotics are reimbursed: Development of an insurance framework for funding new antibiotics based on a policy of risk mitigation. Health Policy 2017, 121, 1025–1030.
- N. Sabtu; D. A. Enoch; N. M. Brown; Antibiotic resistance: what, why, where, when and how?. British Medical Bulletin 2015, 116, 105-113, 10.1093/bmb/ldv041.
- Barrajon-Catalan, E.; Menendez-Gutierrez, M.P.; Falco, A.; Carrato, A.; Saceda, M.; Micol, V. Selective death of human breast cancer cells by lytic immunoliposomes: Correlation with their her2 expression level. Cancer Lett. 2010, 290, 192–203.
- Falco, A.; Barrajón-Catalán, E.; Menéndez-Gutiérrez, M.P.; Coll, J.; Micol, V.; Estepa, A. Melittin-loaded immunoliposomes against viral surface proteins, a new approach to antiviral therapy. Antivir. Res. 2013, 97, 218–221.
- Yi, H.Y.; Chowdhury, M.; Huang, Y.D.; Yu, X.Q. Insect antimicrobial peptides and their applications. Appl. Microbiol. Biotechnol. 2014, 98, 5807–5822.
- Lee, M.; Bang, K.; Kwon, H.; Cho, S. Enhanced antibacterial activity of an attacin-coleoptericin hybrid protein fused with a helical linker. Mol. Biol. Rep. 2013, 40, 3953–3960.
- Ali, S.M.; Siddiqui, R.; Ong, S.K.; Shah, M.R.; Anwar, A.; Heard, P.J.; Khan, N.A. Identification and characterization of antibacterial compound(s) of cockroaches (Periplaneta americana). Appl. Microbiol. Biotechnol. 2017, 101, 253–286.
- Arora, S.; Baptista, C.; Lim, C.S. Maggot metabolites and their combinatory effects with antibiotic on Staphylococcus aureus. Ann. Clin. Microbiol. Antimicrob. 2011, 10, 6.
- Bruniera, F.R.; Ferreira, F.M.; Saviolli, L.R.M.; Bacci, M.R.; Feder, D.; Pedreira, M.; Peterlini, M.A.; Azzalis, L.A.; Junqueira, V.B.; Fonseca, F.L.A. The use of vancomycin with its therapeutic and adverse effects: A review. Eur. Rev. Med. Pharm. Sci. 2015, 19, 694–700.
- Eirich, J.; Orth, R.; Sieber, S.A. Unraveling the protein targets of vancomycin in living S. aureus and E. faecalis cells. J. Am. Chem. Soc. 2011, 133, 12144–12153.
- Tripathi, A.; Schofield, M.M.; Chlipala, G.E.; Schultz, P.J.; Yim, I.; Newmister, S.A.; Nusca, T.D.; Scaglione, J.B.; Hanna, P.C.; Tamayo-Castillo, G.; et al. Baulamycins a and b, broad-spectrum antibiotics identified as inhibitors of siderophore biosynthesis in Staphylococcus aureus and Bacillus anthracis. J. Am. Chem. Soc. 2014, 136, 1579–1586.
- Feng, Z.; Chakraborty, D.; Dewell, S.B.; Reddy, B.V.; Brady, S.F. Environmental DNA-encoded antibiotics fasamycins a and b inhibit fabf in type ii fatty acid biosynthesis. J. Am. Chem. Soc. 2012, 134, 2981–2987.
- Maffioli, S.I.; Fabbretti, A.; Brandi, L.; Savelsbergh, A.; Monciardini, P.; Abbondi, M.; Rossi, R.; Donadio, S.; Gualerzi, C.O. Orthoformimycin, a selective inhibitor of bacterial translation elongation from streptomyces containing an unusual orthoformate. ACS Chem. Biol. 2013, 8, 1939–1946.
