Drug-resistant bacteria pose a serious threat to human health worldwide. Current antibiotics are losing efficacy and new antimicrobial agents are urgently needed. Living organisms are an invaluable source of antimicrobial compounds. The antimicrobial activity of the most representative natural products of animal, bacterial, fungal and plant origin are reviewed in this article. Their activity against drug-resistant bacteria, their mechanisms of action, the possible development of resistance against them, their role in current medicine and their future perspectives are discussed. Natural compounds of heterogeneous origins have been shown to possess antimicrobial capabilities, including against antibiotic-resistant bacteria. The most commonly found mechanisms of antimicrobial action are related to protein biosynthesis and alteration of cell walls and membranes. Various natural compounds, especially phytochemicals, have shown synergistic capacity with antibiotics. There is little literature on the development of specific resistance mechanisms against natural antimicrobial compounds. New technologies such as -omics, network pharmacology and informatics have the potential to identify and characterize new natural antimicrobial compounds in the future. This knowledge may be useful for the development of future therapeutic strategies.
- Natural antimicrobial
- Antimicrobial resistance
- Polyphenols
- Future medicine
- Natural origin
- Antibacterial compound
- Phytochemicals
1. Introduction
Antibiotic resistance is an example of the enormous capacity for natural evolution and adaptation of bacteria to different environments[1][2] [7,8]. Although this process seems inevitable, humans have accelerated it through various anthropogenic activities[3][4] [9,10]. The causes behind the increase in the number of antimicrobial-resistant bacteria in recent years include the misuse of antibiotics in humans and animals, inadequate[3][4][5] control of infections in hospitals and clinics or poor hygiene and sanitation[3][4][5] [9–11]. In addition to the causes mentioned, the problem worsens as there is a drought in the discovery of new antibiotics. The increase in resistance rates in bacteria leads to a decrease in the effectiveness of existing antibiotics, making research in this field unattractive to companies that decide to invest in other types of fields with greater chances of success and benefits[6][7] [12,13]. This concerning trend can be observed in Figure 1.
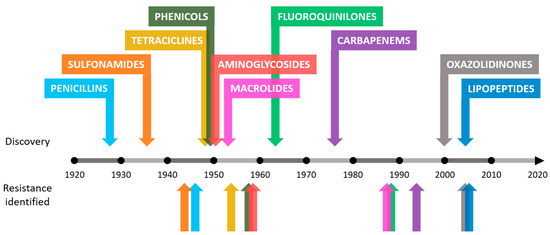
Figure 1. Approximate dates of discovery of new classes of antibiotics and identification of bacterial resistance.
In view of this scenario, research on alternative or complementary therapies to traditional antibiotics has emerged strongly. Antimicrobial products of natural origin have been positioned as compounds of great scientific interest due to their enormous chemical variety and intrinsic properties that have promoted their study as a possible therapeutic tool in recent years.
2. Main Classes of Natural Antimicrobial Products
NPs are extremely diverse in terms of their chemical structures, properties and mechanisms of action. These agents can be classified according to their original source: animal, bacterial, fungal or vegetal.
2.1. Animal origin
Animals have colonized virtually the entire planet Earth. For thousands of years, they have lived closely with different kinds of bacteria and have faced not a few pathogenic microorganisms. Evolution has shaped animal defense systems to deal with these microscopic threats. In recent years, attention has been focused on identifying which molecules confer resistance and allow certain animals to live in hostile environments with high pollution and pathogenic load, as is the case with certain insects such as cockroaches.
Currently, animals, and especially insects, are one of the main sources of antimicrobial proteins or peptides (AMPs). Since the discovery of AMPs in 1974, more than 150 new AMPs have been isolated or identified, the majority being cationic peptides between 20 and 50 residues in length. These molecules mainly have antimicrobial capacity mediated by disruption of the bacterial plasma membrane, most probably by forming pores or ion channels[8] [28]. Some AMPs also have shown antifungal, antiparasitic or antiviral properties[9] [29]. These AMPs can be divided into four subfamilies with different structures and sequences: the α-helical peptides, such as cecropin, which has a broad spectrum of antimicrobial activity against bacteria of both Gram-positive and Gram-negative bacteria; cysteine-rich peptides, such as insect defensins, which are mainly active against Gram-positive bacteria; proline-rich peptides, such as lebocins, which are active against both Gram-positive and Gram-negative bacteria and some fungi; and finally glycine-rich peptides or proteins, such as attacin, which are effective against Gram-negative bacteria and especially against Escherichia coli. These AMPs present a promising basis for the development of medical therapies, however, additional work must be developed to make them more powerful and stable[10] [30]. Moreover, the intrinsic antimicrobial capacity of AMPs can be enhanced by a fusion of peptides to create more potent hybrid ones, such as in the case of attacin from Spodoptera exigua and a coleoptericin-like protein from Protaetia brevitarsis seulensis, which, when fused, exhibited a greater antimicrobial capacity than its two original peptides[11] [31].