- David L. Hawksworth; Robert Lücking; Fungal Diversity Revisited: 2.2 to 3.8 Million Species. The Fungal Kingdom 2017, 5, 79-95, 10.1128/microbiolspec.funk-0052-2016.
- King, A.M.; Reid-Yu, S.A.; Wang, W.; King, D.T.; De Pascale, G.; Strynadka, N.C.; Walsh, T.R.; Coombes, B.K.; Wright, G.D. Aspergillomarasmine a overcomes metallo-beta-lactamase antibiotic resistance. Nature 2014, 510, 503–506.
- Patrick Ymele-Leki; Shugeng Cao; Jared Sharp; Kathleen G. Lambert; Alexander J. McAdam; Robert N. Husson; Giselle Tamayo-Castillo; Jon Clardy; Paula I. Watnick; A High-Throughput Screen Identifies a New Natural Product with Broad-Spectrum Antibacterial Activity. PLoS ONE 2012, 7, e31307, 10.1371/journal.pone.0031307.
- M. Karaman; E. Jovin; R. Malbaša; M. Matavuly; M. Popović; Medicinal and edible lignicolous fungi as natural sources of antioxidative and antibacterial agents. Phytotherapy Research 2010, 24, 1473-1481, 10.1002/ptr.2969.
- Chandra, H.; Bishnoi, P.; Yadav, A.; Patni, B.; Mishra, A.P.; Nautiyal, A.R. Antimicrobial resistance and the alternative resources with special emphasis on plant-based antimicrobials—A review. Plants 2017, 6, 16.
- Quideau, S.; Deffieux, D.; Douat-Casassus, C.; Pouysegu, L. Plant polyphenols: Chemical properties, biological activities, and synthesis. Angew. Chem. Int. Ed. Engl. 2011, 50, 586–621.
- Harvey, A.L.; Edrada-Abel, R.; Quinn, R.J. The re-emergence of natural products for drug discovery in the genomics era. Nat. Rev. Drug Discov. 2015, 14, 111–129.
- Radulovic, N.S.B.; Blagojevic, P.D.; Stojanovic-Radic, Z.Z.; Stojanovic, N.M. Antimicrobial plant metabolites: Structural diversity and mechanism of action. Curr. Med. Chem. 2013, 20, 932–952.
- Coban, I.; Toplan, G.G.; Ozbek, B.; Gurer, C.U.; Sariyar, G. Variation of alkaloid contents and antimicrobial activities of papaver rhoeas l. Growing in turkey and northern cyprus. Pharm. Biol. 2017, 55, 1894–1898.
- Nugraha, A.S.; Damayanti, Y.D.; Wangchuk, P.; Keller, P.A. Anti-infective and anti-cancer properties of the annona species: Their ethnomedicinal uses, alkaloid diversity, and pharmacological activities. Molecules 2019, 24, 4419.
- Tian, J.; Ban, X.; Zeng, H.; He, J.; Huang, B.; Wang, Y. Chemical composition and antifungal activity of essential oil from Cicuta virosa L. Var. Latisecta celak. Int. J. Food Microbiol. 2011, 145, 464–470.
- Tariq, S.; Wani, S.; Rasool, W.; Shafi, K.; Bhat, M.A.; Prabhakar, A.; Shalla, A.H.; Rather, M.A. A comprehensive review of the antibacterial, antifungal and antiviral potential of Essential oils and their chemical constituents against drug-resistant microbial pathogens. Microb. Pathog. 2019, 134, 103580.
- Yang, Y.; Zhang, T. Antimicrobial activities of tea polyphenol on phytopathogens: A review. Molecules 2019, 24, 816. [Google Scholar] [CrossRef]
- Bouarab Chibane, L.; Degraeve, P.; Ferhout, H.; Bouajila, J.; Oulahal, N. Plant antimicrobial polyphenols as potential natural food preservatives. J. Sci. Food Agric. 2019, 99, 1457–1474.
- Bostanghadiri, N.; Pormohammad, A.; Chirani, A.S.; Pouriran, R.; Erfanimanesh, S.; Hashemi, A. Comprehensive review on the antimicrobial potency of the plant polyphenol resveratrol. Biomed. Pharm. 2017, 95, 1588–1595.
- Alvarez-Martinez, F.J.; Barrajon-Catalan, E.; Encinar, J.A.; Rodriguez-Diaz, J.C.; Micol, V. Antimicrobial capacity of plant polyphenols against gram-positive bacteria: A comprehensive review. Curr. Med. Chem. 2018, 27, 2576–2606.
- Su, Y.; Ma, L.; Wen, Y.; Wang, H.; Zhang, S. Studies of the in vitro antibacterial activities of several polyphenols against clinical isolates of methicillin-resistant Staphylococcus aureus. Molecules 2014, 19, 12630–12639.
- Mokhtar, M.; Ginestra, G.; Youcefi, F.; Filocamo, A.; Bisignano, C.; Riazi, A. Antimicrobial activity of selected polyphenols and capsaicinoids identified in pepper (Capsicum annuum L.) and their possible mode of interactio. Curr. Microbiol. 2017, 74, 1253–1260.
- Caselli, A.; Cirri, P.; Santi, A.; Paoli, P. Morin: A promising natural drug. Curr. Med. Chem. 2016, 23, 774–791.
- Tomas-Menor, L.; Barrajon-Catalan, E.; Segura-Carretero, A.; Marti, N.; Saura, D.; Menendez, J.A.; Joven, J.; Micol, V. The promiscuous and synergic molecular interaction of polyphenols in bactericidal activity: An opportunity to improve the performance of antibiotics? Phytother. Res. 2015, 29, 466–473.
- Bai, L.; Takagi, S.; Ando, T.; Yoneyama, H.; Ito, K.; Mizugai, H.; Isogai, E. Antimicrobial activity of tea catechin against canine oral bacteria and the functional mechanisms. J. Vet. Med. Sci. 2016, 78, 1439–1445.
- Cushnie, T.P.; Hamilton, V.E.; Lamb, A.J. Assessment of the antibacterial activity of selected flavonoids and consideration of discrepancies between previous reports. Microbiol. Res. 2003, 158, 281–289.
- Cushnie, T.P.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356.
- Yunfeng Xu; Chao Shi; Qian Wu; Zhiwei Zheng; Peifeng Liu; Guanghui Li; Xiaoli Peng; Xiaodong Xia; Antimicrobial Activity of Punicalagin Against Staphylococcus aureus and Its Effect on Biofilm Formation. Foodborne Pathogens and Disease 2017, 14, 282-287, 10.1089/fpd.2016.2226.
- Jonathan W. Betts; Amir S. Sharili; Lynette M. Phee; David W. Wareham; In VitroActivity of Epigallocatechin Gallate and Quercetin Alone and in Combination versus Clinical Isolates of Methicillin-ResistantStaphylococcus aureus. Journal of Natural Products 2015, 78, 2145-2148, 10.1021/acs.jnatprod.5b00471.
- Marion Zwingelstein; Micheline Draye; Jean-Luc Besombes; Christine Piot; Gregory Chatel; Viticultural wood waste as a source of polyphenols of interest: Opportunities and perspectives through conventional and emerging extraction methods. Waste Management 2020, 102, 782-794, 10.1016/j.wasman.2019.11.034.
- Laura Tomás-Menor; Aránzazu Morales-Soto; Enrique Barrajón-Catalán; Cristina Roldán-Segura; Antonio Segura-Carretero; Vicente Micol; Correlation between the antibacterial activity and the composition of extracts derived from various Spanish Cistus species. Food and Chemical Toxicology 2013, 55, 313-322, 10.1016/j.fct.2013.01.006.