The study of antimicrobial molecules existent in cockroaches (Periplaneta americana) has revealed that extracts derived from its brain have a great antimicrobial capacity against MRSA and neuropathogenic E. coli K1. Although not all the components of the extract could be accurately identified, a great variety of molecules with known biological activity were found, such as isoquinolines, flavanones, sulfonamides and imidazone among others. A hypothesis about the production of this antimicrobial cocktail in the cockroach brain suggests that there could be a constitutive expression of these antimicrobials to protect the animal’s neural system, since it is the central axis of its survival and a key piece to protect when it is lived in an environment of high pollution and exposure to pathogens and even superbugs[12] [32]. Another example of insect producing antimicrobial molecules against resistant bacteria is Lucilia cuprina blowfly maggots. The extract obtained from excretions and secretions from maggots showed mild bacterial growth inhibition. However, using subinhibitory concentrations of this extract in combination with the antibiotic ciprofloxacin enhanced its activity, further delaying the appearance of bacteria resistant to it. The properties of this extract, including the presence of defensins and phenylacetaldehyde, make maggot debridement therapy a promising tool in the treatment of MRSA-infected wounds acquired in hospital[13] [33].
2.2. Bacterial origin
Bacteria are the most prolific source of NPs with antimicrobial activity found so far, especially those of the actinomycetes class. Their great diversity, competitiveness and colonization capacity have led them to the development of secondary metabolites capable of giving them great advantages over other bacterial species. As described in previous sections, the detection and isolation of these bacterial antimicrobial NPs propelled medical science vertiginously in the middle of the last century. Some of the most relevant are described below.
Some of the most important antimicrobial molecules produced by bacteria of the actinomyces class are: vancomycin, baulamycin, fasamycin A and orthoformimycin. Vancomycin is a naturally occurring tricyclic glycopeptide extracted from Streptococcus orientalis that has reaped great success as an antibiotic against Gram-positive bacteria, especially against threats that are resistant to other treatments such as MRSA and penicillin-resistant pneumococci among others[14] [45]. Vancomycin forms hydrogen bonds with the terminal dipeptide of the nascent peptidoglycan chain during biosynthesis of the bacterial cell wall. This union prevents the action of penicillin-binding proteins (PBPs), interrupting further wall formation and finally activating autolysin-triggered cell rupture and cell death[15] [46]. Another important bacterial NP is produced by actinomyces is baulamycin, which is an isolated molecule of the marine bacterium Streptomyces tempisquensis that can inhibit the biosynthesis of iron-chelating siderophores in S. aureus (targeting staphylopherrin B) and Bacillus anthracis (targeting petrobactin), helping to treat MRSA and anthrax infections, respectively. In addition, it was also able to inhibit the growth of Gram-negative bacteria such as S. flexneri and E. coli, turning baulamycin and its derivatives into potential broad-spectrum antibiotics[16] [47]. Fasamycin A is a polyketide isolated from Streptomyces albus that shows specific antimicrobial activity against Gram-positive bacteria such as vancomycin-resistant Enterococci (VRE) and MRSA with MIC values of 0.8 and 3.1 µg/mL, respectively. This molecule targets FabF in the initial condensation step of the elongation cycle from the lipidic biosynthetic bacterial metabolism[17] [48]. Orthoformimycin is a molecule produced by S. griseus which can inhibit bacterial translation by more than 80% in the case of E. coli. Although the mechanism of action is not clear now, one hypothesis is the decoupling of mRNA and aminoacyl-tRNA in the bacterial ribosome[18] [49].