- Debasmita Dubey; Rabindra N. Padhy; Antibacterial activity of Lantana camara L. against multidrug resistant pathogens from ICU patients of a teaching hospital. Journal of Herbal Medicine 2013, 3, 65-75, 10.1016/j.hermed.2012.12.002.
- Dubey, D.; Sahu, M.C.; Rath, S.; Paty, B.P.; Debata, N.K.; Padhy, R.N. Antimicrobial activity of medicinal plants used by aborigines of kalahandi, orissa, india against multidrug resistant bacteria. Asian Pac. J. Trop. Biomed. 2012, 2, S846–S854.
- Sahu, M.C.; Padhy, R.N. In vitro antibacterial potency of Butea monosperma Lam. Against 12 clinically isolated multidrug resistant bacteria. Asian Pac. J. Trop. Dis. 2013, 3, 217–226.
- Mak, S.; Nodwell, J.R. Actinorhodin is a redox-active antibiotic with a complex mode of action against gram-positive cells. Mol. Microbiol. 2017, 106, 597–613.
- Lin, Z.; Xu, X.; Zhao, S.; Yang, X.; Guo, J.; Zhang, Q.; Jing, C.; Chen, S.; He, Y. Total synthesis and antimicrobial evaluation of natural albomycins against clinical pathogens. Nat. Commun. 2018, 9, 3445.
- Pramanik, A.; Stroeher, U.H.; Krejci, J.; Standish, A.J.; Bohn, E.; Paton, J.C.; Autenrieth, I.B.; Braun, V. Albomycin is an effective antibiotic, as exemplified with Yersinia enterocolitica and Streptococcus pneumoniae. Int. J. Med. Microbiol. 2007, 297, 459–469.
- Singh, M.; Chang, J.; Coffman, L.; Kim, S.J. Solid-state nmr characterization of amphomycin effects on peptidoglycan and wall teichoic acid biosyntheses in Staphylococcus aureus. Sci. Rep. 2016, 6, 31757.
- Kang, A.D.; Smith, K.P.; Eliopoulos, G.M.; Berg, A.H.; McCoy, C.; Kirby, J.E. Invitro apramycin activity against multidrug-resistant Acinetobacter baumannii and Pseudomonas aeruginosa. Diagn. Microbiol. Infect. Dis. 2017, 88, 188–191.
- Liu, J.; Smith, P.A.; Steed, D.B.; Romesberg, F. Efforts toward broadening the spectrum of arylomycin antibiotic activity. Bioorg. Med. Chem. Lett. 2013, 23, 5654–5659.
- Jingjing Zhong; Zhili Lu; Jianlu Dai; Weiqing He; Identification of two regulatory genes involved in carbomycin biosynthesis in Streptomyces thermotolerans. Archives of Microbiology 2017, 199, 1023-1033, 10.1007/s00203-017-1376-z.
- Yipeng Wang; Jing Hong; Xiuhong Liu; Hailong Yang; Rui Liu; Jing Wu; Aili Wang; Donghai Lin; Ren Lai; Snake Cathelicidin from Bungarus fasciatus Is a Potent Peptide Antibiotics. PLOS ONE 2008, 3, e3217, 10.1371/journal.pone.0003217.
- Ramar Perumal Samy; Matheswaran Kandasamy; Ponnampalam Gopalakrishnakone; Bradley G. Stiles; E.G Rowan; Dávid Becker; Muthu K. Shanmugam; Gautam Sethi; Vincent T. K. Chow; Wound Healing Activity and Mechanisms of Action of an Antibacterial Protein from the Venom of the Eastern Diamondback Rattlesnake (Crotalus adamanteus). PLoS ONE 2014, 9, e80199, 10.1371/journal.pone.0080199.
- Zheng, Z.; Tharmalingam, N.; Liu, Q.; Jayamani, E.; Kim, W.; Fuchs, B.B.; Zhang, R.; Vilcinskas, A.; Mylonakis, E. Synergistic efficacy of aedes aegypti antimicrobial peptide cecropin a2 and tetracycline against Pseudomonas aeruginosa. Antimicrob. Agents 2017, 61, e00617–e00686.