2.3. Fungal origin
Fungi are eukaryotic-type living things, such as mushrooms, yeasts, and molds. Currently, the existence of some 120,000 species of fungi has been accepted, however, it is estimated that the number of different species of fungi present on earth could be between 2.2 and 3.8 million[19] [58]. This relatively unexplored kingdom is a source of antimicrobial NPs and has great potential to be studied in the future as new species are discovered and identified.
Aspergillomarasmine A is a polyaminoacid naturally produced by Aspergillus versicolor capable of inhibiting antibiotic resistance enzymes in Gram-negative pathogenic bacteria, such as Enterobacteriaceae, Acinetobacter spp., Pseudomonas spp. and Klebsiella pneumoniae. This compound has been used successfully to reverse resistance in mice infected with meropenem-resistant K. pneumoniae thanks to the NDM-I protein, making the bacterium sensitive to the antibiotic and ending the infection[20] [59].
Mirandamycin is a quinol of fungal origin capable of inhibiting the growth of both Gram-negative and Gram-positive bacteria, being more effective against the latter group, including antibiotic-resistant strains such as MRSA or carbapenemase-producing K. pneumoniae. Its mechanism of action consists in the inhibition of the bacterial metabolism of sugars, interfering with their fermentation and transport[21] [60].
There is evidence of the antibacterial capacity of various fungal species against Gram-positive bacteria. Extracts of Ganoderma lucidum, Ganoderma applanatum, Meripilus giganteus, Laetiporus sulphureus, Flammulina velutipes, Coriolus versicolor, Pleurotus ostreatus and Panus tigrinus demonstrated antimicrobial activity in Kirby–Bauer assays against Gram-positive bacteria, such as S. auerus and B. luteus[22] [61].
2.4. Plant origin
Plants are a great source of biomolecules with various interesting properties for humans thanks to their enormous diversity and proven safety for human health[23] [65]. Being sessile organisms, evolution has shaped its metabolism to produce certain molecules to cope with external aggressions and infections, since they cannot flee or defend themselves[24] [66]. The Dictionary of Natural Products lists approximately 200,000 secondary plant metabolites, of which 170,000 have unique chemical structures[25] [67]. Some of the families of molecules with antimicrobial capacity produced by plants are alkaloids, terpenoids, and polyphenols[26] [68].
Plants that have been used in traditional medicine in various countries of the world for thousands of years. They are currently being studied at the molecular and functional level, rediscovering their properties and explaining their mechanisms of action.
Alkaloids have been shown to possess antimicrobial capacity against various bacterial species. Although studies of the antimicrobial capacity of pure alkaloids are limited, there are several studies on the antimicrobial activity of plant extracts that contain alkaloids as their main components. Different extracts rich in alkaloids obtained from Papaver rhoeas have shown activity against S. aureus, Staphylococcus epidermidis and K. pneumoniae, the main active component being roemerine[27] [69]. Raw alkaloid-rich extracts of Annona squamosa seeds and Annona muricata root have also shown moderate antimicrobial capacity against E. coli and S. aureus[28]. [70].
Terpenoids, along with other families of compounds, are part of plant essential oils, many of which possess antimicrobial activity. Various in vitro studies affirm that terpenoids do not possess significant antimicrobial activity per se[29] [71]. However, they can contribute to the antimicrobial activity of complete essential oils thanks to their hydrophobic nature and a low molecular weight that allow them to disrupt the cell wall and facilitate the action of the rest of the active components[30] [72].
Polyphenols are molecules present in plants with a function of defense against stress and have one or more phenolic groups in their chemical structure as a common feature. There is abundant literature on the antimicrobial capacity of polyphenols and extracts of plants rich in them that have bactericidal and bacteriostatic capacity against many pathogens, both Gram-positive and Gram-negative. The potential use of polyphenols as antimicrobials is widely studied to be applied in different areas such as agriculture[31] [73], food preservation[32] [74] and medicine[33] [75].
There are several subfamilies within the group of polyphenols according to their differentiated chemical structures: flavonoids, hydrolyzable tannins, lignans, phenolic acids and stilbenes. In turn, the flavonoid group can be subdivided into other subfamilies: anthocyanidins, flavanones, flavones, flavonols and isoflavones[34] [76]. Examples of flavonoids with antimicrobial activity are quercetin[35] [77], kaempferol[36] [78], morin[37] [79], myricetin[38] [80] epigallocatechin gallate[39] [81] or galangin[40] [82] among many others[34][41] [76,83]. Other known polyphenols with good antimicrobial activity are punicalagin, which exerts both antibacterial and antibiofilm effect against S. aureus[38][42] [80,84], and resveratrol, which has antimicrobial activity against a wide range of bacteria[33] [75].
In addition to synergy with antibiotics, there are also studies that point to the synergy between the polyphenols themselves, such as that between EGCg and quercetin against MRSA, attributed to a co-permeabilization process that would facilitate the activity of the compounds inside of the cell[43] [89]. Synergic activity has also been found between the polyphenols quercetin-3-glucoside, punicalagin, ellagic acid and myricetin in different proportions and combinations against S. aureus CECT 59[38] [80].
Apart from the antimicrobial use of concrete molecules of plant origin, the use of complex extracts made from different parts of plants is common and effective. Plant extracts have a great diversity in their composition, since even from the same plant multiple completely different extracts can be obtained varying the extraction conditions. Time, temperature, solvents, pressure and other parameters such as the use of ultrasound or microwave have a huge impact on the final extract composition[44] [90]. There is numerous evidence of the antimicrobial activity of plant extracts[34][45] [76,91] and the synergistic effect that exists between different phytochemicals[38] [80] when acting against different bacteria. An example of a plant extract with potent activity against AMR bacteria are extracts from Lantana camara leaves against clinical isolates of MRSA, Streptococcus pyogenes, VRE, Acinetobacter baumannii, Citrobacter freundii, Proteus mirabilis, Proteus vulgaris and P. aeruginosa[46] [92]. The ethanolic extracts of Anthocephalus cadamba, Pterocarpus santalinus and Butea monosperma Lam. they have also demonstrated antimicrobial activity against MDR clinical isolates of 10 different microbial species: S. aureus, Acinetobacter sp., C. freundii, Chromobacterium violeceum, E. coli, Klebsiella sp., Proteus sp., P. aeruginosa, Salmonella typhi and Vibrio cholerae[47][48] [93,94]. In the case of B. monosperma Lam., antimicrobial activity was also found in the extract made with hot water from leaf.
2.5. Summary
As a summary, Table 1 contains all the NPs mentioned above together with their producing organism, type, target bacteria, mechanism of action, main use and references. Figure 2 shows the main molecular targets of the most relevant antimicrobial NPs.
Table 1. Alphabetically ordered natural products (NPs) with their properties and capabilities. Asterisk (*) means no antimicrobial activity alone.
Natural Product | Productor Organism | Type of Organism | Activity Against | Mechanism of Action | Main Use | Reference | ||||||||||||||
Actinorhodin | Streptomyces coelicolor | Actinomycete | Gram-positive, including multidrug-resistant S. aureus | ROS production inside bacterial cells | Research |
[49] |
[95] |
|||||||||||||
Albomycin | Streptomyces sp. ATCC 700974 | Actinomycete | Gram-negative and Gram-positive, including MRSA | Seryl t-RNA synthetase inhibition | Medicine |
[96,97] |
||||||||||||||
Amphomycin | Streptomyces canus | Actinomycete | Gram-positive, including MRSA, VRE and MDR S. pneumoniae | Inhibition of peptidoglycan and wall teichoic acid biosyntheses | Medicine |
[52] |
[98] |
|||||||||||||
Apramycin | Streptoalloteicus hindustanus | Actinomycete | Gram-negative, including MDR A. baumannii and P. areuginosa | Inhibition of protein synthesis | Veterinary |
[53] |
[99] |
|||||||||||||
Arlomycins | Streptomyces sp. Tü 6075 | Actinomycete | Gram-positive and Gram-negative | Inhibition of type I bacterial signal peptidase | In research for medical use |
[54] |
[100] |
|||||||||||||
Aspergillomarasmine A* | A. versicolor | Fungus | Sensitivizes carbapenem-resistant bacteria | Inhibition of bacterial metallo-β-lactamases | In research for medical use |