- Gustaferro, C.A.; Steckelberg, J.M. Cephalosporin antimicrobial agents and related compounds. Mayo Clin. Proc. 1991, 66, 1064–1073.
- Brites, L.M.; Oliveira, L.M.; Barboza, M. Kinetic study on cephamycin c degradation. Appl. Biochem. Biotechnol. 2013, 171, 2121–2128.
- Schwarz, S.; Kehrenberg, C.; Doublet, B.; Cloeckaert, A. Molecular basis of bacterial resistance to chloramphenicol and florfenicol. FEMS Microbiol. Rev. 2004, 28, 519–542.
- Allen, N.E.; Nicas, T.I. Mechanism of action of oritavancin and related glycopeptide antibiotics. FEMS Microbiol. Rev. 2003, 26, 511–532.
- Hakami, A.Y.; Sari, Y. Beta-lactamase inhibitor, clavulanic acid, attenuates ethanol intake and increases glial glutamate transporters expression in alcohol preferring rats. Neurosci. Lett. 2017, 657, 140–145.
- Eustáquio, A.S.; Gust, B.; Luft, T.; Li, S.-M.; Chater, K.F.; Heide, L. Clorobiocin biosynthesis in streptomyces. Chem. Biol. 2003, 10, 279–288.
- Samuels, D.S.; Garon, C.F. Coumermycin a1 inhibits growth and induces relaxation of supercoiled plasmids in borrelia burgdorferi, the lyme disease agent. Antimicrob. Agents Chemother. 1993, 37, 46–50.
- Fedorko, J.; Katz, S.; Allnoch, H. In vitro activity of coumermycin a. Appl. Microbiol. 1969, 18, 869–873.
- Cercenado, E. Espectro antimicrobiano de dalbavancina. Mecanismo de acción y actividad in vitro frente a microorganismos gram positivos. Enferm. Infecc. Y Microbiol. Clín. 2017, 35, 9–14.
- Heidary, M.; Khosravi, A.D.; Khoshnood, S.; Nasiri, M.J.; Soleimani, S.; Goudarzi, M. Daptomycin. J. Antimicrob. Chemother. 2018, 73, 1–11.
- Chu, C.; Deng, J.; Man, Y.; Qu, Y. Green tea extracts epigallocatechin-3-gallate for different treatments. BioMed Res. Int. 2017, 2017, 5615647.
- Li, Z.; He, M.; Dong, X.; Lin, H.; Ge, H.; Shen, S.; Li, J.; Ye, R.D.; Chen, D. New erythromycin derivatives enhance beta-lactam antibiotics against methicillin-resistant Staphylococcus aureus. Lett. Appl. Microbiol. 2015, 60, 352–358.
- Falagas, M.E.; Vouloumanou, E.K.; Samonis, G.; Vardakas, K.Z. Fosfomycin. Clin. Microbiol. Rev. 2016, 29, 321–347.
- Curbete, M.M.; Salgado, H.R. A critical review of the properties of fusidic acid and analytical methods for its determination. Crit. Rev. Anal. Chem. 2016, 46, 352–360.
- Wargo, K.A.; Edwards, J.D. Aminoglycoside-induced nephrotoxicity. J. Pharm. Pr. 2014, 27, 573–577.
- Wenzel, M.; Rautenbach, M.; Vosloo, J.A.; Siersma, T.; Aisenbrey, C.H.; Zaitseva, E.; Laubscher, W.E.; Rensburg, W.; Behrends, J.C.; Bechinger, B.; et al. The multifaceted antibacterial mechanisms of the pioneering peptide antibiotics tyrocidine and gramicidin s. mBio 2018, 9, e00802-18. [Google Scholar] [CrossRef]
- Wei, L.; Gao, J.; Zhang, S.; Wu, S.; Xie, Z.; Ling, G.; Kuang, Y.Q.; Yang, Y.; Yu, H.; Wang, Y. Identification and characterization of the first cathelicidin from sea snakes with potent antimicrobial and anti-inflammatory activity and special mechanism. J. Biol. Chem. 2015, 290, 16633–16652. [Google Scholar] [CrossRef]
- Guerrero, M.C.; Modolell, J. Hygromycin a, a novel inhibitor of ribosomal peptidyltransferase. Eur. J. Biochem. 1980, 107, 409–414.
- Arsic, B.; Barber, J.; Cikos, A.; Mladenovic, M.; Stankovic, N.; Novak, P. 16-membered macrolide antibiotics: A review. Int. J. Antimicrob. Agents 2018, 51, 283–298.
- Hoerr, V.; Duggan, G.E.; Zbytnuik, L.; Poon, K.K.; Grosse, C.; Neugebauer, U.; Methling, K.; Loffler, B.; Vogel, H.J. Characterization and prediction of the mechanism of action of antibiotics through nmr metabolomics. BMC Microbiol. 2016, 16, 82.
- Beretta, G. Novel producer of the antibiotic kirromycin belonging to the genus actinoplanes. J. Antibiot. 1993, 46, 1175–1177.
- Wolf, H.; Chinali, G.; Parmeggiani, A. Kirromycin, an inhibitor of protein biosynthesis that acts on elongation factor tu. Proc. Natl. Acad. Sci. USA 1974, 71, 4910–4914.
- Spizek, J.; Rezanka, T. Lincomycin, clindamycin and their applications. Appl. Microbiol. Biotechnol. 2004, 64, 455–464.
- Kurabachew, M.; Lu, S.H.; Krastel, P.; Schmitt, E.K.; Suresh, B.L.; Goh, A.; Knox, J.E.; Ma, N.L.; Jiricek, J.; Beer, D.; et al. Lipiarmycin targets rna polymerase and has good activity against multidrug-resistant strains of mycobacterium tuberculosis. J. Antimicrob. Chemother. 2008, 62, 713–719.
- Sonia I. Maffioli; Attilio Fabbretti; Letizia Brandi; Andreas Savelsbergh; Paolo Monciardini; Monica Abbondi; Rossana Rossi; Stefano Donadio; Claudio O. Gualerzi; Orthoformimycin, a Selective Inhibitor of Bacterial Translation Elongation from Streptomyces Containing an Unusual Orthoformate. ACS Chemical Biology 2013, 8, 1939-1946, 10.1021/cb4004095.
- Yuriy Rebets; Tania Lupoli; Yuan Qiao; Kathrin Schirner; Regis Villet; David Hooper; Daniel Kahne; Suzanne Walker; Moenomycin Resistance Mutations inStaphylococcus aureusReduce Peptidoglycan Chain Length and Cause Aberrant Cell Division. ACS Chemical Biology 2013, 9, 459-467, 10.1021/cb4006744.
- Chao Dai; Yibao Ma; Zhenhuan Zhao; Ruiming Zhao; Qian Wang; Yingliang Wu; Zhijian Cao; Wenxin Li; Mucroporin, the First Cationic Host Defense Peptide from the Venom of Lychas mucronatus. Antimicrobial Agents and Chemotherapy 2008, 52, 3967-3972, 10.1128/aac.00542-08.
- Catlyn Blanchard; Lauren Brooks; Andrew Beckley; Jennifer Colquhoun; Stephen Dewhurst; Paul M. Dunman; Neomycin Sulfate Improves the Antimicrobial Activity of Mupirocin-Based Antibacterial Ointments. Antimicrobial Agents and Chemotherapy 2015, 60, 862-872, 10.1128/aac.02083-15.