[20] |
[59] |
|||||||||||||
Carbomycin | Streptomyces halstedii | Actinomycete | Gram-positive and Mycoplasma | Inhibition of protein synthesis | Medicine |
[55] |
[101] |
|||||||||||||
Cathelicidin-BF | Bungarus fasciatus | Reptile | Mainly Gram-negative, including MDR strains | Damage in microbial cytoplasmic membrane | Research |
[56] |
[41] |
|||||||||||||
CaTx-II | C. adamanteus | Reptile | Gram-positive and Gram-negative | Membrane pore formation and cell wall disintegration | Research |
[57] |
[42] |
|||||||||||||
Cecropin A | Aedes aegypti | Insect | Gram-negative | Disruption of the cytoplasmic membrane | In research for medical use |
[58] |
[102] |
|||||||||||||
Cephalosporin | Cephalosporium acremonium | Fungus | Gram-positive and Gram-negative | Inhibition of cell wall synthesis | Medicine |
[59] |
[103] |
|||||||||||||
Cephamycin C | Streptomyces clavuligerus | Actinomycete | Gram-positive and Gram-negative | Inhibition of cell wall synthesis | Medicine and veterinary |
[60] |
[104] |
|||||||||||||
Chloramphenicol | Streptomyces venezuelae | Actinomycete | Gram-positive and Gram-negative | Inhibition of protein synthesis | Medicine and veterinary |
[61] |
[105] |
|||||||||||||
Chloroeremomycin | Amycolatopsis orientalis | Actinomycete | Gram-positive, including VRE | Inhibition of bacterial cell wall formation | Medicine |
[62] |
[106] |
|||||||||||||
Clavulanic acid* | S. clavuligerus | Actinomycete | Sensitivizes β-lactam-resistant bacteria | β-lactamase inhibitor | Medicine and veterinary |
[63] |
[107] |
|||||||||||||
Clorobiocin | Strteptomyces roseochromogenes | Actinomycete | Gram-positive | Inhibitors of DNA gyrase | Medicine |
[64] |
[108] |
|||||||||||||
Coumermycin | Streptomyces rishiriensis | Actinomycete | Mainly Gram-positive | Inhibition of DNA gyrase | Research |
[109,110] |
||||||||||||||
Dalbavancin | Nonomuraea sp. | Actinomycete | Gram-positive, including MRSA | Inhibition of cell wall synthesis | Medicine |
[67] |
[111] |
|||||||||||||
Daptomycin | Streptomyces roseosporus | Actinomycete | Gram-positive | Inhibition of protein, DNA and RNA synthesis | Medicine |
[68] |
[112] |
|||||||||||||
Epigallocatechin gallate | Abundant in Camellia sinensis | Plant | Gram-positive and Gram-negative | Damage in microbial cytoplasmic membrane | In research for medical use |
[81,113] |
||||||||||||||
Erythromycin | Saccharopolyspora erythraea | Actinomycete | Gram-positive | Inhibition of protein synthesis | Medicine |
[70] |
[114] |
|||||||||||||
Fosfomycin | Streptomyces wedmorensis | Actinomycete | Gram-positive and Gram-negative | Inhibition of cell wall synthesis | Medicine |
[71] |
[115] |
|||||||||||||
Fusidic acid | Fusidium coccineus | Fungus | Gram-positive, including MRSA | Inhibition of protein synthesis | Medicine |
[72] |
[116] |
|||||||||||||
Gentamicin | Micromonospora purpurea | Actinomycete | Gram-negative | Inhibition of protein synthesis | Medicine |
[73] |
[117] |
|||||||||||||
Gramicidin S | B. subtilis | Bacillales | Gram-positive and Gram-negative | Delocalizes peripheral membrane proteins involved in cell division and cell envelope synthesis | Medicine |
[74] |
[118] |
|||||||||||||
Hc-CATH | Hydrophis cyanocinctus | Reptile | Gram-positive and Gram-negative | Damage in microbial cytoplasmic membrane | Research |
[75] |
[119] |
|||||||||||||
Hygromycin | Streptomyces hygroscopicus | Actinomycete | Gram-positive | Inhibition of protein synthesis | Veterinary and research |
[76] |
[120] |
|||||||||||||
Josamycin | Streptomyces narbonensis | Actinomycete | Gram-positive, certain Gram-negative and mycoplasma | Inhibition of protein synthesis | Medicine |
[77] |
[121] |
|||||||||||||
Kanamycin | Streptomyces kanamyceticus | Actinomycete | Mainly Gram-negative and certain Gram-positive | Inhibition of protein synthesis | Medicine |
[78] |
[122] |
|||||||||||||
Kirromycin | Streptomyces collinus | Actinomycete | Anaerobes, neisseriae and streptococci | Inhibition of protein synthesis | Research |
[123,124] |
||||||||||||||
Lincomycin | Streptomyces lincolnensis | Actinomycete | Gram-positive | Inhibition of protein synthesis | Medicine |
[81] |
[125] |
|||||||||||||
Lipiaramycin | Dactosporangium aurantiacum | Actinomycete | Gram-positive and Mycobacterium, including MDR strains | Inhibition of early transcription | Medicine |
[82] |
[126] |
|||||||||||||
Melittin | A. mellifera | Insect | Gram-positive and Gram-negative, including MDR strains | Damage in microbial cytoplasmic membrane | Medicine |
[83] |
[39] |
|||||||||||||
Mirandamycin | Endophytic fungus isolated from the twig of Neomirandea angularis | Fungus | Gram-negative and Gram-positive, including MRSA | Inhibition of bacterial quinol oxidase/ROS production | In research for medical use |
[21] |
[60] |
|||||||||||||
Moenomycin | Streptomyces ghanaensis | Actinomycete | Gram-positive | Inhibition of cell wall synthesis | Veterinary |
[84] |
[127] |
|||||||||||||
Morin | Moraceae family | Plant | Gram-positive and Gram-negative | Inhibition of adhesion to host tissue and DNA helicase | Food technology |
[37] |
[79] |
|||||||||||||
Mucroporin | Lychas mucronatus | Arachnid | Gram-positive and Gram-negative, including MDR strains | Damage in microbial cytoplasmic membrane | Research |
[85] |
[128] |
|||||||||||||
Neomycin | S. fradiae | Actinomycete | Gram-positive and Gram-negative | Inhibition of ribonuclease P | Medicine |
[86] |
[129] |
|||||||||||||
Orthoformimycin | S. griseus | Actinomycete | Gram-positive and Gram-negative | Inhibition of protein synthesis | In research for medical use |
[18] |
[49] |
|||||||||||||
Oxytetracycline | Streptomyces rimosus | Actinomycete | Gram-positive and Gram-negative | Inhibition of protein synthesis | Aquaculture |
[87] |
[130] |
|||||||||||||
Penicillins | Penicillium crysogenum | Fungus | Gram-positive and Gram-negative | Inhibition of cell wall synthesis and activation of the endogenous autolytic system | Medicine |
[88] |
[131] |
|||||||||||||
Pleuromutalin | Clitopilus scyphoides | Fungus | Gram-positive, Gram-negative and Mycoplasma | Inhibition of translation | Veterinary |
[89] |
[132] |
|||||||||||||
Polymyxin | Paenibacillus polymyxa | Bacillales | Mainly Gram-negative (including MDR) and certain Gram-positive | Disruption of the cytoplasmic membrane | Medicine |
[90] |
[133] |
|||||||||||||
Pristinamycin | Streptomyces pristinaespiralis | Actinomycete | Gram-positive, including MRSA | Inhibition of protein synthesis | Medicine |
[91] |
[134] |
|||||||||||||
Punicalagin | Abundant in Punica granatum | Plant | Gram-positive and Gram-negative | Damage in microbial cytoplasmic membrane | Food technology |
[80,84] |
||||||||||||||
Quercetin | Ubiquitous in plants | Plant | Gram-positive and Gram-negative | Damage in the structure of the bacterial cell wall and cell membrane | In research for medical use |
[92] |
[135] |
|||||||||||||
Ramoplanin | Actinoplanes sp. ATCC 33076 | Actinomycete | Gram-positive, including MDR strains | Inhibition of cell wall synthesis | Medicine |
[93] |
[136] |
|||||||||||||
Resveratrol | Abundant in grapes, berries and legumes | Plant | Gram-positive and Gram-negative, including MDR strains | Inhibition of motility, adhesion, quorum sensing, biofilm formation, flagellar gene expression and hemolytic activity | Medicine |