- J.F. Leal; I.S. Henriques; A. Correia; E.B.H. Santos; V.I. Esteves; Antibacterial activity of oxytetracycline photoproducts in marine aquaculture's water. Environmental Pollution 2017, 220, 644-649, 10.1016/j.envpol.2016.10.021.
- Alan J. Wright; The Penicillins. Mayo Clinic Proceedings 1999, 74, 290-307, 10.4065/74.3.290.
- Susanne Paukner; Rosemarie Riedl; Pleuromutilins: Potent Drugs for Resistant Bugs—Mode of Action and Resistance. Cold Spring Harbor Perspectives in Medicine 2016, 7, a027110, 10.1101/cshperspect.a027110.
- Michael J. Trimble; Patrik Mlynárčik; Milan Kolář; Robert E.W. Hancock; Polymyxin: Alternative Mechanisms of Action and Resistance. Cold Spring Harbor Perspectives in Medicine 2016, 6, a025288, 10.1101/cshperspect.a025288.
- Eden C. Cooper; Nigel Curtis; Noel Cranswick; Amanda Gwee; Pristinamycin: old drug, new tricks?. Journal of Antimicrobial Chemotherapy 2014, 69, 2319-2325, 10.1093/jac/dku167.
- Shengan Wang; Jiaying Yao; Bo Zhou; Jiaxin Yang; Maria T. Chaudry; Mi Wang; Fenglin Xiao; Yao Li; Wenzhe Yin; Bacteriostatic Effect of Quercetin as an Antibiotic Alternative In Vivo and Its Antibacterial Mechanism In Vitro. Journal of Food Protection 2017, 81, 68-78, 10.4315/0362-028x.jfp-17-214.
- Mercedes De La Cruz; Ignacio González; Craig A. Parish; Russell Onishi; José R. Tormo; Jesús Martín; Fernando Peláez; Debbie Zink; Noureddine El Aouad; Fernando Reyes; et al.Olga GenilloudFrancisca Vicente Production of Ramoplanin and Ramoplanin Analogs by Actinomycetes. Frontiers in Microbiology 2017, 8, 343, 10.3389/fmicb.2017.00343.
- Heinz G. Floss; Tin-Wein Yu; RifamycinMode of Action, Resistance, and Biosynthesis. Chemical Reviews 2005, 105, 621-632, 10.1021/cr030112j.
- Virginie Nahoum; Sherri Spector; Patrick J Loll; Structure of ristocetin A in complex with a bacterial cell-wall mimetic. Corrigendum.. Acta Crystallographica Section D Biological Crystallography 2011, 67, 592-592, 10.1107/S0907444909018344.
- Katarina Bílikova; Sheng-Chang Huang; I-Ping Lin; Jozef Šimuth; Chi-Chung Peng; Structure and antimicrobial activity relationship of royalisin, an antimicrobial peptide from royal jelly of Apis mellifera. Peptides 2015, 68, 190-196, 10.1016/j.peptides.2015.03.001.
- Paul Sweeney; Cormac D. Murphy; Patrick Caffrey; Exploiting the genome sequence of Streptomyces nodosus for enhanced antibiotic production. Applied Microbiology and Biotechnology 2015, 100, 1285-1295, 10.1007/s00253-015-7060-9.
- Li-Ling Yang; Ming-Yue Zhan; Yu-Li Zhuo; Yue-Min Pan; Yang Xu; Xiu-Hong Zhou; Pei-Jin Yang; Hong-Li Liu; Zi-Hao Liang; Xiao-Dan Huang; et al.Xiao-Qiang YuXiang-Jun Rao Antimicrobial activities of a proline-rich proprotein from Spodoptera litura. Developmental & Comparative Immunology 2018, 87, 137-146, 10.1016/j.dci.2018.06.011.
- William J. Holloway; Spectinomyein. Medical Clinics of North America 1982, 66, 169-173, 10.1016/s0025-7125(16)31450-x.
- E Rubinstein; N Keller; Spiramycin renaissance. Journal of Antimicrobial Chemotherapy 1998, 42, 572-576, 10.1093/jac/42.5.572.
- Hattie E. Webb; Frederick J. Angulo; Sophie Granier; H. Morgan Scott; Guy H. Loneragan; Illustrative examples of probable transfer of resistance determinants from food animals to humans: Streptothricins, glycopeptides, and colistin. F1000Research 2017, 6, 1805, 10.12688/f1000research.12777.1.
- V Ramos-Martín; A Johnson; L McEntee; N Farrington; K Padmore; P Cojutti; F Pea; M N Neely; W W Hope; Pharmacodynamics of teicoplanin against MRSA. Journal of Antimicrobial Chemotherapy 2017, 72, 3382-3389, 10.1093/jac/dkx289.
- Losee L. Ling; Tanja Schneider; Aaron J. Peoples; Amy L. Spoering; Ina Engels; Brian P. Conlon; Anna Mueller; Till F. Schäberle; Dallas E. Hughes; Slava S Epstein; et al.Michael JonesLinos LazaridesVictoria A. SteadmanDouglas R. CohenCintia R. FelixK. Ashley FettermanWilliam P. MillettAnthony G. NittiAshley M. ZulloChao ChenKim Lewis A new antibiotic kills pathogens without detectable resistance. Nature 2015, 517, 455-459, 10.1038/nature14098.
- Fabian Nguyen; Agata L. Starosta; Stefan Arenz; Daniel Sohmen; Alexandra Dönhöfer; Daniel N. Wilson; Tetracycline antibiotics and resistance mechanisms. Biological Chemistry 2014, 395, 559-575, 10.1515/hsz-2013-0292.
- Krisztina M. Papp-Wallace; Andrea Endimiani; Magdalena A. Taracila; Robert A. Bonomo; Carbapenems: Past, Present, and Future. Antimicrobial Agents and Chemotherapy 2011, 55, 4943-4960, 10.1128/aac.00296-11.
- K. C. Nicolaou; How Thiostrepton Was Made in the Laboratory. Angewandte Chemie International Edition 2012, 51, 12414-12436, 10.1002/anie.201205576.
- Meenakshi Bothra; Rakesh Lodha; Madhulika Kabra; Tobramycin for the treatment of bacterial pneumonia in children. Expert Opinion on Pharmacotherapy 2012, 13, 565-571, 10.1517/14656566.2012.656090.
- Kazuki Yamamoto; Satoshi Ichikawa; Tunicamycin: chemical synthesis and biosynthesis. The Journal of Antibiotics 2019, 72, 924-933, 10.1038/s41429-019-0200-1.
- Lingli Huang; Haiyang Zhang; Mei Li; Ijaz Ahmad; Yulian Wang; Zonghui Yuan; Pharmacokinetic-pharmacodynamic modeling of tylosin against Streptococcus suis in pigs. BMC Veterinary Research 2018, 14, 1-11, 10.1186/s12917-018-1645-3.
- Cynthia A. Hernández-Aponte; Jesus Silva-Sanchez; Veronica Quintero-Hernandez; Adela Rodríguez-Romero; Cipriano Balderas; Lourival D Possani; Georgina B. Gurrola; Vejovine, a new antibiotic from the scorpion venom of Vaejovis mexicanus. Toxicon 2011, 57, 84-92, 10.1016/j.toxicon.2010.10.008.
- Mikael Holm; Anneli Borg; Måns Ehrenberg; Suparna Sanyal; Molecular mechanism of viomycin inhibition of peptide elongation in bacteria. Proceedings of the National Academy of Sciences 2016, 113, 978-983, 10.1073/pnas.1517541113.
- Kenneth M. Bischoff; Yanhong Zhang; Joseph O. Rich; Fate of virginiamycin through the fuel ethanol production process. World Journal of Microbiology and Biotechnology 2016, 32, 1-7, 10.1007/s11274-016-2026-3.