[33] |
[75] |
|||||||||||||
Rifamycin | Amycolatopsis mediterranei | Actinomycete | Gram-positive and certain Gram-negative | Inhibition of DNA-dependent RNA synthesis | Medicine | [94] | [137] |
|||||||||||||
Ristocetin A | A. orientalis | Actinomycete | Gram-positive, including MRSA | Inhibition of cell wall synthesis | Medicine |
[95] |
[138] |
|||||||||||||
Royalisin | Apis melifera | Insect | Mainly gram-positive | Damage in the structure of the bacterial cell wall and cell membrane | Research |
[96] |
[37] |
|||||||||||||
Skyllamycins | Streptomyces sp. KY 11784 | Actinomycete | Gram-positive | Inhibition of biofilm formation | In research for medical use |
[97] |
[139] |
|||||||||||||
SlLebocin1 | Spodoptera litura | Insect | Gram-positive and Gram-negative | Damage in microbial cytoplasmic membrane or cell division inhibition | Research |
[98] |
[140] |
|||||||||||||
Spectinomycin | Streptomyces spectabilis | Actinomycete | Gram-positive and Gram-negative | Inhibition of protein synthesis | Medicine |
[99] |
[141] |
|||||||||||||
Spiramycin | Streptomyces ambofaciens | Actinomycete | Gram-positive and Gram-negative | Inhibition of protein synthesis | Medicine |
[100] |
[142] |
|||||||||||||
Streptothricin | Streptomyces (multiple species) | Actinomycete | Gram-positive and Gram-negative | Inhibition of protein synthesis | Veterinary and plant production |
[101] |
[143] |
|||||||||||||
Teicoplanin | Actinoplanes teichomyceticus | Actinomycete | Gram-positive, including MRSA | Inhibition of bacterial cell wall synthesis | Medicine |
[102] |
[144] |
|||||||||||||
Teixobactin | Eleftheria terrae | Betaproteobacteria | Gram-positive, including MRSA | Causes digestion of the cell wall by autolysins | Medicine |
[103] |
[55] |
|||||||||||||
Tetracycline | Streptomyces rimosus | Actinomycete | Gram-positive and Gram-negative | Inhibition of protein synthesis | Medicine |
[104] |
[145] |
|||||||||||||
Thienamycin | Streptomyces cattleya | Actinomycete | Gram-positive and Gram-negative | Inhibition of bacterial cell wall synthesis | Derivates used in medicine | [105] | [146] |
|||||||||||||
Thiostrepton | Streptomyces azureus | Actinomycete | Gram-positive and Gram-negative | Inhibition of protein synthesis | Veterinary and research |
[106] |
[147] |
|||||||||||||
Tobramycin | Streptoalloteicus hindustanus | Actinomycete | Gram-negative | Inhibition of protein synthesis and membrane destabilization | Medicine |
[107] |
[148] |
|||||||||||||
Tunicamycin | Streptomyces chartreusis | Actinomycete | Gram-positive | Inhibition of peptidoglycan and lipopolysaccharide synthesis | Research |
[108] |
[149] |
|||||||||||||
Tylosin | S. fradiae | Actinomycete | Gram-positive and Mycoplasma | Inhibition of protein synthesis | Veterinary |
[109] |
[150] |
|||||||||||||
Vancomycin | S. orientalis | Actinomycete | Gram-positive, including MRSA | Inhibition of bacterial cell wall synthesis | Medicine |
[14] |
[45] |
|||||||||||||
Vejovine | V. mexicanus | Arachnid | Gram-negative, including MDR | Damage in microbial cytoplasmic membrane | Research |
[110] |
[44] |
|||||||||||||
Viomycin | Streptomyces sp. 11861 | Actinomycete | MDR Mycobacterium | Inhibition of protein synthesis | Medicine |
[111] |
[151] |
|||||||||||||
Virginiamycin | Streptomyces virginiae | Actinomycete | Gram-positive | Inhibition of protein synthesis | Agriculture and industry |
[112] |
[152] |
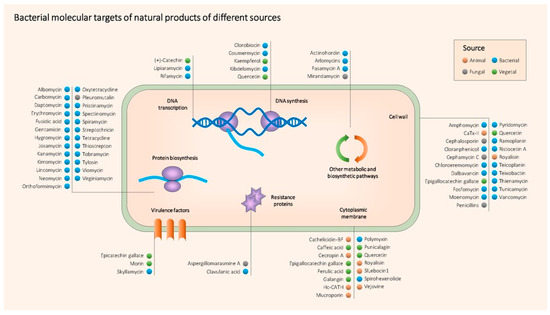
Figure 2. Main known molecular targets of antimicrobial NPs described in this entry.
